Abstract
Mesial temporal lobe epilepsy (MTLE) is a common medically refractory neurological disease. Deep brain electrical stimulation (DBS) of grey matter has been used for MTLE with limited success. However, stimulation of a white matter tract connecting the hippocampi, the ventral hippocampal commissure (VHC), with low frequencies that simulate interictal discharges has shown promising results, with seizure reduction greater than 98% in bilateral hippocampi during stimulation and greater than 50% seizure reduction in bilateral hippocampi after treatment. A major hurdle to the implementation and optimization of this treatment is that the mechanisms of seizure reduction by low frequency electrical stimulation (LFS) are not known. The goal of this study is to understand how commissural fibre tract stimulation reduces bilateral hippocampal epileptic activity in an in vitro slice preparation containing bilateral hippocampi connected by the VHC. It is our hypothesis that electrical stimuli induce hyperpolarization lasting hundreds of milliseconds following each pulse which reduces spontaneous epileptic activity during each inter-stimulus interval (ISI). Stimulus-induced long-lasting-hyperpolarization (LLH) can be mediated by GABAB inhibitory post-synaptic potentials (IPSPs) or slow after-hyperpolarization (sAHP). To test the role of LLH in effective bilateral seizure reduction by fibre tract stimulation, we measured stimulus-induced hyperpolarization during LFS of the VHC using electrophysiology techniques. Antagonism of the GABAB IPSP and/or sAHP diminished stimulus-induced hyperpolarization concurrently with LFS efficacy (greater than 50% reduction). Blocking both the GABAB IPSP and sAHP simultaneously eliminated the effect of electrical stimulation on seizure reduction entirely. These data show that LFS of the VHC is an effective protocol for bilateral hippocampal seizure reduction and that its efficacy relies on the induction of long-lasting hyperpolarization mediated through GABAB IPSPs and sAHP. Based on this study, optimization of the timing of LFS and LFS-induced-LLH may lead to improved outcomes from DBS treatments for human epilepsy.
Key points
Deep brain electrical stimulation (DBS) is a promising treatment for mesial temporal lobe epilepsy (MTLE). However, treatment optimization and clinical application are limited by the fact that the mechanisms of seizure reduction by electrical stimulation remain unknown.
We have shown that low frequency electrical stimulation (LFS) of a white matter target connecting the hippocampi effectively reduces chemically induced epileptic activity in bilateral hippocampi.
LFS induces long-lasting hyperpolarization (1–2 s) in the inter-stimulus interval that protects cells from seizure activity.
This long-lasting hyperpolarization is mediated by (1) GABAB IPSPs and (2) the slow afterhyperpolarization (sAHP). Its magnitude, as measured by amplitude and area, is correlated with LFS efficacy of seizure reduction.
Understanding the mechanisms of LFS could have therapeutic applications for seizure reduction in patients with MTLE.
Introduction
Mesial temporal lobe epilepsy (MTLE), which is characterized by seizures of the hippocampus and surrounding structures, is very common and often refractory to medical treatment with drug therapy (King et al. 1995; Swanson, 1995; Barbarosie & Avoli, 1997; Jallon, 1997; Calcagnotto et al. 2000; Avoli et al. 2002; Spencer, 2002; Duncan et al. 2006). Surgical resection offers better results, rendering 65–75% of selected patients free of seizures. However, many patients do not meet selection criteria and removal of important brain tissues is of concern (Blume, 2006; Blume & Parrent, 2006). Deep brain electrical stimulation (DBS) provides a less invasive, reversible and customizable alternative to surgical resection (Velasco et al. 2000a, b; Durand & Bikson, 2001; Vonck et al. 2002, 2005; Kakiuchi et al. 2006; Morrell, 2006, 2011; Boon et al. 2007; Sunderam et al. 2010). Specifically, low-frequency electrical stimulation (LFS) at frequencies mimicking interictal frequencies has been shown to decrease neural excitability in vitro (Jerger & Schiff, 1995; Durand & Bikson, 2001; Khosravani et al. 2003; D’Arcangelo et al. 2005; Schiller & Bankirer, 2007; Toprani et al. 2008, 2010; Toprani & Durand, 2013), in animal models (Barbarosie & Avoli, 1997; Avoli, 2001; Velisek et al. 2002; Benabid et al. 2005; Goodman et al. 2005; Kile et al. 2010; Rashid et al. 2011; Tang & Durand, 2012) and in patients (Kinoshita et al. 2005; Kakiuchi et al. 2006; Schrader et al. 2006; Yamamoto et al. 2006). However, this treatment is still recent and its mechanisms of action remain largely unknown. Understanding how DBS reduces seizures could enable safer implementation and patient-specific optimization of this promising new treatment. It is therefore the goal of this study to investigate the mechanisms of seizure reduction by DBS.
To date, the mechanisms to explain the antiepileptic effects of LFS are highly speculative. Several hypotheses have been proposed, including: (1) depression of synaptic excitatory response; (2) potassium-mediated depolarization block; (3) glial–neuronal interplay; (4) decreased excitatory/increased inhibitory synaptic neurotransmission; and (5) reduction in the excitability of neurons (Durand & Bikson, 2001; McIntyre et al. 2004; Schiller & Bankirer, 2007). Many of these hypotheses have been explored, but the mechanism has not been solved. Short-term synaptic depression of excitatory neurotransmission during LFS has been observed in cortical slices (Schiller & Bankirer, 2007) and may occur in the hippocampus as well. Potassium-mediated axonal block has been observed during high frequency stimulation (HFS) of hippocampal fibre tracts (Jensen & Durand, 2007, 2009), but its role in LFS has not been examined. Glial cells are known to interact with neurons during seizure generation (Tian et al. 2005; Lanerolle et al. 2010; Reato et al. 2012). It is possible there is glial–neuronal interplay mediating seizure reduction by LFS as well. There are many reasons to suspect increased inhibition may be important. Enhanced GABA-mediated inhibition has been proposed as a theory to explain the mechanisms of seizure reduction by LFS (Lopez-Meraz et al. 2004; D’Arcangelo et al. 2005), although it has been shown that GABAA is not necessary for seizure reduction by LFS in cortical slices (Schiller & Bankirer, 2007). Reduction in neuronal excitability may occur through stimulation-induced hyperpolarization. Low-frequency stimulation evokes long-lasting hyperpolarization (LLH) on the order of 1–2 s in healthy slices predominantly through two mechanisms: (1) induction of the GABAB IPSPs (Dutar & Nicoll, 1988a, b) and (2) enhancement of the slow afterhyperpolarization (sAHP; Alger & Nicoll, 1980a, b).
Because of the slow time course, the GABAB IPSP shows pronounced summation upon repetitive stimulation, in contrast to the fast GABAA IPSP. GABAB receptors on the postsynaptic neuron are located extrasynaptically. As a result, they are only activated when the concentration of GABA is high enough to ‘spill over’ the synaptic cleft (Dutar & Nicoll, 1988a; Otis & Mody, 1992), which can happen in the following two ways: (1) repetitive activation of the synapse and (2) simultaneous activation of a number of release sites, conditions which are both met by LFS and result in pooling of GABA in the extrasynaptic space. Synchronous rhythmic activity, such as LFS, leads to simultaneous recruitment of pre- and postsynaptic GABAB receptors, thereby sculpting the pattern of activity.
Like the GABAB IPSP, the slow afterhyperpolarization (sAHP) limits the firing frequency of neurons and is responsible for generating spike-frequency adaptation, especially in response to sustained or repetitive depolarization (Madison & Nicoll, 1984; Lancaster & Nicoll, 1987; Storm, 1987; Sah, 1996; Shah & Haylett, 2000; Shah et al. 2001; Villalobos et al. 2004; Vatanparast & Janahmadi, 2009), as occurs with LFS. It is activated by the calcium influx during somatic depolarization (Lancaster et al. 2001), especially by bursts of action potentials, which suggests it could be a prominent factor in mediating the antiepileptic effects of LFS by generating long-lasting hyperpolarization during which further action potentials are less likely.
We previously reported an LFS protocol that reduced bilateral 4-aminopyridine (4-AP)-induced seizure-like activity during and after LFS application in an amplitude- and frequency-dependent manner (Toprani & Durand, 2013). The in vitro study introduced a new slice preparation that maintains anatomical and functional connectivity of the hippocampi solely through an intact ventral hippocampal commissure (VHC) and utilized 4-AP to model MTLE (Tapia & Sitges, 1982; Perreault & Avoli, 1991; Traub et al. 1996). A chronic open-loop LFS protocol implemented at a fixed frequency of 1 Hz, which was determined to be the interictal frequency in this preparation by spectral analysis, was applied to the hippocampal commissures for broad bilateral hippocampal seizure reduction in the previous study. The goal of the current study was to understand the mechanism of bilateral seizure reduction by the published LFS protocol. We approached this by testing several potential mechanisms of seizure reduction by LFS, which can be broadly characterized as: (1) depression of synaptic excitatory response; (2) potassium-mediated depolarization block; (3) glial–neuronal interplay; (4) decreased excitatory/increased inhibitory synaptic neurotransmission; and (5) reduction in the excitability of neurons. In particular, we focused on decreased neuronal excitability by LFS-induction of long-lasting hyperpolarization (LLH) composed of (1) the GABAB IPSP and (2) the sAHP.
Methods
Ethical approval and animal handling
All procedures in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of Case Western Reserve University, Cleveland, OH, USA. Seventy-one Sprague-Dawley rats from Charles River, Wilmington, MA, USA (12–21 days) were used for this study. Animals were housed according to IACUC guidelines. All rats were anaesthetized using ethyl ether or isofluorane before decapitation for brain harvesting.
Functionally connected bilateral hippocampal slice preparation
The brain was removed and placed in cold (3–4°C) oxygenated (O2 95%, CO2 5%) sucrose-rich artificial cerebrospinal fluid (ACSF), consisting of (in mm): 220 sucrose, 3 KCl, 1.25 NaH2PO4, 2 MgSO4, 26 NaHCO3, 2 CaCl2 and 10 g l−1 d-glucose (pH 7.45). The cerebellum was detached and the ventral surface of the brain was secured in a vibrating-blade microtome (VT1000S, Leica, Nussloch, Germany) containing sucrose-based cold, oxygenated ACSF. A previously reported bilateral hippocampal slice preparation was utilized in which the hippocampi, entorhinal cortices (EC; for contribution to seizure generation (Barbarosie & Avoli, 1997) and VHC were preserved after other tissues were carefully dissected away (Toprani & Durand, 2013). Axial slices 750 or 350 μm thick were cut and immediately preserved in oxygenated ACSF consisting of (in mm): 124 NaCl, 3.75 KCl, 1.25 KH2PO4, 2 MgSO4, 26 NaHCO2, 2 CaCl2 and 10 d-glucose for at least 60 min before being transferred to an interface-recording chamber (Harvard Apparatus, Holliston, MA, USA). Slice health was confirmed by extracellular field recordings of evoked potentials from CA3 and CA1 larger than 1 mV for all preparations in ACSF that persist over the course of the experiment. For intracellular recordings, pyramidal cell health was confirmed by comparing recorded cellular properties, including: (1) resting membrane potential; (2) membrane time constant; (3) input resistance; (4) action potential amplitude; and (5) action potential duration to those reported by Buckmaster et al. (1993) for pyramidal cells. Cells were rejected if values for any of these properties were outside of the reported range. Measurements were obtained as described in that paper (Buckmaster et al. 1993). The axonal anatomy of the VHC was verified in all preparations by bilateral evoked potentials elicited by a single stimulus in the VHC tract (Toprani & Durand, 2013). Evoked responses in ACSF had a mean value of 3 mV ± 1.7 mV with a single vertex (n= 20) and were obtained from left and right hippocampi.
Seizure generation
Epileptic activity was generated using 4-AP (100 μm) in ACSF because it enhanced synaptic activity without bias for any neurotransmitter (Tapia & Sitges, 1982; Rutecki et al. 1987; Perreault & Avoli, 1991; Traub et al. 1996). Alternative seizure models including magnesium-free ACSF (Jones, 1989) and bicuculline methiodide (BMI; 10 μm) in ACSF (Borck & Jefferys, 1999) were also used. Effective seizure generation included regular interictal-like and ictal-like waveforms, as quantified previously (Toprani & Durand, 2013), both of which are shown in Fig. 1C. Seizures, or ictal activity, were defined as high-frequency spiking activity (>10 Hz) lasting at least 1 s of variable amplitude. To develop seizures in vitro using only 4-AP without cutting axons that may serve as brakes on seizures but are involved in the circuitry we are studying (Barbarosie & Avoli, 1997) or introducing confounding stimuli, we optimized the experimental protocol to facilitate the generation of electrographic seizures. This was accomplished by: (1) including the adjacent EC, which may play a role in hippocampal seizure development (Barbarosie & Avoli, 1997); (2) using thick (750 μm) slices; (3) using younger, therefore smaller as well as more seizure-prone, animals (12 to 21 days) and, most importantly; (4) maintaining both hippocampi along with their axonal connections to one another to enable interchange as well as reinforcement of epileptic activity.
Figure 1. LFS reduces 4-AP-induced epileptiform activity in a bilateral hippocampi–VHC slice preparation.
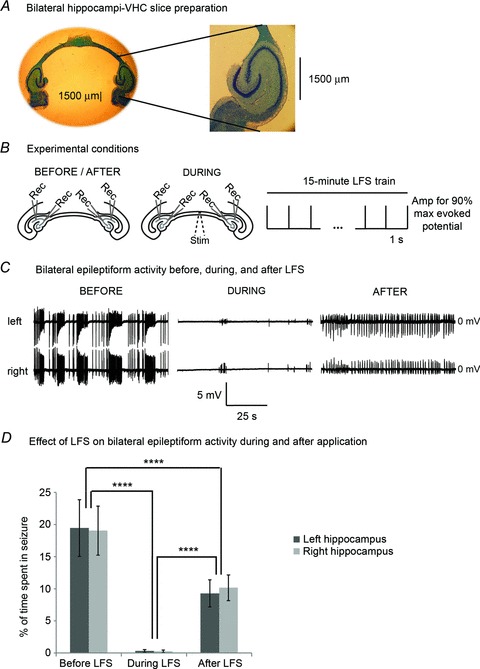
A, an example of a bilateral slice preparation that has been stained with Cresyl Violet for nuclei and Luxol Fast Blue for myelinated axons of the VHC is shown to the left. The demarcation of the VHC axonal tract exiting the fimbria/fornix of the hippocampus is clearly shown on an expanded scale to the right. B, experimental conditions are shown on a schematic diagram of the bilateral slice preparation. Baseline epileptic activity is recorded intracellularly/extracellularly from left and right CA3/CA1 for at least 15 min once a steady state of seizure activity is reached, after which LFS is applied to the VHC for 15 min. Recovery from LFS is recorded for at least 15 min in the same way. C, truncated traces of synchronized left and right recordings before, during and after LFS are shown. D, this LFS protocol significantly reduced ictal epochs by 99 ± 1% and 98 ± 1% in the left and right hippocampi, respectively, during treatment (n= 32). Seizures are significantly reduced by 42 ± 15% on the left and 40 ± 12% on the right after LFS was turned off (P < 0.0001, ANOVA; P < 0.0001, Tukey-Kramer). Bar graphs represent data means ± SD. ****P < 0.0001.
Long-lasting hyperpolarization reduction
Stimulation-induced long lasting hyperpolarization was reduced using pharmacological antagonists of GABAB receptors as well as the sAHP. GABAB antagonists included 2-OH-saclofen (500 μm; Kabashima et al. 1997; Parnas et al. 1999) and CGP-55845-HCl (1 μm), a potent, selective GABAB antagonist (Urwyler et al. 2005; Kirmse & Kirischuk, 2006). The medium after-hyperpolarization (mAHP) and sAHP were blocked non-selectively using clotrimazole (10 μm; Shah et al. 2001). UCL-2077 (10 μm) was used for selective and specific block of the sAHP (Shah et al. 2006).
Electrical recording
Extracellular field recordings were made using glass microelectrodes (1–4 MΩ) filled with 150 mm NaCl. Intracellular sharp electrode recordings were made using glass microelectrodes (90–130 MΩ) filled with 1 m KCl. Orthodromic evoked potentials and/or action potentials from VHC stimulation were recorded in the CA1 stratum pyramidale while antidromic evoked potentials and/or action potential were simultaneously recorded from the CA3 stratum pyramidale. This procedure was described and responses to stimulation of the VHC were shown in our previous work (Toprani & Durand, 2013). All signals were amplified using an Axoclamp-2A microelectrode amplifier (Axon Instruments, Union City, CA, USA), low-pass filtered (5 kHz) and further amplified by a FLA-01 (Cygnus Technology, Southport, NC, USA), then stored on a DT-200 digital tape recorder (Microdata Instrument, South Plainfield, NJ, USA) as well as on computer via an optical data acquisition program (44.1 kHz sampling rate, Audio Companion, Roni Music, http://www.RoniMusic.com).
Stimulation parameters
CA3 and CA1 evoked responses were periodically elicited by stimulation of the VHC using a monopolar tungsten electrode at a rate of 0.33 Hz in monophasic cathodic pulses (100 μs pulse duration, current intensity to elicit 90% of the maximum evoked potential). Pulse trains were generated using a function generator (Grass S88 Stimulator, Grass Technologies, Middleton, WI, USA) and converted to a current by stimulus isolator units (A-M systems, Sequim, WA, USA). The LFS protocol consisted of monophasic cathodic pulses (100 μs pulse duration) at a current intensity to elicit 90% of the maximum evoked potential applied at 1 Hz to the VHC while recording intracellularly or extracellularly from CA3 and CA1. LFS efficacy and cellular responses to different stimulation frequencies (1, 3 and 10 Hz) were compared with and without long-lasting hyperpolarization reduction.
Experimental setup and analysis
To study the contribution of stimulus-induced long-lasting hyperpolarization to the mechanism of seizure reduction by LFS, we quantified the amplitude and area of the long-lasting hyperpolarization evoked by LFS in non-epileptic and epileptic control slices bathed in 100 μm 4-AP. We then applied two different GABAB antagonists (2-OH-saclofen, 500 μm; CGP-55845-HCl, 1 μm) as well as two different sAHP antagonists (clotrimazole 10 μm; UCL-2077 10 μm) or GABAB and sAHP antagonists together and measured the change in each of the following variables compared to non-epileptic (ACSF) as well as epileptic (4-AP) controls: (1) reduction in percentage of seizure time by LFS; (2) distribution of activity in the inter-stimulus interval (ISI); (3) long-lasting hyperpolarization amplitude measured at the negative peak after 100 ms so that GABAA effects are not included; and (4) long-lasting hyperpolarization area. The same variables were quantified for each of the different seizure models (4-AP; low-magnesium ACSF; and BMI) and all of the stimulation frequencies tested. Recordings were obtained from bilateral hippocampi functionally connected by the VHC to ensure observed effects are not localized to one side or focus. All data were analysed using MATLAB (MathWorks, Natick, MA, USA) and Lab Chart v7 (AD Instruments, Dunedin, New Zealand). Stimulus artifacts were removed using a template-subtraction algorithm which subtracts artifacts and evoked responses, but spares non-evoked activity that has been validated and is described in a previous paper (Toprani & Durand, 2013). Data are presented as means ± SD, where n represents the number of slices from different animals. Two-tailed Student's t tests and/or ANOVA (P < 0.05) were applied for statistical comparison. Throughout the text, P values are represented as follows: *represents changes between treatment and control groups such that *indicates P < 0.05; **indicates P < 0.01; ***indicates P < 0.001; and ****indicates P < 0.0001.
Results
LFS of the VHC reduces bilateral 4-AP-induced epileptiform activity
To test the effect of fibre tract stimulation of the VHC on bilateral hippocampal activity simultaneously, we utilized a slice preparation consisting of both hippocampi interconnected by VHC fibres with all other tissues removed (Fig. 1A). The anatomical and functional connectivity of this slice preparation has been verified (Toprani & Durand, 2013). 4-AP (100 μm) was added to ACSF solution and bath applied onto slices to induce reliable hippocampal electrographic seizure-like activity for a minimum of 30 min prior to recording. With a modified protocol to facilitate seizure generation (see Methods), spontaneous epileptic activity was similar in bilateral CA3 and CA1 pyramidal cells and lasted > 8 h. In particular, two types of epileptic activity occurred that were recorded extracellularly as well as intracellularly (Fig. 1C): (1) low frequency (<5 Hz) periodic spikes or bursts that began 10–15 min after bath application of 4-AP with variable field potential amplitudes from 0.5 to 10 mV and frequencies between 0.2 and 5 Hz lasting between 30 and 550 ms and (2) high-frequency oscillations (>10 Hz) of variable amplitude occurring every 1–3 min with each episode lasting 3–60 s that generally occurred regularly 1–3 h after 4-AP application. These events were similar to those reported in Toprani & Durand (2013), which were classified as (1) interictal spikes or bursts and (2) ictal or seizure-like episodes based on their power spectral densities. Since epileptic activity is a property of network interplay, these events were best observed as field potential recordings. Figure 1C shows a typical example of extracellular recordings containing ictal and interictal epochs, which were highly correlated between left and right hippocampi.
This slice preparation consisting of only bilateral hippocampi and ECs connected by the VHC is a suitable model for the analysing the mechanisms of reduction of epileptic activity in bilateral hippocampi with stimulation of their interconnecting axon tract, the VHC, since these crucial structures are maintained without the influence of any other tissue. The stimulus location was chosen for its potential to affect bilateral hippocampal communication (Fig. 1). The frequency was selected based on experiments showing that the average interictal frequency approximates 1 Hz in the in vitro preparations. The duration of the stimulation was set to 15 min based on the observation that seizures occur at every 97 ± 34 s, on average, in this model. The stimulation amplitude was chosen to elicit 90% of the maximum possible evoked response, at which there were no electrophysiological signs of slice deterioration. In this model, 1 Hz VHC stimulation at amplitudes that generated 90% of the maximal orthodromic evoked response (100–500 μA) for 15 min was sufficient to generate seizure reduction in bilateral hippocampi (CA3/CA1) in 32 slices during LFS, as was evident by the diminished spontaneous activity in the traces shown in Fig. 1C after artifact/evoked potential reduction was applied (Tang & Durand, 2012; Toprani & Durand, 2013). The effect persisted after LFS. Interictal activity at the stimulation frequency remained pronounced in the post-stimulation period and seizures were reduced, as shown in the traces. Highly correlated interictal and ictal activity occurred prior to stimulation in left and right hippocampi. During LFS, the percentage of seizure time was reduced by 99 ± 1% in the left hippocampus and 98 ± 1% in the right hippocampus with a lasting post-LFS reduction in percentage seizure time of 42 ± 15% on the left and 40 ± 12% on the right (P < 0.0001, ANOVA; P < 0.0001, Tukey-Kramer; Fig. 1D) that could be sustained without baseline seizure recurrence for hours.
Seizure reduction does not depend on long-term depression or depolarization block
In order to determine if the effect of low frequency stimulation was mediated by long-term depression (LTD), the tissue response to the stimulating pulse was monitored throughout the experiments. Orthodromic evoked field and intracellular responses were elicited by test pulses every 5 s from bilateral CA1 in slices bathed in 4-AP solution prior to starting LFS. During 15 min of 1 Hz stimulation of the VHC at amplitudes that elicited 90% of the maximal evoked response, the amplitude of bilateral orthodromic evoked responses and the number of evoked action potentials in CA1 were monitored for each stimulus pulse as shown by the diagram in Fig. 1B. Evoked potential amplitudes were calculated as the difference between the average potential of points (a, b) and the potential at point (c), as shown in the inset of Fig. 2A. The mean evoked potential amplitude in 4-AP was 5 ± 3.5 mV, which was hyperactive compared to the evoked potential in ACSF with a mean amplitude of 3 ± 1.7 mV. The orthodromic CA1 evoked potential amplitude was normalized per slice and averaged over 19 trials. After removing the first 2 min of stimulation to allow for acclimation as previously described (Toprani & Durand, 2013), there were no significant differences in evoked potential amplitude across the stimulation period (P > 0.99 using ANOVA). Furthermore, the mean evoked potential amplitude during LFS was not significantly different from that prior to LFS (P > 0.5, Student's t test). These results suggest LTD was not induced by the 1 Hz stimulation protocol and is not the mechanism of seizure reduction.
Figure 2. LTP/LTD do not occur in CA1 pyramidal cells during this LFS protocol.
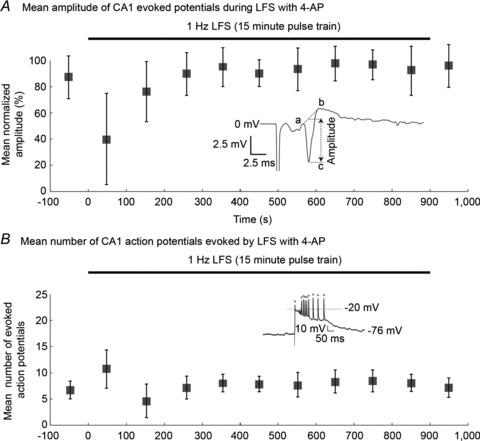
The LFS protocol, applied to the VHC while recording from bilateral CA1 as shown in Fig. 1B, did not induce significant changes in evoked responses (n= 19). Evoked field potentials (A) or action potentials (B) were recorded from CA1 before, during and after 1 Hz LFS in slices with 4-AP. A, the average normalized evoked potential amplitude did not change across the stimulation protocol (P > 0.99, ANOVA). Evoked potential amplitudes were determined as the average potential of (a, b) minus the potential of (c), as shown in the inset. B, similarly, the mean number of action potentials elicited by each electrical stimulus remained relatively consistent throughout the LFS protocol (P= 0.99, ANOVA). Responses to LFS with potential amplitudes above –20 mV following each stimulus were counted as action potentials as shown in the inset. Box plots represent data means ± SD.
We next tested the hypothesis that LFS could cause a depolarizing block by analysing the distribution of action potentials following stimulation using intracellar recording methods. During LFS, an action potential was detected as a post-stimulus peak voltage greater than −20 mV, as shown in the inset of Fig. 2B: 7 ± 2.7 Action potentials were elicited by each stimulus during the LFS train in 4-AP, compared to 2.4 ± 1.2 for an equal stimulus in ACSF. In 4-AP, elicited action potentials occurred atop a paroxysmal depolarizing shift, with return to baseline before the next stimulus. After again allowing 2 min for acclimation to LFS, the mean number of action potentials elicited by each pulse did not show differences throughout the LFS protocol (P= 0.99 using ANOVA). The average number of action potentials evoked during LFS was not significantly different from that prior to LFS initiation (P > 0.5, Student's t test). Action potentials were reliably produced by each stimulus. The mean normalized evoked potentials and number of elicited action potentials did not change throughout LFS treatment in healthy control slices (ACSF) either (data not shown). The steady amplitude of evoked responses throughout the course of LFS (1) demonstrate the bilateral hippocampal slices were viable throughout the entire stimulation period; (2) shows that there was not significant potentiation or depression of the response in this model; and (3) indicates that sustained depolarization that would cause ‘depolarization block’ did not occur in left or right hippocampi with VHC stimulation.
Glial cells do not exhibit voltage shifts in response to LFS
Glial cells can be indicators of cellular ionic changes capable of decreasing neural excitability such as shifts in potassium concentration. Therefore, intracellular recordings were obtained from 10 glial cells bathed in 4-AP (100 μm) before and during a 15 min 1 Hz LFS train. Glial cells were identified by the following three criteria: (1) low resting potential of −85 ± 7 mV; (2) no action potential with 5 nA current injection; (3) no spontaneous firing of action potentials. Simultaneous field potential recordings were obtained from CA1 cell layers as shown in Fig. 3A. Prior to LFS, glial cells showed marked depolarization (27 ± 3.5 mV) that correlated with ictal epochs recorded from field potentials. During LFS, glial cells throughout the bilateral slice preparation exhibited minimal voltage changes (2 ± 1.3 mV). An example of this is shown in Fig. 3B and data from 10 slices are summarized in Fig. 3C. The glial cell responses, measured as voltage change (in mV), during epilepsy and LFS were compared. There were significant differences (P < 0.0001, Student's paired t test) between the glial cell responses to epilepsy and to LFS. Hyperactive evoked field potentials typical of 4-AP were reliably recruited by each stimulus as an indicator of current injection during LFS. The lack of appreciable glial cell responses during LFS does not support large underlying potassium shifts or glial-neural signalling as the major contributors to the mechanism of seizure reduction by LFS, although they cannot be ruled out by this analysis.
Figure 3. Glial cells do not exhibit voltage shifts in response to LFS.
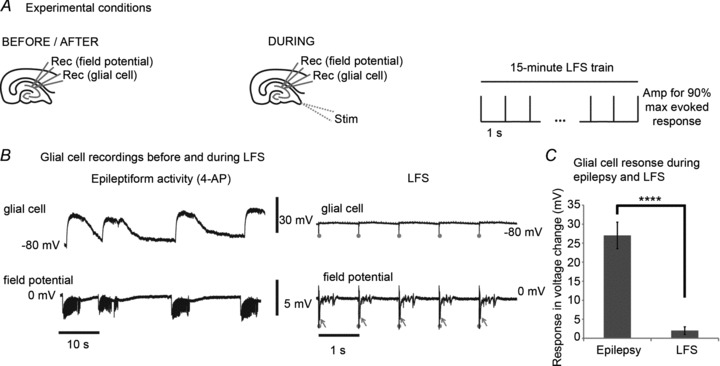
A, intracellular recordings were obtained from 10 glial cells bathed in 4-AP (100 μm) before and during a 15 min 1 Hz LFS train. Simultaneous field potential recordings were obtained from CA1. B, prior to LFS, glial cells showed marked depolarization that correlated with ictal epochs recorded from field potentials. During LFS, glial cells did not exhibit appreciable voltage changes. In contrast, hyperactive evoked field potentials that are typical in 4-AP were reliably recruited by each stimulus. Stimuli are marked by grey dots and the evoked potentials are shown by arrows. C, the glial cell responses, measured as voltage change in mV, during epilepsy and LFS are compared. There is a significant difference (P < 0.0001, Student's paired t test) between the glial cell response to epilepsy and to LFS. Bar graphs represent data means ± SD. ****P < 0.0001.
Effective LFS changes the distribution of inter-stimulus activity
Next, we looked at how LFS affected the distribution of epileptic activity. Extracellularly recorded CA1 signals from 20 slices that received 15 min of 1 Hz LFS were divided into sections containing only ictal epochs, only interictal epochs, or activity recorded during LFS. Examples of each are shown in Fig. 4. Samples of 50 s of each type of activity were randomly selected from all 20 slices to assess how epileptic activity was distributed across 1 s intervals, representative of the inter-stimulus interval, with and without LFS. Peaks were detected in each case if they were greater than 1 standard deviation from the mean amplitude. Time was counted from the first detected peak of a seizure or interictal burst per recording in non-stimulated segments and from the first stimulus artifact during LFS. The time distribution of peaks across a 1 s interval was plotted for 1000 s of ictal-only recordings, interictal-only recordings and LFS recordings, respectively, taken across slices. In the absence of LFS, spontaneous 4-AP-induced epileptic activity (ictal or interictal) appeared evenly distributed in time. Indeed, there were no significant differences in number of ictal or interictal peaks across four equal time blocks of the ISI (P > 0.05, ANOVA; Fig. 4B). In contrast, LFS entrained activity, as evidenced by minimization of non-evoked activity during stimulation (bottom panel of Fig. 4B). During LFS, most peaks occurred during the first part of the ISI (P < 0.0001, ANOVA; post hoc analysis using Tukey-Kramer). In combination with the data presented in Fig. 1 and 2, this analysis showed that the LFS protocol significantly reduced epileptic activity in bilateral hippocampi and that the pattern of activity was significantly affected during LFS such that a bursting evoked response occurred after each stimulus while non-stimulated field potentials were minimized. These results suggest the existence of an inhibitory period following the evoked responses to each stimulus during LFS. To further study this phenomenon, two additional seizure models were utilized to evaluate seizure reduction by LFS.
Figure 4. LFS changes the distribution of inter-stimulus activity.
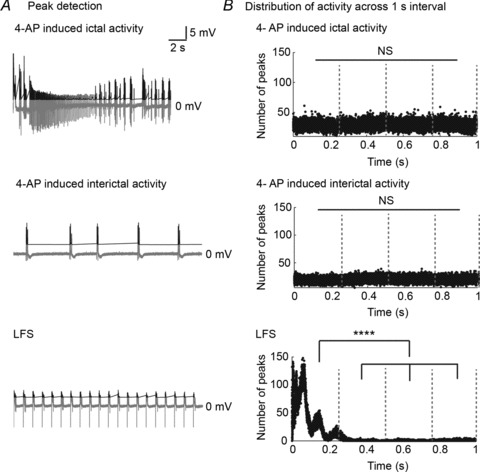
Epileptic activity is recorded extracellularly from left and right CA3/CA1 after which LFS is applied to the VHC (15 min each) as shown in Fig. 1B. A, 20 s of a 4-AP-induced ictal epoch (top panel), interictal epoch (middle panel) and LFS (bottom panel) are shown (grey traces). Field potential peaks were detected in each case if they were greater than 1 standard deviation from the mean height, as shown (black traces). B, the distribution of peaks across a 1 s interval from 1000 s in total (50 s randomly selected from 20 slices) of ictal recordings (top panel), interictal recordings (middle panel) and recordings during 1 Hz LFS (bottom panel) is shown. Sorting into 1 s intervals begins at the first ictal or interictal spike detected, respectively, or at the stimulus artifact, where applicable. There is no significant difference in number of ictal or interictal peaks across four equal time-blocks of the ISI (P > 0.05, ANOVA). During LFS, more peaks occur during the first part of the ISI (P < 0.0001, ANOVA; post hoc analysis using Tukey-Kramer). ****P < 0.0001.
Redistribution of inter-stimulus activity by LFS is robust in several models of epilepsy
Previously, we showed that reduction of epileptiform activity by the described LFS protocol was robust in three seizure models (n= 14, 9, 5, respectively): 4-AP; bicuculline methiodide (BMI); and magnesium-free ACSF (Toprani & Durand, 2013). For this study, we asked what could be learned about the mechanism of LFS given that it effectively reduced seizures generated by these distinct methods. To answer this question, we analysed the distribution of epileptic activity during the ISI as described above for the 4-AP seizure model. The distribution of peaks across the 1 s ISIs from 1000 s (50 s randomly selected from each slice) of recordings during 1 Hz LFS in BMI and magnesium-free LFS are shown (Fig. 5B). Sortation into 1 s intervals is from each stimulus artifact. During LFS, the majority of peaks occurred in the first half of the ISI in all three epilepsy models in which LFS was effective (P < 0.0001, ANOVA; post hoc analysis using Tukey-Kramer). There were no significant differences between percentage of total peaks per half of the ISI between the three different epilepsy models (P > 0.5, ANOVA). Therefore, the percentages of total peaks per half of the ISI were averaged across models and compared (Fig. 5C). On average across models, 99 ± 0% of peaks occurred within the first half of the ISI in these seizure models. This was significantly different from the 1 ± 1% of peaks that occurred in the second half of the ISI during LFS in these models (****P < 0.0001, Student's t test). Taken together, these data suggest that effective LFS changes the distribution of inter-stimulus activity such that there is a large evoked response directly following stimuli that is followed by a period of inhibition which is independent of GABAA mechanisms.
Figure 5. LFS changes the distribution of inter-stimulus activity in several epilepsy models.
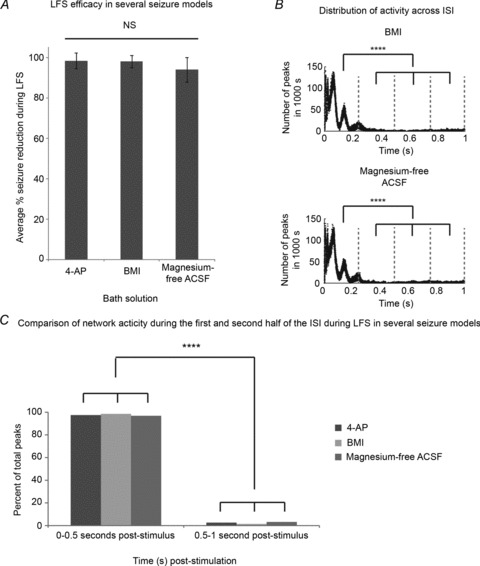
Trains of 15 min 1 Hz LFS were applied to bilateral hippocampi–VHC slices after recording baseline epileptic activity for at least 15 min as shown in Fig. 1B in either 4-AP (100 μm), bicuculline methiodide (BMI, 10 μm), or magnesium-free ACSF. A, LFS efficacy is not different in three distinct seizure models (P= 0.682, ANOVA). Seizure reduction during electrical stimulation across groups is distributed as follows (n= 14, 9 and 5, respectively): 97%± 8% in 100 μm 4-AP; 97%± 6% in 10 μm BMI; and 94%± 12% in magnesium-free ACSF. B, the distribution of peaks across the 1 s inter-stimulus intervals (ISI) from 1000 s (50 s randomly selected from 20 slices) of recordings during 1 Hz LFS in BMI (top panel) and magnesium-free LFS (bottom panel) are shown. Sorting into 1 s intervals begins at each stimulus artifact. During LFS, more peaks occur during the first part of the ISI (P < 0.0001, ANOVA; post hoc analysis using Tukey-Kramer) in each of these seizure models. C, the percentage of total peaks that occur in the first and second half of the ISI during 1 Hz LFS is shown for each of the three distinct epilepsy models. There are no significant differences between percentage of total peaks per half of the ISI between the three different epilepsy models (P > 0.5, ANOVA). Therefore, the percentages of total peaks per half of the ISI were averaged across models and compared. On average across models, 99 ± 0% of peaks occur within the first half of the ISI in these seizure models where LFS is effective. This is significantly different from the 1 ± 1% of peaks that occur in the second half of the ISI during LFS in these models (P < 0.0001, Student's t test). ****P < 0.0001.
LFS induces hyperpolarization during application
The results so far indicate the existence of an inhibitory period following the evoked potentials of each LFS stimulus. It is our hypothesis that the inhibitory window is due to membrane hyperpolarization induced by LFS. While applying 1 Hz LFS trains to the VHC of bilateral hippocampi–VHC slices bathed in 4-AP, intracellular recordings were obtained from bilateral CA1 before, during and after 15 min of LFS. 60 s of representative activity recorded intracellularly from CA1 in 4-AP before (left), during (middle) and after (right) LFS are shown in Fig. 6A. (A 1 s trace during LFS in 4-AP is shown in Fig. 8A.) Hyperpolarization of the cell membrane occurred following stimulation pulses during LFS. The minimum membrane voltages (Vm) within 1 s time windows were determined before, during and after LFS (15 min each) for each sample (n= 18) and are shown across the entire 45 min experiment period for 1 slice in Fig. 6B. The minimum voltage per stimulus occurred, on average, 560 ± 200 ms after each electrical pulse. The mean ± SD of minimum Vm was determined every 3 min before, during and after LFS (15 min each) for each slice and averaged across 18 slices. A decrease in mean minimum Vm approximating 10 mV occurred during LFS compared to baseline. Indeed there were significant differences in the average minimum Vm between groups (P < 0.005 using ANOVA). Post hoc analysis using the Tukey-Kramer test showed that average minimum Vm was reduced during LFS, but was the same before and after LFS (LSD = 5.37, P < 0.05 and 7.19, P < 0.01). Several points can be made about the measured hyperpolarization: (1) it was induced by electrical stimulation; (2) its duration was > 500 ms, with a peak at around 500 ms; (3) hyperpolarization occurred for as long as LFS was applied; (4) it was widespread, occurring in several hippocampal subfields bilaterally.
Figure 6. LFS induces hyperpolarization during application.
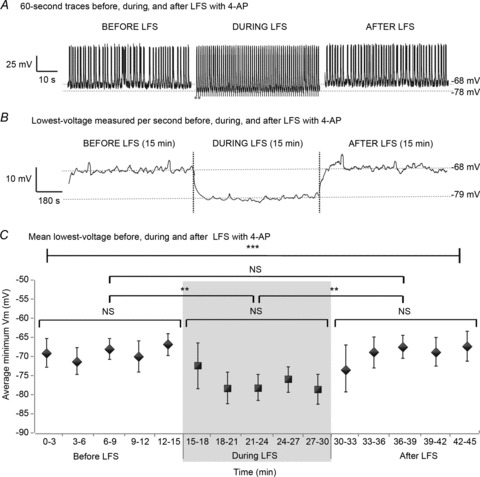
Trians of 15 min 1 Hz LFS were applied to bilateral hippocampi–VHC slices after recording baseline epileptic activity for at least 15 min as shown in Fig. 1B in 4-AP (100 μm). A, 60 s of representative activity recorded intracellularly from CA1 in 4-AP before (left), during (middle) and after (right) LFS are shown. Stimuli are marked by grey dots. During LFS, cells appear to reach more negative voltages than at baseline. B, the minimum Vm per second was determined before, during and after LFS (15 min each) for each sample (n= 18) and is shown across the entire 45 min experiment period for 1 slice. C, the mean ± SD of minimum Vm before, during and after LFS (15 min each) was determined every 3 min for each slice and averaged across 18 slices. There is a significant difference in the average minimum Vm during stimulation compared to before or after LFS (P < 0.005, ANOVA). Post hoc analysis using the Tukey test shows that average minimum Vm is reduced during LFS, but is the same before and after LFS (LSD = 5.37, P < 0.05 and 7.19, P < 0.01). Box plots represent data means ± SD. **P < 0.01; ***P < 0.001.
Figure 8. GABAB/sAHP antagonists decrease stimulus-induced long-lasting hyperpolarization in epileptic slices.
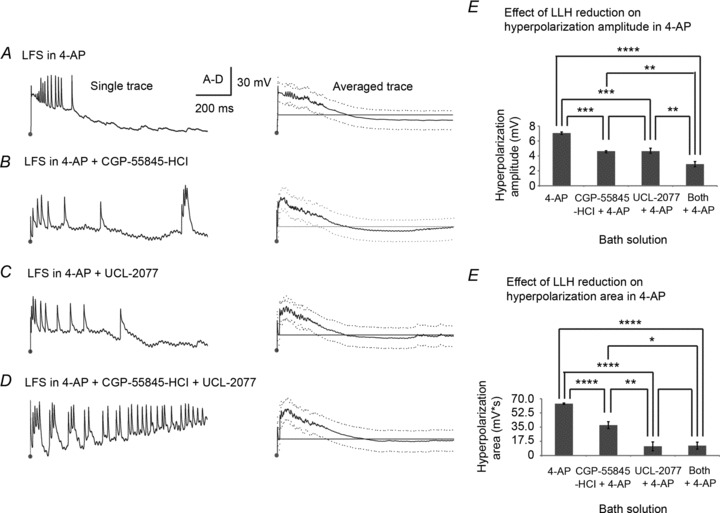
LFS (1 Hz) was applied to the VHC of bilateral hippocampi–VHC slices while recording intracellularly from bilateral CA3/CA1 pyramidal cells as shown in Fig. 7A in either (1) 4-AP (n= 4); (2) CGP-55845-HCl (1 μm) in 4-AP (n= 3); (3) UCL-2077 (10 μm) in 4-AP (n= 3); or (4) both CGP-55845-HCl + UCL-2077 in 4-AP (n= 3). A–D, a single trace showing evoked as well as non-evoked activity that occurs in the inter-stimulus interval (ISI) during 1 Hz electrical stimulation in each solution is shown in the left column. Traces are 1 s with stimulation marked by dots. Activity in the ISI is averaged across slices for each solution. The average (continuous line) ± SD (dotted line) is shown in the right column with the sample mean resting potential in grey. E, peak hyperpolarization amplitudes in each solution are measured as the lowest voltage of the averaged signal in the ISI after 100 ms per slice minus the resting potential of that cell. The mean hyperpolarization amplitudes across slices per solution are: (1) 7.1 ± 0.3 mV; (2) 4.6 ± 0.3 mV; (3) 4.7 ± 0.7 mV; and (4) 2.9 ± 0.7 mV for (1) 4-AP (n= 4); (2) CGP-55845-HCl in 4-AP (n= 3); (3) UCL-2077 in 4-AP (n= 3); and (4) both CGP-55845-HCl + UCL-2077 in 4-AP (n= 3), respectively. Hyperpolarization amplitude differs across groups (P < 0.0001, ANOVA, LSD (Tukey-Kramer) at P < 0.01 = 0.79 mV). F, hyperpolarization area is measured per solution as the sum of the averaged voltage potentials lower than the resting potential in the ISI minus the resting potential of that cell. The mean hyperpolarization areas across slices per solution are: (1) 63.8 ± 1.7 mV s; (2) 37.3 ± 8.7 mV s; (3) 12.1 ± 11.0 mV s; and (4) 11.3 ± 8.7 mV s for (1) 4-AP; (2) CGP-55845-HCl in 4-AP; (3) UCL-2077 in 4-AP; and (4) CGP-55845-HCl + UCL-2077 in 4-AP, respectively. There are significant differences in hyperpolarization area between groups (P < 0.0001, ANOVA, LSD (Tukey-Kramer) at P < 0.01 = 9.35 mV s). Bar graphs represent data means ± SD. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
LLH reduction by GABAB/sAHP antagonists globally decreases stimulus-induced hyperpolarization during LFS in non-epileptic slices
Hyperpolarization occurred during LFS in this model (Fig. 6) and may mediate the period of inhibition following each stimulus pulse that prevented spontaneous epileptic activity from occurring (Fig. 4 and 5). We have already excluded the role of fast hyperpolarization mediated by GABAA as underlying seizure reduction by LFS (Fig. 5). We next focused on mechanisms of slow or long-lasting hyperpolarization (LLH) that might be activated throughout bilateral hippocampi by LFS to facilitate seizure reduction during stimulation. Leading candidates for LFS-induced LLH on a time scale of >500 ms include (1) the GABAB–mediated slow IPSP and (2) the sAHP. We analysed the mechanism behind LFS-induced hyperpolarization by antagonizing each of these pathways separately as well as together in the bilateral hippocampi–VHC slice preparation. To accomplish this, CGP-55845-HCl (1 μm) and UCL-2077 (10 μm) were used to selectively and specifically inhibit the GABAB IPSP and sAHP, respectively. We first studied their effects on stimulation-induced hyperpolarization in non-epileptic slices bathed in ACSF (Fig. 7) and then in our standard seizure model, 4-AP (Fig. 8).
Figure 7. GABAB/sAHP antagonists decrease stimulus-induced long-lasting hyperpolarization in non-epileptic slices.
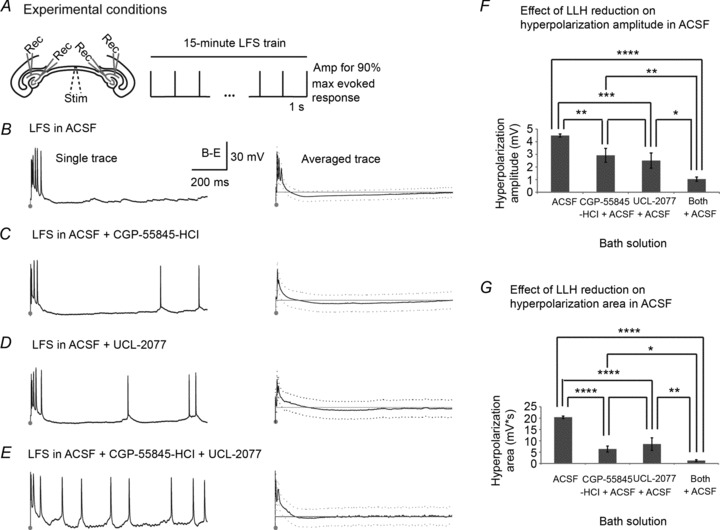
A, 1 Hz LFS was applied to the VHC of bilateral hippocampi–VHC slices while recording intracellularly from bilateral CA3/CA1 pyramidal cells in either (1) ACSF (n= 7); (2) CGP-55845-HCl (1 μm) in ACSF (n= 8); (3) UCL-2077 (10 μm) in ACSF (n= 7); or (4) both CGP-55845-HCl + UCL-2077 in ACSF (n= 6). B–E, a single trace showing evoked as well as non-evoked activity that occurs in the inter-stimulus interval (ISI) during 1 Hz electrical stimulation in each solution is shown in the left column. Traces are 1 s with stimulation marked by dots. For each solution, averaged traces (continuous line) ± SD (dotted line) with sample mean resting potentials (grey line) are shown in the right column. F, peak hyperpolarization amplitudes in each solution are measured as the lowest voltage of the averaged signal in the ISI after 100 ms per slice minus the resting potential of that cell. The mean hyperpolarization amplitudes across slices per solution are: (1) 4.5 ± 0.3 mV; (2) 2.9 ± 1.1 mV; (3) 2.5 ± 1.2 mV; and (4) 1.0 ± 0.3 mV for (1) ACSF; (2) CGP-55845-HCl in ACSF; (3) UCL-2077 in ACSF; and (4) both CGP-55845-HCl + UCL-2077 in ACSF, respectively, which are significantly different (P < 0.0001, ANOVA, LSD (Tukey-Kramer) at P < 0.01 = 1.5 mV). G, hyperpolarization area is measured in each solution as the sum of the averaged voltage potentials lower than the resting potential in the ISI minus the resting potential of that cell. The mean hyperpolarization areas across slices per solution are: (1) 20.4 ± 1.1 mV s; (2) 6.4 ± 2.6 mV s; (3) 8.6 ± 5.5 mV s; and (4) 1.3 ± 0.8 mV s for (1) ACSF; (2) CGP-55845-HCl in ACSF; (3) UCL-2077 in ACSF; and (4) both CGP-55845-HCl + UCL-2077 in ACSF, respectively, which are significantly different (P < 0.0001 using ANOVA, LSD (Tukey-Kramer) at P < 0.01 = 5.15 mV s). Bar graphs represent data means ± SD. ****P < 0.0001.
Intracellular CA3/CA1 recordings from left and right hippocampi were established and monitored for at least 15 min for healthy resting membrane potentials (−65 ± 11 mV) and spontaneous action potentials that coincided with extracellular interictal/ictal activities as well as evoked action potentials, all with amplitudes around 67 ± 10 mV and duration of 4 ± 2 ms. Cell membrane time constants averaged 15 ± 12 with input resistances of 57 ± 23 MΩ. Upon cell validation, 1 Hz LFS was applied to the VHC fibre tract while continuous intracellular recordings were maintained in each of 4 solutions: (1) ACSF (n= 7); (2) CGP-55845-HCl in ACSF (n= 8); (3) UCL-2077 in ACSF (n= 7); and (4) both CGP-55845-HCl and UCL-2077 in ACSF (n= 6). Single traces of the activity in the ISI as well as averaged traces across slices are shown for each solution in Fig. 7. As shown in Fig. 7B, LFS elicited a burst of action potentials in ACSF followed by long-lasting hyperpolarization that recovered to baseline by the end of the 1 s ISI. In most cases, spontaneous action potentials did not occur during the ISI in ACSF alone. The addition of CGP-55845-HCl or UCL-2077 shortened the duration and amplitude of the stimulus-induced hyperpolarization. As a result, non-evoked spontaneous spiking activity occurred during the ISI in these solutions (Fig. 7C and D) with most prominence in solutions containing both CGP-55845-HCl and UCL-2077, which coincided with the smallest LLH (Fig. 7E). The change in hyperpolarization due to GABAB/sAHP antagonists was quantified as (1) change in hyperpolarization amplitude, measured as the lowest voltage of the averaged signal in the ISI after 100 ms per slice minus the resting potential of that cell (Fig. 7F); and (2) change in hyperpolarization area, measured in each solution as the sum of the averaged voltage potentials lower than the resting potential in the ISI minus the resting potential of that cell (Fig. 7G). Hyperpolarization amplitude was diminished by GABAB/sAHP antagonists and differed significantly across groups (P < 0.0001 using ANOVA, LSD (Tukey-Kramer) at P < 0.01 = 1.5 mV) such that there were significant differences between ACSF control and all solutions containing GABAB/sAHP antagonists (P < 0.01 compared to a solution with a single GABAB or sAHP antagonist and P < 0.0001 compared to a solution with both GABAB and sAHP antagonists). Hyperpolarization amplitude was also smaller in solutions containing both GABAB and sAHP antagonists than in those with just one (P < 0.05). There were significant differences in hyperpolarization area between groups (P < 0.0001 using ANOVA, LSD (Tukey-Kramer) at P < 0.01 = 5.15 mV s). Notably, there were very significant differences in hyperpolarization area between ACSF control and all solutions containing any LLH reducers (P < 0.0001). Hyperpolarization area was also smaller in solutions containing both types of LLH reducers than in those with just one (P < 0.05). This analysis was repeated with 2-OH-saclofen (500 μm) and clotrimazole (10 μm) to antagonize the GABAB IPSP and medium and slow afterhyperpolarizations (m- and sAHPs) for validation of the results with very similar findings (Supplemental Fig. S1). Therefore, inhibition of the GABAB IPSP or the sAHP decreased stimulus-induced hyperpolarization throughout bilateral hippocampi in non-epileptic slices and combined inhibition of both further reduced the stimulation-induced hyperpolarization.
LLH reduction with GABAB/sAHP antagonists globally decreases stimulus-induced hyperpolarization during LFS in epileptic slices
We next analysed the effects of LLH reduction on bilateral stimulus-induced hyperpolarization in 4-AP (Fig. 8). The same experimental procedure and solutions were used with the addition of 4-AP (100 μm) to all solutions: (1) 4-AP (n= 4); (2) CGP-55845-HCl in 4-AP (n= 3); (3) UCL-2077 in 4-AP (n= 3); and (4) both CGP-55845-HCl and UCL-2077 in 4-AP (n= 3). While the effects of GABAB/sAHP antagonists on hyperpolarization amplitude and area were similar in 4-AP relative to ACSF, they had to be interpreted differently as each stimulus elicited many action potentials (7 ± 2.7 compared to 2.4 ± 1.2 in ACSF, as shown in Fig. 2), with a resultant large hyperpolarization (−7.05 ± 0.31 mV in 4-AP compared to -4.49 ± 0.25 mV in ACSF), as described previously for other seizure models (Alger & Nicoll, 1980a, b). Action potentials occurred atop a paroxysmal depolarizing shift lasting 300–500 ms, which can be seen for each example in Fig. 8A–D. Stimulus-induced hyperpolarization did not begin until half of the ISI had elapsed and, as a result, did not fully recover to baseline resting potential during 1 Hz LFS in 4-AP. In contrast, when CGP-55845-HCl, UCL-2077, or both were added to 4-AP, the resting potential fully recovered before every subsequent pulse. Hyperpolarization amplitude differed across groups (P < 0.0001 using ANOVA, LSD (Tukey-Kramer) at P < 0.01 = 0.79 mV), with significant differences between 4-AP control and all solutions containing GABAB/sAHP antagonists (P < 0.01 compared to a solution with a single GABAB or sAHP antagonist and P < 0.0001 compared to a solution with both GABAB and sAHP antagonists). Hyperpolarization amplitude was also smaller in solutions containing both types of LLH reducers than in those with just one, as seen in ACSF (P < 0.05). There were again significant differences in hyperpolarization area between groups (P < 0.0001, ANOVA, LSD (Tukey-Kramer) at P < 0.01 = 9.35 mV s) with very significant differences in hyperpolarization area between ACSF control and all solutions containing any LLH reducers (P < 0.0001). Therefore, inhibition of the GABAB IPSP and/or sAHP decreased stimulus-induced hyperpolarization throughout bilateral hippocampi in a 4-AP epilepsy model. Knowing this effect, any changes in LFS efficacy with the addition of LLH reducers to the 4-AP epilepsy model can be interpreted in this context.
LFS induced hyperpolarization for the duration of its application in 4-AP (Fig. 6). We next assessed whether the reduction in long-lasting hyperpolarization by CGP-55845-HCl and UCL-2077 persisted throughout the 15 min LFS protocol (Fig. 9). Twenty bilateral slices were bathed in either 1 μm CGP-55845-HCl (10 slices) or 10 μm UCL-2077 (10 slices). Trains of 15 min 1 Hz LFS were applied after recording baseline epileptic activity for at least 15 min in each of the solutions. Samples of 60 s of representative activity recorded intracellularly from CA1 in CGP-55845-HCl before (left), during (middle) and after (right) LFS are shown for CGP-55845-HCl in Fig. 9A. This procedure was repeated with UCL-2077 with similar results (not shown). During LFS with LLH reduction, cells did not reach more negative voltages than at baseline, unlike in 4-AP alone. In fact, the minimum Vm for 1 s time windows before, during and after LFS (15 min each) for each sample was similar across the entire 45 min experiment period, as shown for one example with CGP-55845-HCl in Fig. 9B. To quantify this, the mean ± SD of minimum Vm per 3 min before, during and after LFS (15 min each) was determined per slice and averaged across slices for each solution (Fig. 9C). There were no significant differences in the average minimum Vm before, during and after LFS when LLH was antagonized by either CGP-55845-HCl or UCL-2077 (P > 0.1, ANOVA). Therefore, diminishing LLH by CGP-55845-HCl and UCL-2077 removed the nearly 10 mV stimulus-induced hyperpolarization that occurred in 4-AP (Fig. 6) for the duration of the LFS protocol. These data suggest that the shift in lowest-voltage during stimulation trains is indeed caused by LLH. The effect of removing stimulus-induced LLH on seizure reduction by LFS was examined next.
Figure 9. LFS-induced long-lasting hyperpolarization is diminished by GABAB/sAHP antagonists.
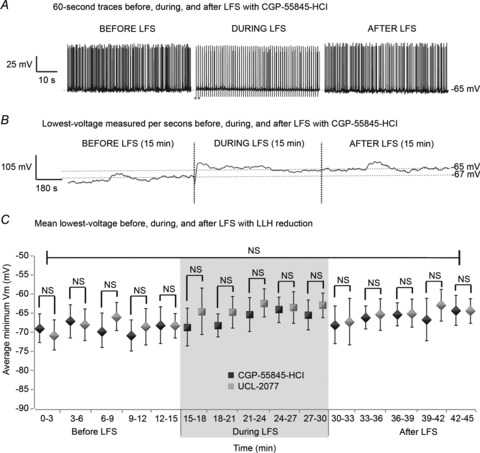
Trains of 15 min 1 Hz LFS were applied to bilateral hippocampi–VHC slices after recording baseline epileptic activity for at least 15 min as shown in Fig. 1B in (1) CGP-55845-HCl (1 μm) or (2) UCL-2077 (10 μm), followed by at least 15 min of recovery from LFS. A, 60 s of representative activity recorded intracellularly from CA1 in CGP-55845-HCl before (left), during (middle) and after (right) LFS are shown. This procedure was repeated with UCL-2077 with similar results (not shown). During LFS, cells do not appear to reach more negative voltages than at baseline in these conditions. B, the minimum Vm per second was determined before, during and after LFS (15 min each) for each sample (n= 10 per solution) and is shown across the entire 45 min experiment period for 1 slice washed in CGP-55845-HCl. C, the mean ± SD of minimum Vm before, during and after LFS (15 min each) was determined per slice every 3 min and averaged across slices for each solution. There are no significant differences in mean lowest voltage at any time point between slices bathed in CGP-55845-HCl or UCL-2077 (P≫ 0.05, Student's t test). There are no significant differences in the average minimum Vm before, during or after LFS in solutions containing either CGP-55845-HCl or UCL-2077(P > 0.1 using ANOVA).
LLH reduction with GABAB/sAHP antagonists decreases LFS efficacy
So far, we have shown that LFS induces LLH through GABAB signalling and sAHP. However, it is still not clear that LLH is necessary for seizure reduction by LFS. In order to address this question, we recorded seizures (extracellular recordings) from bilateral hippocampal slices before, during and after LFS with and without LLH reducers (Fig. 10). Baseline seizure characteristics were obtained from left and right extracellular CA3/CA1 recordings after recording stable seizures for at least 30 min in each solution (n= 32; 16; 16; and 12 for: 4-AP only; CGP-55845-HCl + 4-AP; UCL-2077 + 4-AP; and CGP-55845-HCl + UCL-2077 + 4-AP, respectively). Seizures occurred 13 ± 5% of the time in CGP-55845-HCl and 15 ± 8% of the time in UCL-2077, which was less than in 4-AP where baseline seizures occurred 19 ± 5% of the time (P < 0.05, Student's t test). After obtaining baseline recordings for at least 15 min, LFS was applied for 15 min, followed by at least 15 min of recording the after-effects. Left and right hippocampal activity was nearly identical and was therefore pooled for comparative analysis between groups, as tested and described previously (Toprani & Durand, 2013). There were significant differences in average percentage seizure reduction during LFS between groups (P < 0.0001 using ANOVA). CGP-55845-HCl and UCL-2077, as well as their combination, reduced LFS efficacy relative to that in 4-AP alone (P < 0.001, Tukey-Kramer post hoc analysis). With the addition of CGP-55845-HCl, only 23 ± 18% of total seizures were prevented during LFS in comparison to 97 ± 3% in 4-AP alone. Similarly, only 16 ± 5% of seizures were prevented by LFS in UCL-2077 + 4-AP, while there was nearly no effect of LFS (0 ± 7% seizure reduction) when CGP-55845-HCl and UCL-2077 were added in combination to 4-AP. Furthermore, the addition of both CGP-55845-HCl and UCL-2077 to 4-AP reduced LFS efficacy significantly more than either one alone (P < 0.01 when compared to CGP-55845-HCl + 4-AP only and P < 0.05 when compared to UCL-2077 + 4-AP only, Tukey-Kramer post hoc analysis).
Figure 10. LLH reduction with GABAB/sAHP antagonists reduces LFS efficacy.
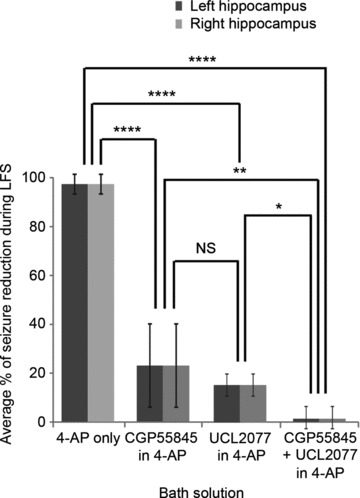
Trains of 15 min 1 Hz LFS trains were applied to the VHC of bilateral hippocampi–VHC slices after recording baseline epileptic activity extracellularly from CA3/CA1 bilaterally for at least 15 min as shown in Fig. 1B in either (1) 4-AP only (100 μm); (2) CGP-55845-HCl (1 μm) + 4-AP; (3) UCL-2077 (10 μm) + 4-AP; or (4) CGP-55845-HCl + UCL-2077 + 4-AP. The average percentage of seizure reduction during LFS was quantified for left and right hippocampi in each solution (n= 32, 16, 16 and 12 for: 4-AP only; CGP55854 + 4-AP; UCL-2077 + 4-AP; and CGP-55845-HCl + UCL-2077 + 4-AP, respectively.) There are significant differences in average percentage seizure reduction during LFS between groups (P < 0.0001 using ANOVA). CGP-55845-HCl and UCL-2077, as well as their combination, reduce LFS efficacy relative to that in 4-AP alone. Furthermore, the addition of both CGP-55845-HCl and UCL-2077 to 4-AP reduces LFS efficacy significantly more than either one alone as determined using Tukey-Kramer post hoc analysis. Bar graphs represent data means ± SD. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
These experiments were repeated using alternative GABAB/sAHP antagonists to verify the results (Supplemental Fig. S2). 2-OH-saclofen (500 μm) and clotrimazole (10 μm) were used to inhibit the GABAB IPSP and the m/sAHP, respectively. Baseline seizure characteristics were similar to those with CGP-55845-HCl and UCL-2077. Seizures occurred for 11 ± 4% of the time in 2-OH-saclofen and 21 ± 11% of the time in clotrimazole prior to LFS. Seizure reduction during electrical stimulation was distributed across groups as follows (n= 14, 20 and 13, respectively): 97 ± 4% in 4-AP; 41 ± 11.5% in 2-OH-saclofen; and 14 ± 8% in clotrimazole. There were significant differences in seizure reduction between groups (P < 0.0001, ANOVA). Post hoc analyses using the Scheffe criterion (P < 0.0001) showed that GABAB antagonism with 2-OH-saclofen and m/sAHP antagonism with clotrimazole diminished LFS efficacy, as found with CGP-55845-HCl and UCL-2077. In combination, these data suggest that intact GABAB signalling and the m/sAHP, are necessary for seizure reduction by this LFS protocol.
Inter-stimulus activity increases when LLH is reduced with GABAB/sAHP antagonists
To understand how decreased LLH led to increased epileptic activity during LFS, we examined the distribution of action potentials during the ISI with CGP-55845-HCl; UCL-2077; and CGP-55845-HCl + UCL-2077; compared to ACSF control (n= 7, 8, 7; and 6, respectively) as well as 4-AP control (n= 4, 3, 3 and 3, respectively). For this analysis, 15 min 1 Hz LFS trains were applied to the VHC of bilateral hippocampi–VHC slices while recording intracellularly from CA3/CA1. The mean number of action potentials (peaks with magnitude greater than 40 mV that reached a voltage higher than −20 mV) that occurred (1) 100–325 ms post stimuli; (2) 325–550 ms post stimuli; (3) 550–775 ms post stimuli; or (4) 775–1000 ms post stimuli during LFS trials was determined for each solution (Table 1). The first 100 ms of the ISI were omitted as most evoked responses occurred in this timeframe and would overshadow any non-evoked activity during the ISI. Since there was a long-lasting paroxysmal depolarizing shift (300–500 ms) in 4-AP-based solutions, some residual evoked activity was captured by this analysis. In ACSF-only control, very few action potentials occurred during any point of the ISI while LFS was applied. Spontaneous action potentials that did occur in ACSF happened at the end of the ISI (mainly in the 775–1000 ms time block). When LLH was blocked with either CGP-55845-HCl or UCL-2077 in ACSF, an increase in spontaneous action potentials was observed, especially during the latter half of the ISI. When LLH was dually blocked with CGP-55845 and UCL-2077, action potentials were prevalent throughout the ISI. In 4-AP-only control, evoked responses were prolonged, as can be seen by the high number of action potentials still occurring in the first time block (100–325 ms). As in ACSF, LLH reducers increased the number of action potentials across the ISI with the highest number and most uniform distribution when both GABAB and sAHP antagonists were added together. The differences in the total number of non-evoked action potentials that occurred during LFS between solutions are quantified in Fig. 11. There were significant differences in the number of action potentials during LFS between groups in ACSF as well as in 4-AP (P < 0.0001 using ANOVA). Differences between pairs of groups were determined using Tukey-Kramer post hoc analysis and were significant for ACSF or 4-AP controls compared to either or both LLH reducers (P < 0.01) as well as for both LLH reducers compared to just one in either ACSF or 4-AP (P < 0.0001). This analysis showed that decreasing LLH mediated by the GABAB IPSP or the sAHP during the ISI of LFS significantly increased non-evoked spontaneous cellular activity during LFS. This increase in non-evoked activity correlated with the decrease in LFS efficacy that was observed with the addition of GABAB/sAHP antagonists (Fig. 10).
Table 1.
GABAB/sAHP antagonists increase the number of peaks (mean ± SD) per time interval of the ISI during 1 Hz LFS
| Time interval (ms) | ACSF | ACSF + CGP-55845-HCl | ACSF + UCL-2077 | ACSF + both | 4-AP in ACSF | 4-AP in ACSF + CGP-55845-HCl | 4-AP in ACSF + UCL-2077 | 4-AP in ACSF + both |
|---|---|---|---|---|---|---|---|---|
| 100–325 | 15 ± 20 | 247 ± 138 | 743 ± 1,099 | 743 ± 1,056 | 1689 ± 146 | 1481 ± 175 | 1610 ± 562 | 1793 ± 824 |
| 325–550 | 17 ± 20 | 355 ± 201 | 181 ± 399 | 1,93 ± 1,940 | 12 ± 13 | 640 ± 342 | 771 ± 226 | 1953 ± 1,009 |
| 550–775 | 131 ± 90 | 757 ± 446 | 577 ± 1,030 | 2001 ± 2,339 | 0 ± 0 | 307 ± 252 | 590 ± 145 | 1985 ± 853 |
| 775–1000 | 348 ± 123 | 840 ± 334 | 1005 ± 1,441 | 1936 ± 1,940 | 0 ± 1 | 250 ± 197 | 574 ± 158 | 1835 ± 668 |
15 min 1 Hz LFS trains were applied to the VHC of bilateral hippocampi–VHC slices while recording extracellularly from CA3/CA1 bilaterally in either (1) ACSF or 4-aminopyridine-only (4-AP, 100 μm); (2) 1 μm CGP-55845-HCl + either ACSF or 4-AP in ACSF; (3) 10 μm UCL-2077 + either ACSF or 4-AP; or (4) both CGP-55845-HCl + UCL-2077 in either ACSF or 4-AP. The mean number of action potentials (peaks with magnitude greater than 40 mV that reach a voltage higher than −20 mV) that occur (1) 100–325 ms post stimuli; (2) 325–550 ms post stimuli; (3) 550–775 ms post stimuli; or (4) 775-1000 ms post stimuli during LFS trials is shown in ACSF ± GABAB/sAHP antagonists and 4-AP ± GABAB/sAHP antagonists.
Figure 11. GABAB/sAHP antagonists increase inter-stimulus activity.
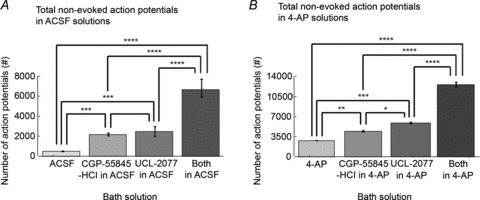
Trains of 15 min 1 Hz LFS were applied to the VHC of bilateral hippocampi–VHC slices while recording extracellularly from CA3/CA1 bilaterally as shown in Fig. 7A in either (1) ACSF or 4-AP (100 μm); (2) CGP-55845-HCl (1 μm) + either ACSF only or 4-AP in ACSF; (3) UCL-2077 (10 μm) and either ACSF or 4-AP; or (4) both CGP-55845-HCl + UCL-2077 in either ACSF or 4-AP. The total number of non-evoked action potentials (peaks with magnitude greater than 40 mV that reach a voltage higher than -20 mV) that occur during the LFS train is averaged across slices in all solutions in ACSF (n= 7, 8, 7 and 6, respectively; A) as well as 4-AP (n= 4, 3, 3 and 3, respectively; B). There are significant differences in number of action potentials during LFS between groups in ACSF as well as 4-AP (P < 0.0001 using ANOVA). Differences between pairs of groups are determined using Tukey-Kramer post hoc analysis. Bar graphs represent data means ± SD. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
Higher stimulation frequencies can rescue LFS efficacy when LLH is reduced with GABAB/sAHP antagonists
If indeed LFS sculpted the pattern of inter-stimulus activity by maximizing cell hyperpolarization in between pulses via mechanisms of LLH, then one could predict that the impaired seizure suppression that occurred when LLH was decreased in duration and/or peak amplitude could be rescued by increased frequency of stimulation. This hypothesis was tested by applying higher-frequency electrical stimulation to slices where the mechanisms of LLH have been antagonized in an effort to re-couple the ISI duration to the length of the effective hyperpolarization. As shown in Fig. 12, the average percentage of seizure reduction was quantified for left and right hippocampi after applying 15 min of 1; 3; and 10 Hz LFS trains to the VHC of bilateral hippocampi–VHC slices following at least 15 min of extracellularly recorded bilateral epileptic activity in either (1) CGP-55845-HCl (1 μm) + 4-AP; (2) UCL-2077 (10 μm) + 4-AP; or (3) both CGP-55845-HCl + UCL-2077 + 4-AP (n= 5 for each condition). Left and right results were similar and were therefore pooled for comparisons. LFS at 1 Hz prevented far less than half of all seizures in all solutions containing LLH antagonists, with only 4 ± 12% seizure reduction by 1 Hz LFS in slices with both CGP-55845-HCl and UCL-2077. Increasing the stimulation frequency to 3 Hz provided somewhat improved seizure reduction, while applying 10 Hz stimulation even to slices with GABAB/sAHP antagonism rescued LFS efficacy completely. At 1 Hz, but not at 3 and 10 Hz, there were significant differences in average percentage seizure reduction during LFS between groups of different LLH reducers + 4-AP (P < 0.05 using ANOVA). There were significant differences in average seizure reduction during LFS with different stimulation frequencies in solutions of GABAB/sAHP antagonists (P < 0.0001 using ANOVA).
Figure 12. Higher stimulation frequencies can rescue LFS efficacy with LLH reduction with GABAB/sAHP antagonists.
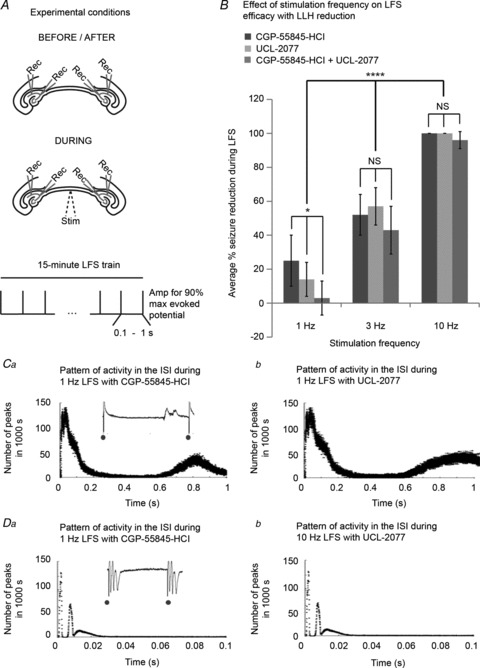
A, 15 min 1; 3; and 10 Hz LFS trains were applied to the VHC of bilateral hippocampi–VHC slices after recording baseline epileptic activity extracellularly from CA3/CA1 bilaterally for at least 15 min as shown in Fig. 1B in either (1) CGP-55845-HCl (1 μm) + 4-AP; (2) UCL-2077 (10 μm) + 4-AP; or (3) both CGP-55845-HCl + UCL-2077 + 4-AP. B, the average percentage of seizure reduction during LFS was quantified for left and right hippocampi in each solution during 1; 3; and 10 Hz LFS trials (n= 5 for each condition). Left and right results were pooled for comparisons. At 1 Hz, but not at 3 and 10 Hz, there are significant differences in average percentage seizure reduction during LFS between groups of different LLH antagonists + 4-AP (P < 0.05 using ANOVA). There are significant differences in average seizure reduction during LFS with different stimulation frequencies in solutions of LLH antagonists (P < 0.0001 using ANOVA). The distribution of extracellular peaks of activity, detected as described previously, over the 1 s ISI during 1 Hz LFS is shown for (Ca) CGP-55845-HCl and (Cb) UCL-2077. There is evoked activity as well as some spontaneous activity that occurs during the second half of the ISI. The distribution of extracellular peaks of activity, detected as described previously, over the 0.1 s ISI during 10 Hz LFS is shown for (Da) CGP-55845-HCl and (Db) UCL-2077. Only evoked activity occurs. Bar graphs represent data means ± SD. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001.
To understand this effect, the distribution of epileptic activity during the ISI was assessed as in Fig. 4 and 5. The distribution of extracellular peaks of activity, detected as described previously, over the 1 s ISI during 1 Hz LFS is shown in Fig. 12C for CGP-55845-HCl and UCL-2077. There was evoked activity following the stimuli as seen in prior examples where LFS was effective. However, in each solution where LLH was reduced with GABAB/sAHP antagonists, spontaneous non-evoked activity also occurred during the second half of the ISI that was not seen in seizure models in which 1 Hz LFS was effective. This analysis was repeated for the distribution of extracellular peaks over the 0.1 s ISI during 10 Hz LFS with CGP-55845-HCl and UCL-2077 (Fig. 12D). With this shortened ISI duration, only evoked activity occurred even in the presence of GABAB/sAHP antagonists. The suppression of spontaneous activity during the ISI with higher frequency stimulation probably explains the improved efficacy of higher frequency stimulation in these seizure models. Even though LLH was reduced, this interval was short enough that methods of short-hyperpolarization (GABAA IPSPs and fast AHP) may be able to ‘protect’ the ISI.
In order to validate these findings, we repeated these trials using alternative methods of GABAB/sAHP antagonism: 2-OH-saclofen (500 μm) and clotrimazole (10 μm) (Supplementary Fig. 3). The LFS protocol was tested as described in each of these solutions using LFS frequencies of 1; 3; and 10 Hz (n= 8, 3 and 3, respectively) to see if efficacy could be rescued. Mean ± SD percentage of seizure reduction is presented for each frequency. One-way ANOVA showed significant differences between groups of frequencies (P < 0.0001), which suggests LFS efficacy could be rescued by utilizing high stimulation frequencies in the presence of LLH reduction with GABAB/sAHP antagonists. Epileptiform activity recurred between stimuli when GABAB IPSPs or m/sAHPs were reduced concurrent with decreased LFS efficacy under those conditions. The finding that inter-stimulus epileptiform activity could be prevented in solutions containing GABAB/sAHP antagonists by increasing stimulation frequency, which also rescued protocol efficacy, is supportive of the hypothesized mechanism of LFS.
Discussion
In this study, we utilize a bilateral hippocampal slice preparation to test the hypothesis that low frequency electrical stimulation of the VHC axonal tract induces long-lasting hyperpolarization in hippocampal pyramidal cells bilaterally and that LLH is necessary for seizure reduction by LFS. LFS-induced LLH occurs through two major mechanisms: (1) the GABAB IPSP and (2) the sAHP. During the application of LFS, the pattern of inter-stimulus activity is sculpted in a predictable pattern when LFS is effectively reducing seizures such that a hyperactive bursting response to stimulation is followed by minimized activity during the second half of the ISI. It is our hypothesis that stimulus-induced hyperpolarization is responsible for the inhibitory effect. We have shown that LFS induces hyperpolarization on a 1–2 s scale mediated by the GABAB IPSP and sAHP and antagonists of these mechanisms significantly diminish this LFS-induced hyperpolarization along with the seizure reduction efficacy of LFS (Figs 7–9). In the case when both mechanisms are blocked, seizure reduction by LFS is abolished (Fig. 10).
The anti-epileptic potential of GABAB receptors is underscored by the presence of spontaneous generalized seizures in GABAB receptor knock-out mice (Prosser et al. 2001; Schuler et al. 2001) as well as the induction of partial and convulsive seizures by GABAB receptor antagonists (Vergnes et al. 1997; Leung et al. 2005). In the hippocampus, GABA release from single interneurons has been shown to modulate sodium spike frequency (Miles & Wong, 1984; Miles et al. 1996), to inhibit dendritic calcium spikes (Miles & Wong, 1984), to synchronize pyramidal cells and to set the pace of oscillations (Cobb et al. 1995; Scanziani, 2000). It has been shown that GABAB receptors are up-regulated during LFS resulting in decreased hippocampal seizures caused by amygdala kindling (Wu et al. 2013). Other groups have also speculated that an increase in after-discharge threshold following LFS may be responsible as well (Goodman et al. 2005).
Although the sAHP is also implicated as a player in epilepsy, its precise role in cell excitability and central nervous system function is minimally studied since (1) the molecular identity of the channel underlying the sAHP is unknown, although it has been shown to be a small conductance potassium channel and (2) prior to the introduction of UCL-2077, there were no specific inhibitors of the sAHP (Shah et al. 2006). Nonetheless, lack of a developed sAHP, especially in children, is linked to childhood febrile seizures. The sAHP limits firing to a sustained depolarizing input (Alger & Nicoll, 1980a; Hotson & Prince, 1980; Madison & Nicoll, 1984). Furthermore, previous studies have shown that using neurotransmitters to suppress the sAHP increase cell excitability, measured as the number of spikes during a depolarizing pulse (Storm, 1990; Sah & Faber, 2002). Slow calcium-activated potassium currents responsible for the sAHP help to terminate epileptic interictal bursts in immature CA3 pyramidal neurons (Sipila et al. 2006). It is plausible they play the same role when interictal bursts are simulated electrically as in this LFS protocol.
Long term depression (LTD) has also been proposed as a mechanism of antiepileptic DBS (Albensi et al. 2004; Schiller & Bankirer, 2007). LTD may be responsible for seizure control by LFS because application of an NMDA antagonist removed both LTD effects and seizure activity during LFS (Albensi et al. 2004. However, that has been disputed on the basis that the effects of LFS remain, even using a stimulus train whose duration is too short to induce LTD (Goodman et al. 2005). Furthermore, seizure reduction by LFS can be seen within 30 s of starting stimulation in our model, a time course that is not consistent with an LTD-mediated mechanism. In addition, LTD is not observed during this stimulation protocol since the evoked potentials did not significantly change in amplitude during LFS nor did the number of action potentials recruited per stimulus (Fig. 2). This is corroborated by a similar VHC stimulation protocol tested in vivo (Tang & Durand, 2012). However, we do not discount that LTD may underlie the long term seizure reduction by LFS. In addition, LTD could play a role in enhancing efficacy of repeated duty cycles.
Another possible mechanism of LFS seizure reduction is axonal block via potassium-mediated depolarization (Jensen & Durand, 2007, 2009). This mechanism was considered since a distinguishing feature of this LFS protocol is stimulation of a white matter tract for bilateral targeting. Intracellular recordings during stimulation in epileptic slices do indicate the presence of paroxysmal depolarization shift (PDS) following each stimulus, as shown in Fig. 2 and 7, during which the occurrence of spontaneous spikes is severely limited. However, the inter-stimulus interval during which seizures are inhibited lasts longer than the duration of the PDS so that cannot explain the effect in its entirety. Furthermore, there is no evidence of depolarization block as cell potentials return to baseline between stimuli and cells respond reliably to stimuli. To the contrary, cells exhibit marked hyperpolarization of nearly 10 mV relative to baseline during LFS in 4-AP (Fig. 6). In addition, spontaneous firing occurs in cells during LFS (Fig. 7, 8 and 11). Finally, glial cells provide an indirect measure of extracellular potassium changes and, while they exhibit significant voltage shifts during seizures (Tian et al. 2005; Gomez-Gonzalo et al. 2010), they do not respond appreciably to low frequency stimulation of the VHC (Fig. 3).
Given their involvement in seizures as well as their role in antiepileptic drugs, increased GABAA-mediated inhibition or decreased glutamatergic excitation are also natural candidates for the mechanism of seizure reduction by LFS. However, we did not find any change in LFS efficacy in the presence of BMI, as reported by Schiller & Bankirer (2007)), so we conclude that GABAA inhibition is not central to seizure reduction by this LFS protocol. The LFS protocol is also equally effective under the condition of enhanced glutamatergic signalling produced by the magnesium-free seizure model, suggesting inhibition of glutamatergic neurotransmission is not a key player.
There are several other potential mechanisms of seizure reduction by LFS not tested in this study. The first of these is the depolarization-activated hyperpolarizing M-current (Marrion, 1997). While this current may be involved in seizure reduction by LFS, it possesses the unique characteristic of sustained activation. This is inconsistent with peak LFS efficacy at 1 Hz or higher frequencies (Toprani & Durand, 2013). This mechanism should remain effective even at lower LFS frequencies. Additionally, adenosine has been called the brain's endogenous anticonvulsant and is responsible for seizure arrest and post-ictal refractoriness (Gouder et al. 2003; Boison, 2006). Adenosine levels can be modulated by electrical stimulation (Unekwe & Savage, 1991; Kakiuchi et al. 2006). Similar to GABAB, adenosine can activate a potassium current through G-protein coupling (Trussel & Jackson, 1987). It can also inhibit calcium currents and, subsequently, neurotransmitter release (Rocher et al. 1999). However, these adenosine responses are typically seen at higher stimulation frequencies than used in this study (Crosson & Gray, 1997). In conclusion, while a number of other mechanisms may play a role in seizure reduction by LFS, this study definitively demonstrates the importance of long-lasting-hyperpolarization mediated by the GABAB IPSP and sAHP as necessary for bilateral hippocampal seizure reduction by LFS of the VHC axon tract.
This study has several limitations. While an in vitro slice preparation of bilaterally coupled hippocampi maintains key network connections of hippocampal CA3 → VHC → contralateral hippocampal CA1 in an environment that can be highly controlled, conclusions cannot be directly generalized. Seizure dynamics differ in other preparations and species. Furthermore, chemical seizures are distinct from physiological seizures, which are complex and variable. Physiological interictal activity is not constant or regular as in this model and a stimulation frequency that correlates with clinical interictal activity may be harder to extrapolate. Furthermore, this protocol may be of limited value in cases where deficiencies in GABAB or sAHP signalling underlie epileptogenicity.
In addition to generalizability, another limitation of the in vitro model is that potential side effects of the LFS protocol cannot be assessed. The sAHP is prolonged during ageing, which correlates with impairments in learning and memory (Moyer et al. 1992; Disterhoft et al. 1996; Giese et al. 1998; Vergara et al. 1998; Weiss et al. 2000). In addition, recent studies have shown implications of interictal spiking activity on hippocampal cognitive function (Kleen et al. 2010; Brown et al. 2012). In particular, Kleen et al showed that interictal spikes slow memory retrieval but do not affect memory encoding or maintenance. So far, minimal memory deficiencies have been reported from DBS (Theodore & Fisher, 2004; Kakiuchi et al. 2006), including LFS in patients (Koubeissi et al. 2012). However, in light of these concerns, we would recommend utilizing LFS protocols during sleep or rest and giving patients the ability to turn off stimulation at any time they may need to maximize memory retrieval. Changes in memory or task-learning should be monitored while implementing DBS in vivo and in clinical trials.
Conclusions
DBS is a promising treatment for MTLE. Specifically, low frequency electrical stimulation that mimics interictal bursts of white matter tracts, like the hippocampal commissures that interconnect bilateral hippocampi, has shown impressive seizure reduction in recent studies using in vitro and in vivo models, as well as human patients with MTLE. In this study, we utilized an in vitro slice preparation containing both hippocampi connected by the VHC to study the mechanisms of bilateral seizure reduction by low frequency electrical stimulation of an under-utilized white matter target, the VHC. Epileptic activity spreads across the VHC and LFS of this single target effectively reduces bilateral seizures in this model without causing depolarization block, LTD, or requiring GABAA receptors. Increasing glutamate signalling does not affect LFS efficacy and glial cells do not exhibit voltage changes during LFS. However, LFS does induce a bursting response to stimulation followed by long-lasting hyperpolarization on the order of 1–2 s. Cell firing is limited during the hyperpolarization which can be reduced by pharmacologically inhibiting the GABAB IPSP or sAHP. Along with reducing the LFS-induced hyperpolarization, these antagonists significantly reduce LFS efficacy. These findings about the behaviour of cells during low frequency electrical stimulation of the hippocampal commissures lend important mechanistic insights about DBS at low frequencies for epilepsy that can facilitate implementation and optimization of this non-invasive treatment solution for MTLE patients.
Translational perspective
Deep brain electrical stimulation (DBS) is an emerging treatment for medically refractory mesial temporal lobe epilepsy (MTLE). Using an in vitro brain slice preparation consisting of bilateral hippocampi connected by a white matter tract, the ventral hippocampal commissure (VHC), we investigated the mechanisms underlying bilateral hippocampal seizure reduction by low frequency electrical stimulation (LFS) of a single white matter stimulation target. Several potential mechanisms of seizure reduction by LFS have been tested, which can be broadly characterized as: (1) depression of synaptic excitatory response; (2) potassium-mediated depolarization block; (3) glial-neuronal interplay; (4) decreased excitatory/increased inhibitory synaptic neurotransmission; and (5) reduction in the excitability of neurons. In particular, decreased neuronal excitability by LFS induction of long-lasting hyperpolarization (LLH) composed of (1) the GABAB slow inhibitory post-synaptic potential (IPSP) and (2) the slow afterhyperpolarization (sAHP) were found to be necessary for seizure reduction by LFS. These insights into the mechanisms of DBS, specifically LFS, promote safer implementation and optimization of this promising therapy. Based on this study, optimization of the timing of LFS and LFS-induced-LLH may lead to improved outcomes from DBS treatments for human epilepsy. Furthermore, potential side-effects of treatment can be predicted and, therefore, prevented. For example, given the role of the sAHP in memory retrieval, patients’ quality of life can be maximized by applying LFS during sleep or rest. Finally, understanding the physiological effects of LFS may guide novel therapeutic applications of DBS, which has the advantages that it is minimally invasive, reversible and does not have systemic effects.
Acknowledgments
The authors would like to thank Dr Yuang Tang for his valuable input. Sheela Toprani was a Howard Hughes Medical Institute Medical Research Fellow.
Glossary
- 4-AP
4-aminopyridine
- ACSF
artificial cerebral spinal fluid
- AHP
afterhyperpolarization
- BMI
bicuculline methiodide
- DBS
deep brain stimulation
- DHC
dorsal hippocampal commissure
- EC
entorhinal cortex
- HC
hippocampal commissures
- HFS
high frequency stimulation
- IACUC
Institutional Animal Care and Use Committee
- IPSP
inhibitory post-synaptic potential
- ISI
inter-stimulus interval
- LFS
low frequency stimulation
- LLH
long-lasting hyperpolarization
- LTD
long term depression
- mAHP
medium after-hyperpolarization
- MTLE
mesial temporal lobe epilepsy
- NS
no significance
- sAHP
slow after-hyperpolarization
- SD
standard deviation
- VHC
ventral hippocampal commissure
Additional information
Competing interests
None declared
Author contributions
S.T. and D.M.D. contributed equally to the conception and design of the experiments. S.T. mostly carried out the collection, analysis and interpretation of data. S.T. and D.M.D. contributed equally to drafting the article or revising it critically for important intellectual content. Both authors approved the final version of the manuscript.
Funding
This work is supported by a Grant from the Walter H Coulter Foundation and NIH grant 3RO1NS060757.
Supplementary material
Supplemental Fig. S1
Supplemental Fig. S2
Supplementary Fig. 3
References
- Albensi BC, Ata G, Schmidt E, Waterman JD, Janigro D. Activation of long-term synaptic plasticity causes suppression of epileptiform activity in rat hippocampal slices. Brain Res. 2004;998:56–64. doi: 10.1016/j.brainres.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Alger BE, Nicoll RA. Epileptiform afterhyperpolarization: calcium-dependent K potential in hippocampal CA1 pyramidal cells. Science. 1980a;210:1122–1124. doi: 10.1126/science.7444438. [DOI] [PubMed] [Google Scholar]
- Alger BE, Nicoll RA. Spontaneous inhibitory post-synaptic potentials in hippocampus: mechanism for tonic inhibition. Brain Res. 1980b;200:195–200. doi: 10.1016/0006-8993(80)91108-7. [DOI] [PubMed] [Google Scholar]
- Avoli Massimo, D’Antuno M, Louvel J, Kohling R, Biagini G, Pumain R, D’Arcangelo G, Tancredi V. Network and pharmacological mechanisms leading to epileptiform synchronization in the limbic system in vitro. Prog Neurobiol. 2002;68:167–207. doi: 10.1016/s0301-0082(02)00077-1. [DOI] [PubMed] [Google Scholar]
- Avoli M. Do interictal discharges promote or control seizures? Experimental evidence from an in vitro model of epileptiform discharge. Epilepsia. 2001;42(Suppl. 3):S2–4. doi: 10.1046/j.1528-1157.2001.042suppl.3002.x. [DOI] [PubMed] [Google Scholar]
- Barbarosie M, Avoli M. CA3-driven hippocampal-entorhinal loop controls rather than sustains in vitro limbic seizures. J Neurosci. 1997;17:9308–9314. doi: 10.1523/JNEUROSCI.17-23-09308.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid AL, Wallace B, Mitrofanis J, Xia C, Piallat B, Fraix V, Batir A, Krack P, Pollak P, Berger F. Therapeutic electrical stimulation of the central nervous system. C R Biol. 2005;328:177–186. doi: 10.1016/j.crvi.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Blume WT. Focal seizures: intractability and semiology. Adv Neurol. 2006;97:17–25. [PubMed] [Google Scholar]
- Blume WT, Parrent AG. Assessment of patients with intractable epilepsy for surgery. Adv Neurol. 2006;97:537–548. [PubMed] [Google Scholar]
- Boison D. Adenosine kinase, epilepsy and stroke; mechanisms and therapies. Trends Pharmacol Sci. 2006;27:652–658. doi: 10.1016/j.tips.2006.10.008. [DOI] [PubMed] [Google Scholar]
- Boon P, Vonck K, De Herdt V, Van Dycke A, Goethals M, Goossens L, Van Zandijcke M, De Smedt T, Dewaele I, Achten R, Wadman W, Dewaele F, Caemaert J, Van Roost D. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007;48:1551–1560. doi: 10.1111/j.1528-1167.2007.01005.x. [DOI] [PubMed] [Google Scholar]
- Borck C, Jefferys JGR. Seizure-like events in disinhibited ventral slices of adult rat hippocampus. J Neurophysiol. 1999;82:2130–2142. doi: 10.1152/jn.1999.82.5.2130. [DOI] [PubMed] [Google Scholar]
- Brown EC, Matsuzaki N, Asano E. The transient effect of interictal spikes from a frontal focus on language-related gamma activity. Epilepsy Behav. 2012;24:497–502. doi: 10.1016/j.yebeh.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckmaster P, Stowbridge BW, Schwartzkroin A. A comparison of rat hippocampal mossy cells and CA3c pyramidal cells. J Neurophysiol. 1993;70:1281–1300. doi: 10.1152/jn.1993.70.4.1281. [DOI] [PubMed] [Google Scholar]
- Calcagnotto ME, Barbarosie M, Avoli M. Hippocampus-entorhinal cortex loop and seizure generation in the young rodent limbic system. J Neurophysiol. 2000;83:3183–3187. doi: 10.1152/jn.2000.83.5.3183. [DOI] [PubMed] [Google Scholar]
- Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization and neuronal activity in the hippocampus by individual GABAergic interneurons. Nature. 1995;378:823–828. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- Crosson CE, Gray T. Response to prejunctional adenosine receptors is dependent on stimulus frequency. Curr Eye Res. 1997;16:359–364. doi: 10.1076/ceyr.16.4.359.10690. [DOI] [PubMed] [Google Scholar]
- D’Arcangelo G, Panuccio G, Tancredi V, Avoli M. Repetitive low-frequency stimulation reduces epileptiform synchronization in limbic neuronal networks. Neurobiol Dis. 2005;19:119–128. doi: 10.1016/j.nbd.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Disterhoft JF, Thompson LT, Moyer JR, Mogul DJ. Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci. 1996;59:413–420. doi: 10.1016/0024-3205(96)00320-7. [DOI] [PubMed] [Google Scholar]
- Duncan JS, Sander JW, Sisodiya SM, Walker MC. Adult epilepsy. Lancet. 2006;367:1087–1100. doi: 10.1016/S0140-6736(06)68477-8. [DOI] [PubMed] [Google Scholar]
- Durand DM, Bikson M. Suppression and control of epileptiform activity by electrical stimulation: a review. Proc IEEE. 2001;89:1065–1082. [Google Scholar]
- Dutar P, Nicoll RA. A physiological role for GABAB receptors in the central nervous system. Nature. 1988a;332:156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- Dutar P, Nicoll RA. Pre and postsynaptic GABAB receptors in the hippocampus have different pharmacological properties. Neuron. 1988b;1:585–591. doi: 10.1016/0896-6273(88)90108-0. [DOI] [PubMed] [Google Scholar]
- Giese KP, Storm JF, Reuter D, Fedorov NB, Shao LR, Leicher T, Pongs O, Silva AJ. Reduced K+ channel inactivation, spike broadening, and after-hyperpolarization in Kv beta 1.1-deficient mice with impaired learning. Learn Mem. 1998;5:257–273. [PMC free article] [PubMed] [Google Scholar]
- Gomez-Gonzalo M, Losi L, Chiavegato A, Zonta M, Cammarota M, Brondi M, Vetri F, Uva L, Pozzan T, Curtis Md, Ratto GM, Carmignoto G. An excitatory loop with astrocytes contributes to drive neurons to seizure threshold. PLoS Biol. 2010;8:1–19. doi: 10.1371/journal.pbio.1000352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman JH, Berger RE, Tcheng TK. Preemptive low-frequency stimulation decreases the incidence of amygdala-kindled seizures. Epilepsia. 2005;46:1–7. doi: 10.1111/j.0013-9580.2005.03804.x. [DOI] [PubMed] [Google Scholar]
- Gouder N, Fritschy J-M, Boison D. Seizure suppression by adenosine A1 receptor activation in a mouse model of pharmacoresistant epilepsy. Epilepsia. 2003;44:877–885. doi: 10.1046/j.1528-1157.2003.03603.x. [DOI] [PubMed] [Google Scholar]
- Hotson JR, Prince DA. A calcium-activated hyperpolarization follows repetitive firing in hippocampal neurons. J Neurophysiol. 1980;43:409–419. doi: 10.1152/jn.1980.43.2.409. [DOI] [PubMed] [Google Scholar]
- Jallon P. The problem of intractability: the continuing need for new medical therapies in epilepsy. Epilepsia. 1997;38(Suppl 9):S37–42. doi: 10.1111/j.1528-1157.1997.tb05203.x. [DOI] [PubMed] [Google Scholar]
- Jensen A, Durand D. Suppression of axonal conduction by sinusoidal stimulation in rat hippocampus in vitro. J Neural Eng. 2007;4:1–16. doi: 10.1088/1741-2560/4/2/001. [DOI] [PubMed] [Google Scholar]
- Jensen A, Durand D. High frequency stimulation can block axonal conduction. Exp Neurol. 2009;220:57–70. doi: 10.1016/j.expneurol.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerger K, Schiff SJ. Periodic pacing an in vitro epileptic focus. J Neurophysiol. 1995;73:876–879. doi: 10.1152/jn.1995.73.2.876. [DOI] [PubMed] [Google Scholar]
- Jones R. Ictal epileptiform events induced by removal of extracellular magnesium in slices of entorhinal cortex are blocked by baclofen. Exp Neurol. 1989;104:155–161. doi: 10.1016/s0014-4886(89)80009-3. [DOI] [PubMed] [Google Scholar]
- Kabashima N, Shibuya I, Ibrahim N, Ueta Y. Inhibition of spontaneous EPSCs and IPSCs by presynaptic GABAB receptors on rat supraoptic magnocellular neurons. J Physiol. 1997;504:113–126. doi: 10.1111/j.1469-7793.1997.113bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakiuchi S, Rall TW, McIlwain H. The effect of electrical stimulation upon the accumulation of adenosine 3′,5′-phosphate in isolated cerebral tissue. J Neurochem. 2006;16:485–491. doi: 10.1111/j.1471-4159.1969.tb06847.x. [DOI] [PubMed] [Google Scholar]
- Khosravani H, Carlen PL, Valazquez JLP. The control of seizure-like activity in the rat hippocampal slice. Biophys J. 2003;84:687–695. doi: 10.1016/S0006-3495(03)74888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kile K, Tian N, Durand D. Low frequency stimulation decreases seizure activity in a mutation model of epilepsy. Epilepsia. 2010;51:1745–1753. doi: 10.1111/j.1528-1167.2010.02679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King D, Spencer SS, McCarthy G, Luby M, Spencer DD. Bilateral hippocampal atrophy in medial temporal lobe epilepsy. Epilepsia. 1995;36:905–910. doi: 10.1111/j.1528-1157.1995.tb01634.x. [DOI] [PubMed] [Google Scholar]
- Kinoshita M, Ikeda A, Matsuhashi M, Matsumoto R, Hitomi T, Begum T, Usui K, Takayama M, Mikuni N, Miyamoto S, Hashimoto N, Shibasaki H. Electric cortical stimulation suppresses epileptic and background activities in neocortical epilepsy and mesial temporal lobe epilepsy. Clin Neurophysiol. 2005;116:1291–1299. doi: 10.1016/j.clinph.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Kirmse K, Kirischuk S. Ambient GABA contrains the strength of GABAergic synapses at Cajal-Retzius cells in the developing visual cortex. J Neurosci. 2006;26:4216–4227. doi: 10.1523/JNEUROSCI.0589-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleen JK, Scott RC, Holmes GL, Lenck-Santini PP. Hipppocampal interictal spikes disrupt cognition in rats. Ann Neurol. 2010;67:250–257. doi: 10.1002/ana.21896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubeissi MZ, Durand D, E EK, Syed T, Miller J, Luders H. Low frequency electrical stimulation of white matter tracts in intractable mesial temporal lobe epilepsy. American Academy, Annual Conference. 2012 [Google Scholar]
- Lancaster B, Hu H, Ramakers GMJ, Storm JF. Interaction between synaptic excitation and slow afterhyperpolarization current in rat hippocampal pyramidal cells. J Physiol. 2001;536:809–823. doi: 10.1111/j.1469-7793.2001.00809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA. Interaction between synaptic excitation and slow afterhyperpolarization in rat hippocampal neurones. J Physiol. 1987;389:187–203. doi: 10.1113/jphysiol.1987.sp016653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanerolle Nd, Lee T, Spencer D. Astrocytes and epilepsy. Neurotherapeutics. 2010;7:424–438. doi: 10.1016/j.nurt.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung LS, Canning KJ, Shen B. Hippocampal afterdischarges after GABA(B)-receptor blockade in the freely moving rat. Epilepsia. 2005;46:203–216. doi: 10.1111/j.0013-9580.2005.35804.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Meraz ML, Neri-Bazan L, Rocha L. Low frequency stimulation modifies receptor binding in rat brain. Epilepsy Res. 2004;59:95–105. doi: 10.1016/j.eplepsyres.2004.02.005. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Savasta M, Goff LKL, Vitek JL. Uncovering the mechanisms of action of deep brain stimulation: activation, inhibition, or both. Clin Neurophysiol. 2004;115:1239–1248. doi: 10.1016/j.clinph.2003.12.024. [DOI] [PubMed] [Google Scholar]
- Madison DV, Nicoll RA. Control of the repetitive discharge of rat CA1 pyramidal neurons in vitro. J Physiol. 1984;354:319–331. doi: 10.1113/jphysiol.1984.sp015378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrion NV. Control of M-Current. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- Miles R, Toth K, Gulyas AI, Hajos N, Freund TF. Differences between somatic and dendritic inhibition in the hippocampus. Neuron. 1996;16:815–823. doi: 10.1016/s0896-6273(00)80101-4. [DOI] [PubMed] [Google Scholar]
- Miles R, Wong RK. Unitary inhibitory synaptic potentials in the guinea-pig hippocampus in vitro. J Physiol. 1984;356:97–113. doi: 10.1113/jphysiol.1984.sp015455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrell M. Brain stimulation for epilepsy: can scheduled or responsive neurostimulation stop seizures. Curr Opin Neurol. 2006;19:164–168. doi: 10.1097/01.wco.0000218233.60217.84. [DOI] [PubMed] [Google Scholar]
- Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77:1295–1304. doi: 10.1212/WNL.0b013e3182302056. [DOI] [PubMed] [Google Scholar]
- Moyer JR, Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age-dependent and concentration-dependent manner. J Neurophysiol. 1992;68:2100–2109. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- Otis TS, Mody I. Differential activation of GABAA and GABAB receptors by spontaneously released transmitter. J Neurophysiol. 1992;67:227–235. doi: 10.1152/jn.1992.67.1.227. [DOI] [PubMed] [Google Scholar]
- Parnas I, Rashkovan G, Ong J, Kerr D. Tonic activation of presynaptic GABAB receptors in the opener neuromuscular junction of crayfish. J Neurophysiol. 1999;81:1184–1191. doi: 10.1152/jn.1999.81.3.1184. [DOI] [PubMed] [Google Scholar]
- Perreault P, Avoli M. Physiology and pharmacology of epileptiform activity induced by 4-aminopyridine in rat hippocampal slices. J Neurophysiol. 1991;65:771–785. doi: 10.1152/jn.1991.65.4.771. [DOI] [PubMed] [Google Scholar]
- Prosser HM, Gill CH, Hirst WD, Grau E, Robbins M, Calver A, Soffin EM, Farmer CE, Lanneau C, Gray J, Schenck E, Warmerdam BS, Clapham C, Reavill C, Rogers DC, Stean T, Upton N, Humphreys K, Randall A, Geppert M, Davies CH, Pangalos MN. Epileptogenesis and enhanced prepulse inhibition in GABAB1-deficient mice. Mol Cell Neurosci. 2001;17:1059–1070. doi: 10.1006/mcne.2001.0995. [DOI] [PubMed] [Google Scholar]
- Rashid S, Pho G, Czigler M, Werz MA, Durand DM. Low frequency stimulation of ventral hippocampal commissures reduces seizures in a rat model of chronic temporal lobe epilepsy. Epilepsia. 2011;53:147–156. doi: 10.1111/j.1528-1167.2011.03348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reato D, Cammarota M, Parra LC, Carmignoto G. Computational model of neuron-astrocyte interactions during focal seizure generation. Front Comput Neurosci. 2012;6:1–14. doi: 10.3389/fncom.2012.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocher A, Gonzalez C, Almaraz L. Adenosine inhibits L-type Ca2+ current and catecholamine release in the rabbit carotid body chemoreceptor cells. Eur J Neurosci. 1999;11:673–681. doi: 10.1046/j.1460-9568.1999.00470.x. [DOI] [PubMed] [Google Scholar]
- Rutecki PA, Lebeda FJ, Johnston D. 4-Aminopyridine produces epileptiform activity in hippocampus and enhances synaptic excitation and inhibition. J Neurophysiol. 1987;57:1911–1924. doi: 10.1152/jn.1987.57.6.1911. [DOI] [PubMed] [Google Scholar]
- Sah P. Ca2+-activated K +currents in neurones: types, pysiological roles and modulation. Trends Neurosci. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Sah P, Faber E. Channels underlying neuronal calcium-activated potassium currents. Prog Neurobiol. 2002;66:345–353. doi: 10.1016/s0301-0082(02)00004-7. [DOI] [PubMed] [Google Scholar]
- Scanziani M. GABA spillover activates postsynaptic GABAB receptors to control rhythmic hippocampal activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Schiller Y, Bankirer Y. Cellular mechanisms underlying antiepileptic effects of low- and high-frequency electrcal stimulation in acute epilepsy in neocortical brain slices in vitro. J Neurophysiol. 2007;97:1887–1902. doi: 10.1152/jn.00514.2006. [DOI] [PubMed] [Google Scholar]
- Schrader LM, Stern JM, Wilson CL, Fields TA, Salamon N, Nuwer MR, Vespa PM, Fried I. Low frequency electrical stimulation through subdural electrodes in a case of refractory status epilepticus. Clin Neurophysiol. 2006;117:781–788. doi: 10.1016/j.clinph.2005.12.010. [DOI] [PubMed] [Google Scholar]
- Schuler V, Luscher C, Blanchet C, Klix N, Sansig G, Klebs K, Schmutz M, Heid J, Gentry C, Urban L, Fox A, Spooren W, Jaton AL, Vigouret J, Pozza M, Kelly PH, Mosbacher J, Froestl W, Kaslin E, Korn R, Bischoff S, Kaupmann K, van der Putten H, Bettler B. Epilepsy, hyperalgesia, impaired memory, and loss of pre- and postsynaptic GABAB responses in mice lacking GABAB(1) Neuron. 2001;31:47–58. doi: 10.1016/s0896-6273(01)00345-2. [DOI] [PubMed] [Google Scholar]
- Shah M, Haylett D. The pharmacology of hSK1 Ca2+-activated K+ channels expressed in mammalian cell lines. Br J Pharmacol. 2000;129:627–630. doi: 10.1038/sj.bjp.0703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Javadzadeh-Tabatabaie M, Benton DC, Ganellin CR, Haylett DG. Enhancement of hippocampal pyramidal cell excitability by the novel selective slow-afterhyperpolarization channel blocker 3-(triphenylmethylaminomethyl)pyridine (UCL2077) Mol Pharmacol. 2006;70:1494–1502. doi: 10.1124/mol.106.026625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah MM, Miscony Z, Javadzadeh-Tabatabaie M, Ganellin CR, Haylett DG. Clotrimazole analogues: effective blockers of the slow afterhyperpolarization in cultured rat hippocampal pyramidal neurones. Br J Pharmacol. 2001;132:889–898. doi: 10.1038/sj.bjp.0703895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipila ST, Huttu K, Voipio J, Kaila K. Intrinsic bursting of immature CA3 pyramidal neurons and consequent giant depolarizing potentials are driven by a persistent Na+ current and terminated by a slow Ca2+-activated K+ current. Eur J Neurosci. 2006;23:2330–2338. doi: 10.1111/j.1460-9568.2006.04757.x. [DOI] [PubMed] [Google Scholar]
- Spencer Susan S. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–227. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol. 1987;385:733–759. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm J. Potassium currents in hippocampal pyramidal cells. Prog Brain Res. 1990;83:161–187. doi: 10.1016/s0079-6123(08)61248-0. [DOI] [PubMed] [Google Scholar]
- Sunderam S, Gluckman B, Reato D, Bikson M. Toward rational design of electrical stimulation strategies for epilepsy control. Epilepsy Behav. 2010;17:6–22. doi: 10.1016/j.yebeh.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson TH. The pathophysiology of human mesial temporal lobe epilepsy. J Clin Neurophysiol. 1995;12:15–22. [PubMed] [Google Scholar]
- Tang Y, Durand DM. A novel electrical stimulation paradigm for the suppression of epileptiform activity in an in vivo model of mesial temporal lobe status epilepticus. IJNS. 2012;22:12500–12501–12514. doi: 10.1142/S0129065712500062. [DOI] [PubMed] [Google Scholar]
- Tapia R, Sitges M. Effect of 4-aminopyridine on transmitter release in synaptosomes. Brain Res. 1982;250:291–299. doi: 10.1016/0006-8993(82)90423-1. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Fisher RS. Brain stimulation for epilepsy. Lancet Neurol. 2004;3:111–118. doi: 10.1016/s1474-4422(03)00664-1. [DOI] [PubMed] [Google Scholar]
- Tian G-F, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Want X, Zielke H, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toprani S, Durand DM. Fiber tract stimulation can reduce epileptiform activity in an in vitro bilateral hippocampal slice preparation. Exp Neurol. 2013;240:28–43. doi: 10.1016/j.expneurol.2012.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toprani S, Najm I, Durand D. Seizure suppression by low frequency electrical stimulation of the fimbria in combined hippocampus-entorhinal cortex slices in rats. Ann Neurol. 2008;64:S4–S64. [Google Scholar]
- Toprani S, Najm I, Durand D. Fiber tract stimulation for bilateral hippocampal seizure prevention. 2010 Neuroscience Meeting Planner. 2010 Program No. 657.29. [Google Scholar]
- Traub RD, Borck C, Colling SB, Jefferys JGR. On the structure of ictal events in vitro. Epilepsia. 1996;37:879–891. doi: 10.1111/j.1528-1157.1996.tb00042.x. [DOI] [PubMed] [Google Scholar]
- Trussel LO, Jackson MB. Dependence of an adenosine-activated potassium current on a GTP-binding protein in mammalian central neurons. J Neurosci. 1987;7:3306–3316. doi: 10.1523/JNEUROSCI.07-10-03306.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unekwe PC, Savage AO. The effects of electrical stimulation, adenosine and adenosine-5′-triphosphate (ATP) on mouse rectal muscle. Pharmacol Res. 1991;23:389–398. doi: 10.1016/1043-6618(91)90053-z. [DOI] [PubMed] [Google Scholar]
- Urwyler S, Gjoni T, Koljatic J, Dupuis D. Mechanisms of allosteric modulation at GABAB receptors by CGP7930 and GS38783: effects on affinities and efficacies of orthosteric ligands with distinct intrinsic properties. Neuropharmacology. 2005;48:343–353. doi: 10.1016/j.neuropharm.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Vatanparast J, Janahmadi M. Contribution of apamin-sensitive SK channels to the firing precision but not to the slow afterhyperpolarization and spike frequency adaptation in snail neurons. Brain Res. 2009:1255. doi: 10.1016/j.brainres.2008.12.003. 57–66. [DOI] [PubMed] [Google Scholar]
- Velasco A, Velasco M, Velasco F, Menes D, Gordon F, Rocha L, Briones M, I M. Subacute and chronic electrical stimulation of the hippocampus on intractable temporal lobe seizures: preliminary report. Arch Med Res. 2000a;31:316–328. doi: 10.1016/s0188-4409(00)00064-3. [DOI] [PubMed] [Google Scholar]
- Velasco M, Velasco F, Velasco A, Boleaga B, Jiminez F, Brito F, Marquez I. Subacute electrical stimulation of the hippocampus blocks intractable temporal lobe seizures and paroxysmal EEG activities. Epilepsia. 2000b;41:158–169. doi: 10.1111/j.1528-1157.2000.tb00135.x. [DOI] [PubMed] [Google Scholar]
- Velisek L, Veliskova J, Stanton PK. Low-frequency stimulation of the kindling focus delays basolateral amygdala kindling in immature rats. Neurosci Lett. 2002;326:61–63. doi: 10.1016/s0304-3940(02)00294-x. [DOI] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Curr Opin Neurol. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Vergnes M, Boehrer A, Simler S, Bernasconi R, Marescaux C. Opposite effects of GABAB receptor antagonists on absences and convulsive seizures. Eur J Pharmacol. 1997;332:245–255. doi: 10.1016/s0014-2999(97)01085-6. [DOI] [PubMed] [Google Scholar]
- Villalobos C, Shakkottai V, Chandy K, Michelhaugh S, Andrade R. SKCa channels mediate the medium but not the slow calcium-activated hyperpolarization in cortical neurons. J Neurosci. 2004;24:3537–3542. doi: 10.1523/JNEUROSCI.0380-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonck K, Boon P, Achten E, De Reuck J, Caemaert J. Long-term amygdalohippocampal stimulation for refractory temporal lobe epilepsy. Ann Neurol. 2002;52:556–565. doi: 10.1002/ana.10323. [DOI] [PubMed] [Google Scholar]
- Vonck K, Boon P, Claeys P, Dedeurwaerdere S, Achten R, Van Roost D. Long-term deep brain stimulation for refractory temporal lobe epilepsy. Epilepsia. 2005;46:98–99. doi: 10.1111/j.1528-1167.2005.01016.x. [DOI] [PubMed] [Google Scholar]
- Weiss C, Presont AR, Oh MM, Schwarz RD, Welty D, Disterhoft JF. The M1 muscarinic agonist CI-1017 facilitates trace eyeblink conditioning in aging rabbits and increases the excitability of CA1 pyramidal neurons. J Neurosci. 2000;20:783–790. doi: 10.1523/JNEUROSCI.20-02-00783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Hong Z, Li Y, Zhou F, Shi J. Effects of low-frequency hippocampal stimulation on gamma-amino butyric acid type B receptor expression in pharmacoresistant amygdaloid kindling epileptic rats. Neuromodulation. 2013;16:105–113. doi: 10.1111/j.1525-1403.2012.00493.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto J, Ikeda A, Kinoshita M, Matsumoto R, Satow T, Takeshita K, Matsuhashi M, Mikuni N, Miyamoto S, Hashimoto N, Shibasaki H. Low-frequency electric cortical stimulation decreases interictal and ictal activity in human epilepsy. Seizure. 2006;15:520–527. doi: 10.1016/j.seizure.2006.06.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


