Abstract
Objectives:
CT offers a three-dimensional solution to the inaccuracies associated with lateral cephalogram-based cephalometric analysis. However, it is associated with significant concerns regarding ionizing radiation exposure. MRI offers a non-ionizing alternative, but this has been less well investigated. We present a novel gradient echo MRI sequence (“Black Bone”) and highlight the potential of this sequence in cephalometric analysis.
Methods:
After regional ethics approval, “Black Bone” imaging was obtained in eight patients in whom lateral cephalograms were available. “Black Bone”, T1 and T2 weighted spin echo imaging were obtained in the mid-sagittal plane, and measurements were compared with those obtained on the lateral cephalogram using both the Advantage Windows Workstation (GE Medical Systems, Buckinghamshire, UK) and the Dolphin® cephalometric software (v. 11.5.04.23, Premium; Dolphin Imaging, Chatsworth, CA) by one assessor. Further assessment was made by scoring the ease of landmark identification on a ten-point scale.
Results:
“Black Bone” imaging surpassed T1 and T2 weighted imaging in terms of cephalometric landmark identification. A number of mid-sagittal cephalometric landmarks could not be clearly identified on T2 weighted imaging, making analysis impossible. Measurements on “Black Bone” demonstrated the smallest discrepancy when compared with those obtained on the lateral cephalogram. The discrepancy seen between measurements completed on mid-sagittal MRI and the lateral cephalogram was compounded by inherent inaccuracies of the lateral cephalogram. The overall mean discrepancy between distance measurements on “Black Bone” imaging and those on the lateral cephalogram was 1–2 mm.
Conclusions:
Overall, “Black Bone” MRI offered an improved method of cephalometric landmark identification over routine MRI sequences, and provides a potential non-ionizing alternative to CT for three-dimensional cephalometrics.
Keywords: cephalometry, magnetic resonance imaging, dentofacial deformities, malocclusion
Introduction
In the 80 years since Broadbent1 first introduced the cephalogram, practice has changed very little. The literature is, however, replete with studies highlighting inaccuracies associated with the cephalogram and cephalometric analysis. Many of the key landmarks are derived from the projection of the bony structures rather than being actual physical points on the skull. The identification of these points is largely dependent on manual identification, with some landmarks being located with more precision than others.2–4 Continued reliance on two-dimensional (2D) representations of anatomy, which disregards the contribution of soft tissues or any asymmetry that may exist in the patient, is somewhat surprising.5–10 Whilst CT and cone beam CT (CBCT) have been seen as potentially valuable for cephalometry, offering methods for three-dimensional (3D) analyses, the fundamental difficulty lies in the lack of comparative norms. The available databases of lateral and frontal cephalograms from normal and treated patient populations were established prior to appreciation of the risks of radiation exposure. Obtaining a similar database with CT and CBCT today would arguably be unethical on safety grounds. The American Academy of Oral and Maxillofacial Radiology, Springfield, IL, recently published clinical recommendations regarding the use of CBCT in orthodontics, with guidelines focussed on minimizing or eliminating radiation exposure in diagnostic imaging.11 Four key messages for orthodontic practice were highlighted: (1) image appropriately by applying imaging selection recommendations, (2) assess the radiation dose risk, (3) minimize patient radiation exposure and (4) maintain professional competency in performing and interpreting CBCT studies.11 The cumulative effects of ionizing radiation were identified as a significant concern, particularly in young patients with benign conditions.
MRI offers a non-ionizing method of imaging such patients, but this modality has received little attention in cephalometrics. The potential of a 3D MRI method of cephalometry with the ability to perform landmark identification in orthogonal planes and on 3D reconstructed images with available normative values could revolutionize the management of patients with facial disproportion.
We have previously reported the “Black Bone” MRI sequence. This gradient echo sequence is optimized to minimize the soft tissue contrast and enhance the bone–soft tissue boundary. The technique uses a short echo time and repetition time with a low flip angle and volume acquisition, resulting in short imaging times (Table 1). The biometric accuracy of the technique and its ability to create 3D reconstructed images of the craniofacial skeleton have been demonstrated.12,13
Table 1.
“Black Bone” MRI parameters
| Parameter | Value |
| Repetition time | 8.6 ms |
| Echo time | 4.2 ms |
| Scan field of view | 24 cm |
| Phase encode | 256 |
| Frequency encode | 256 |
| Receive bandwidth | 31.25 |
| ZIP | 2512 |
| NEX | 2 |
| Echo train length | 1 |
NEX, number of excitations; ZIP, zerofill interpolation.
The aim of this preliminary comparative study was to determine the potential of “Black Bone” and T1 and T2 weighted spin echo MRI, using the lateral cephalogram as the “gold standard.”
Materials and Methods
Patients
Ethical approval was granted by the Berkshire Research Ethics Committee (Berkshire, UK) for MRI examination in patients aged 14 years and older in whom lateral cephalograms were available. Written informed consent from the participants and/or their parents was obtained. Recruitment was completed in two phases: (1) adult volunteers without significant facial disproportion to confirm viability of the study and (2) patients referred for consideration of orthognathic surgery before commencement of orthodontic treatment. Patients who had previously undergone surgery, had completed orthodontic treatment or had fixed orthodontic appliances were excluded. In total, three adult volunteers in Cohort 1 and five patients in Cohort 2 were recruited, with a mean age of 37 years (range, 21–60 years) in Cohort 1 and 19 years (range, 16–27 years) in Cohort 2.
Imaging
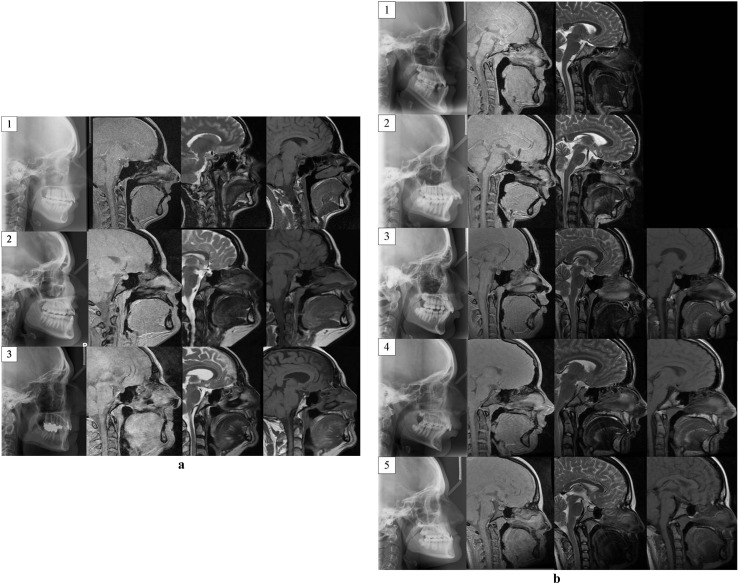
MRI was completed on a 1.5 T magnet. Sagittal T1, T2 weighted spin echo and “Black Bone” images were obtained with image acquisition centred on the mid-sagittal structures to permit direct comparison with lateral radiographs (Figure 1a,b). Patients were asked to bite on their molar teeth to maintain their normal dental occlusion to enhance alignment of the MRI and lateral cephalogram images.
Figure 1.
(a) Lateral cephalogram (left), sagittal “Black Bone” (second from left), sagittal T2 weighted (second from right) and sagittal T1 weighted MRI images of the three volunteers (indicated by numbers 1–3) used for cephalometric analysis. (b) Lateral cephalogram (left), sagittal “Black Bone” (second from left), sagittal T2 weighted (second from right) and sagittal T1 weighted MRI images of the five patients (indicated by numbers 1–5) included for cephalometric analysis. Sagittal T1 imaging was not acquired in Patients 1 and 2
Cephalometric analysis
“Black Bone” data sets were compared with those of the lateral cephalogram using midline anatomical landmarks identifiable on both modalities (Table 2). Analysis was completed manually using Advantage Windows Workstation v. 4.2 (AWW; GE Medical Systems, Buckinghamshire, UK), and with the Dolphin® cephalometric software v. 11.5.04.23 Premium (screen resolution 1680 × 1050 pixels; Dolphin Imaging, Chatsworth, CA).
Table 2.
Distance and angle measurements used for cephalometric analysis
| Angles | Distance |
| SNA | S-N |
| SNB | S-PNS |
| ANB | S-ANS |
| Interincisal angle | S-Gn |
| U1-NA angle | Me-ANS |
| L1-NB angle | N-A |
| N-B | |
| N-Pog | |
| U1-NA | |
| L1-NB | |
| PNS-ANS |
1, incisor; A, A point; ANS, anterior nasal spine; B, B point; Gn, gonion; L, lower; Me, menton; N, nasion; PNS, posterior nasal spine; Pog, pogonion; S, sella; U, upper.
Advantage Windows Workstation
The lateral cephalogram was imported in digital imaging and communications in medicine format into an AWW. The magnification factor of the lateral cephalogram was determined using the integral measuring device. The image was magnified and a 10 mm distance on the device measured on ten occasions and a mean result obtained for the image. This provided the multiplication factor to standardize the measurements obtained on the cephalogram.
The biometric accuracy of the “Black Bone” sequence had previously been determined, confirming that a standardization factor was not required.12
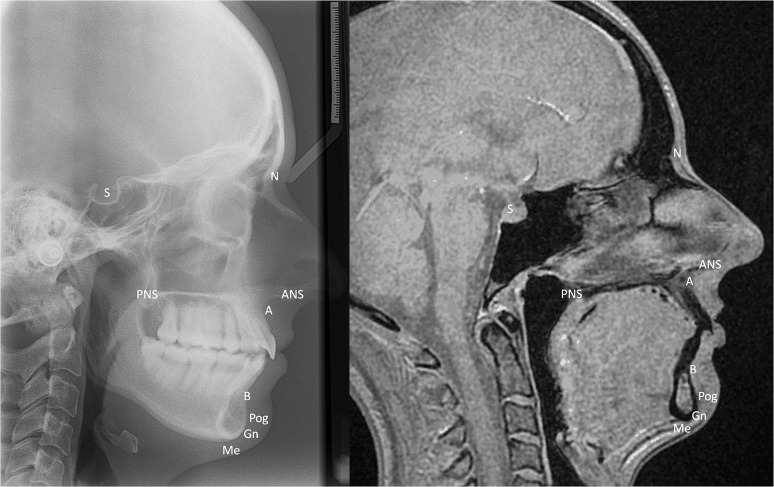
For both the lateral cephalogram and the sagittal MRI image, cephalometric analysis was completed in a standardized manner. For the MRI data sets, the mid-sagittal image in the right lateral view was used for analysis (Figure 2). The cephalometric points were identified on the magnified image and a marker placed; for the midpoint of the pituitary fossa, an oval drawing object was used to correlate with the inferior aspect of the fossa, and the centre of the object marker was used to define the centre of the fossa. The distances and angles between these points were each measured ten times.
Figure 2.
Lateral cephalogram (left) and mid-sagittal “Black Bone” image (right) with cephalometric landmarks identified. A, A point; ANS, anterior nasal spine; B, B point; Gn, gonion; Me, menton; N, nasion; PNS, posterior nasal spine; Pog, pogonion; S, sella
To determine the extent of the discrepancy between measurements obtained from “Black Bone” and the lateral cephalogram, a mean value was calculated across all repeated measures for each distance or angle. The difference in the mean values between the lateral cephalogram and “Black Bone” was subsequently combined for all patients to obtain an overall mean for both the angular and the distance measurements.
For the volunteer data sets (Cohort 1), the ease with which the cephalometric points could be identified was recorded on a ten-point scale from 0 (poorly identifiable) to 10 (easily identifiable). A score was recorded for each of the ten cephalometric points, and these were subsequently combined to provide an overall score for the lateral cephalogram, T1, T2 and “Black Bone” imaging.
All measurements were entered into Microsoft Excel™ (Microsoft Corporation, Redmond, WA) and analysed using SPSS® v. 18.0 (statistical package for the social sciences; IBM Corporation, Somers, NY).
Dolphin cephalometric analysis
Analysis of the lateral cephalogram and sagittal MRI images was completed in a similar manner to that conducted using AWW. To avoid loss of image quality, the relevant digital imaging and communications in medicine image was directly imported into Dolphin; for the MRI data sets, the same mid-sagittal image used on the AWW was used. Calibration of measurements was performed by the software using two identifiable points. For the lateral cephalogram, a 10 mm distance on the integral measuring device was used. For the MRI images, the known field of view was used by marking the horizontal extremes of the image. Since the cephalometric points and measurements did not correspond to a standard cephalometric analysis method, the measurements were identified on Dolphin and combined to produce a new analysis protocol.
The repeatability of landmark identification was assessed with Dolphin for the lateral cephalogram, sagittal “Black Bone” and sagittal T1 imaging from one of the volunteers. Digitization was completed on ten occasions for each image, and the coefficient of variation calculated using the co-ordinates of each landmark from the sella, with alignment along a line between the sella and nasion, with the first digitization as the reference trace.
Comparisons between the lateral cephalogram, “Black Bone” imaging and T1 MRI were made in a similar manner to AWW. Measurements were automatically calculated from the identified cephalometric points by the software. These were exported to Microsoft Excel and analysed using SPSS. Repeatability was assessed using the coefficient of variation (CoV) expressed as a percentage [CoV=standard deviation (SD)/mean×100].
As a result of the findings in the first part of the study, analysis of T2 weighted MRI was not completed with the Dolphin cephalometric software.
All measurements were completed by one assessor. In view of the characteristic appearance of each MRI sequence, it was not possible to blind the assessor to the imaging.
Results
Advantage Windows Workstation
The CoV results for the lateral cephalogram for each of the three volunteers are shown in Table 3. For the angles, those involving the teeth and ANB demonstrated the greatest variability; the distance measurements involving the central incisor teeth also demonstrated increased variability. Comparable findings were seen for “Black Bone” imaging (Table 4). With T1 weighted imaging, there was greater variation seen than with both “Black Bone” and the lateral cephalogram (Table 5). Many of the cephalometric points could not be identified on T2 weighted imaging, making cephalometric analysis impossible. This was demonstrated by the scores obtained for the ease of point identification (Table 6). Overall, for the ten bony landmarks used, there was greater difficulty in identifying the landmarks on both T1 and T2 imaging. It was also noted that whilst the lateral cephalogram was considered to be the gold standard, there were some landmarks that were particularly challenging to identify, such as the posterior nasal spine (PNS) in cases of partially erupted wisdom teeth. Overall, the ease of cephalometric point identification on “Black Bone” was comparable to the lateral cephalogram.
Table 3.
Mean, standard deviation (SD) and coefficient of variation (CoV) results for repeated cephalometric measurements for the three volunteers on lateral cephalogram
| Measurements | Patient | ||||||||
| 1 | 2 | 3 | |||||||
| Mean | SD | CoV | Mean | SD | CoV | Mean | SD | CoV | |
| Angles | |||||||||
| SNA | 83.2 | 0.4 | 0.5 | 89.0 | 0.0 | 0.0 | 85.8 | 0.8 | 0.9 |
| SNB | 78.8 | 0.4 | 0.5 | 90.8 | 0.4 | 0.5 | 81.8 | 0.8 | 0.9 |
| ANB | 4.3 | 0.5 | 10.8 | 1.8 | 0.4 | 22.5 | 4.0 | 0.0 | 0.0 |
| Interincisal | 114.0 | 7.2 | 6.3 | 136.4 | 1.4 | 1.0 | 157.2 | 1.6 | 1.0 |
| U1-NA | 29.6 | 3.9 | 13.2 | 28.4 | 0.8 | 2.9 | 7.6 | 0.5 | 6.5 |
| L1-NB | 32.2 | 4.6 | 14.2 | 16.4 | 1.4 | 8.4 | 13.0 | 1.4 | 11.0 |
| Distances | |||||||||
| U1-NA | 3.7 | 0.3 | 7.4 | 5.8 | 0.4 | 6.9 | 1.4 | 0.3 | 24.7 |
| L1-NB | 5.6 | 0.3 | 4.5 | 0.9 | 0.2 | 24.8 | 2.0 | 0.3 | 14.0 |
| S-N | 68.0 | 0.3 | 0.4 | 63.2 | 0.3 | 0.4 | 68.5 | 0.5 | 0.7 |
| PNS-ANS | 52.9 | 1.2 | 2.2 | 49.2 | 0.8 | 1.6 | 50.5 | 0.6 | 1.2 |
| S-ANS | 84.8 | 1.7 | 2.0 | 79.8 | 0.4 | 0.4 | 87.3 | 0.4 | 0.5 |
| Me-ANS | 62.9 | 0.2 | 0.4 | 62.1 | 0.2 | 0.4 | 62.6 | 0.7 | 1.1 |
| N-Pog | 99.7 | 0.6 | 0.6 | 98.7 | 0.7 | 0.7 | 105.9 | 1.6 | 1.5 |
1, incisor; A, A point; ANS, anterior nasal spine; B, B point; Gn, gonion; L, lower; Me, menton; N, nasion; PNS, posterior nasal spine; Pog, pogonion; S, sella; U, upper.
Table 4.
Mean, standard deviation (SD) and coefficient of variation (CoV) results for repeated cephalometric measurements for the three volunteers on “Black Bone”
| Measurements | Patient | ||||||||
| 1 | 2 | 3 | |||||||
| Mean | SD | CoV | Mean | SD | CoV | Mean | SD | CoV | |
| Angles | |||||||||
| SNA | 83.8 | 1.0 | 1.2 | 91.8 | 0.4 | 0.4 | 84.4 | 0.5 | 0.6 |
| SNB | 79.2 | 0.8 | 1.0 | 88.6 | 0.5 | 0.6 | 80.4 | 0.5 | 0.6 |
| ANB | 4.8 | 0.4 | 8.4 | 3.2 | 0.4 | 12.6 | 4.0 | 0.0 | 0.0 |
| Interincisal | 123.6 | 2.4 | 2.0 | 131.6 | 3.0 | 2.3 | 137.4 | 4.8 | 3.5 |
| U1-NA | 21.8 | 2.1 | 9.5 | 21.2 | 0.8 | 3.6 | 18.4 | 3.8 | 20.4 |
| L1-NB | 31.0 | 2.6 | 8.5 | 24.6 | 3.6 | 14.8 | 21.4 | 2.1 | 9.7 |
| Distances | |||||||||
| U1-NA | 4.6 | 0.6 | 12.3 | 4.7 | 0.3 | 5.8 | 1.3 | 0.3 | 22.2 |
| L1-NB | 6.6 | 0.9 | 13.1 | 1.2 | 0.3 | 27.5 | 4.3 | 0.6 | 13.5 |
| S-N | 70.2 | 0.3 | 0.4 | 56.7 | 0.3 | 0.6 | 67.6 | 0.3 | 0.5 |
| PNS-ANS | 56.1 | 3.5 | 6.3 | 48.8 | 2.4 | 4.9 | 57.4 | 0.3 | 0.6 |
| S-ANS | 86.3 | 2.2 | 2.6 | 78.8 | 0.3 | 0.4 | 86.8 | 0.5 | 0.5 |
| Me-ANS | 64.7 | 0.9 | 1.4 | 60.8 | 0.4 | 0.6 | 63.7 | 0.4 | 0.6 |
| N-Pog | 99.9 | 1.4 | 1.4 | 102.6 | 0.7 | 0.7 | 109.1 | 0.7 | 0.7 |
1, incisor; A, A point; ANS, anterior nasal spine; B, B point; Gn, gonion; L, lower; Me, menton; N, nasion; PNS, posterior nasal spine; Pog, pogonion; S, sella; U, Upper.
Table 5.
Mean, standard deviation (SD) and coefficient of variation (CoV) results for repeated cephalometric measurements for the three volunteers on T1 MRI
| Measurements | Patient | ||||||||
| 1 | 2 | 3 | |||||||
| Mean | SD | CoV | Mean | SD | CoV | Mean | SD | CoV | |
| Angles | |||||||||
| SNA | 83.4 | 2.4 | 2.9 | 93.0 | 0.0 | 0.0 | 90.0 | 0.8 | 0.9 |
| SNB | 80.3 | 1.1 | 1.4 | 90.3 | 0.5 | 0.5 | 84.7 | 1.0 | 1.1 |
| ANB | 4.2 | 1.8 | 44.5 | 2.7 | 0.5 | 18.0 | 5.3 | 0.5 | 9.0 |
| Interincisal | 121.7 | 5.4 | 4.4 | 128.7 | 2.5 | 2.0 | 142.7 | 1.3 | 0.9 |
| U1-NA | 29.3 | 3.8 | 12.8 | 24.7 | 1.7 | 7.0 | 9.7 | 0.5 | 5.0 |
| L1-NB | 26.7 | 6.0 | 22.3 | 24.3 | 2.5 | 10.4 | 24.0 | 1.7 | 6.9 |
| Distances | |||||||||
| U1-NA | 7.9 | 5.0 | 62.4 | 3.2 | 0.6 | 18.5 | 1.4 | 0.4 | 31.4 |
| L1-NB | 6.1 | 0.6 | 9.7 | 2.2 | 0.7 | 33.1 | 3.0 | 0.3 | 10.5 |
| S-N | 69.7 | 2.0 | 2.8 | 59.1 | 0.5 | 0.8 | 65.5 | 0.2 | 0.3 |
| PNS-ANS | 56.6 | 3.8 | 6.8 | 48.6 | 1.5 | 3.1 | 49.7 | 1.9 | 3.9 |
| S-ANS | 87.0 | 3.3 | 3.8 | 80.3 | 1.0 | 1.2 | 86.9 | 0.5 | 0.6 |
| Me-ANS | 66.1 | 1.5 | 2.2 | 61.6 | 0.6 | 0.9 | 65.0 | 0.4 | 0.6 |
| N-Pog | 98.6 | 1.4 | 1.4 | 100.4 | 0.3 | 0.3 | 106.6 | 0.3 | 0.3 |
1, incisor; A, A point; ANS, anterior nasal spine; B, B point; Gn, gonion; L, lower; Me, menton; N, nasion; PNS, posterior nasal spine; Pog, pogonion; S, sella; U, upper.
Table 6.
Mean ± standard deviation (SD) confidence scores for cephalometric point identification on T1, T2, “Black Bone” and lateral cephalogram
| Identification point | T1 | T2 | “Black Bone” | Lateral cephalogram |
| S | 7.4 ± 0.9 | 6.3 ± 2.3 | 8.6 ± 0.5 | 8.3 ± 1.0 |
| N | 4.1 ± 2.7 | 1.6 ± 2.2 | 6.7 ± 2.8 | 6.8 ± 2.2 |
| A | 3.6 ± 2.5 | 1.3 ± 1.8 | 7.4 ± 0.7 | 6.5 ± 1.7 |
| B | 6.9 ± 1.6 | 6.4 ± 0.9 | 8.2 ± 0.4 | 8.3 ± 0.5 |
| PNS | 2.4 ± 2.2 | 0.0 ± 0.0 | 6.1 ± 1.4 | 5.0 ± 2.7 |
| ANS | 2.4 ± 2.2 | 0.4 ± 0.7 | 6.9 ± 0.8 | 4.5 ± 2.6 |
| Gn | 6.8 ± 0.7 | 6.5 ± 1.3 | 8.2 ± 0.8 | 8.5 ± 0.6 |
| Pog | 7.6 ± 0.7 | 7.0 ± 0.9 | 8.7 ± 0.5 | 8.8 ± 0.5 |
| Me | 7.6 ± 0.7 | 7.4 ± 0.7 | 8.7 ± 0.5 | 8.8 ± 0.5 |
| Overall score | 48.8 ± 9.9 | 36.8 ± 2.6 | 69.4 ± 5.3 | 65.3 ± 8.7 |
A, A point; ANS, anterior nasal spine; B, B point; Gn, gonion; Me, menton; N, nasion; PNS, posterior nasal spine; Pog, pogonion; S, sella.
For the three adult volunteers, the overall discrepancy between the “Black Bone” and the lateral cephalogram results was 1.2° ± 0.9° for the angles and 2.1 ± 1.8 mm for the distances. For the five patients, the overall discrepancy was 1.5° ± 1.6° for the angles and 3.0 ± 2.9 mm for the distances. The greatest discrepancy occurred with the measurement between the PNS and anterior nasal spine.
Dolphin cephalometric software
Similar results were obtained with the Dolphin cephalometric software. In terms of repeatability, the greatest variability was seen for the lateral cephalogram for measurements associated with the central incisor teeth. In terms of the bony landmarks, the coefficient of variation for the direct distance between landmark and sella varied between 31% and 68%.
For the repeated measures across the ten digitizations of the “Black Bone” sagittal images, the greatest variability occurred with measurements associated with the teeth. This was because of difficulty in clearly identifying the central incisor outline on one sagittal image.
Digitization of the sagittal T1 weighted MRI images demonstrated higher CoV for the distance and angle measurements than for those of both the lateral cephalogram and “Black Bone”.
Overall, the discrepancy between “Black Bone” and the lateral cephalogram was 2.1° ± 1.7° for the angles and 2.8 ± 2.7 mm for the distances. A greater discrepancy was seen for T1 results than for those of the lateral cephalogram for the same cohort, with an overall discrepancy of 5.0° ± 2.9° for the angles and 3.3 ± 3.2 mm for the distances.
Discussion
It has been demonstrated that “Black Bone” MRI offers a potential alternative to lateral cephalometry with landmarks that can be clearly identified, and with comparable accuracy of the lateral cephalogram. The ease with which the cephalometric landmarks could be identified on “Black Bone” imaging was enhanced by the minimal soft tissue contrast, contributing to improved landmark identification compared with T1 and T2 weighted imaging.
As with any cephalometric study, there are a number of limitations, which should be noted. Most fundamental is the use of the lateral cephalogram as the gold standard. It was not possible to standardize positioning of the patient between MRI and the lateral cephalogram because of the inherent nature of these investigations—one being performed with the patient upright and the other whilst supine. This is one of the benefits of using CBCT for cephalometric analysis.14,15 However, the fundamental concern regarding ionizing radiation exposure with this imaging modality remains. Periago et al16 investigated the accuracy of 3D images acquired using CBCT, reporting a discrepancy of less than 2 mm between the anatomical and the CBCT measurements in all but 2 (10%) measurements. In their article, they suggested that the dose associated with CBCT was of the same magnitude as that obtained from dental radiographs; however, more recently, it has been demonstrated that such assumptions are a gross underestimate.17–19 Kumar et al20 investigated whether CBCT synthesized cephalograms using Dolphin provided the same accuracy as conventional cephalograms. In total, 31 patients treated in the dentofacial deformities programme were recruited and underwent CBCT in addition to routine cephalogram. In total, 12 linear and 5 angular measurements were assessed, and in most cases, no statistical difference was found between the two modalities. Waitzman et al21 used five dry skulls impregnated with small pieces of metal to standardize measurement points to perform eight measurements on both CT and directly on the skulls. They reported a percentage difference between CT and direct measurement of 0.1–3.0%, concluding that craniofacial measurements from CT scans were accurate and reproducible. Similar findings have been reported by a number of authors.22–24 Few studies have directly compared CT or CBCT with the lateral cephalogram.
Overall, it was noted that the level of discrepancy between “Black Bone” and the lateral cephalogram was within a more acceptable range in cases where the cephalometric points were easily identified. Inaccuracy was compounded by the associated errors of measurement between two points, being minimized in the second part of the study by using the Dolphin cephalometric software.
This is the first study to compare MRI-based cephalometric measurement with the lateral cephalogram. Previous work using MRI in cephalometry to date has been in the evaluation of the airway, particularly in obstructive sleep apnoea.25–27 Cotton et al28 reviewed 11 craniometric points on MRI, stating that these could be reliably identified. The points identified included the glabella, bregma, lambda, opisthocranion, opisthion, basion, inion, porion, infraorbital and eurion. Cevidanes et al29 evaluated the repeatability, measurement error and validity of locating anatomical landmarks on high-resolution MRI with 1 mm isotropic voxel resolution. In total, ten anatomical landmarks were identified in the coronal, axial and sagittal planes of four head MRI. Information regarding the sequence used was not included, and to our knowledge, this work has not been published. Plooij et al,30 in their review article, concluded that MRI was unsuitable for image fusion for monitoring and follow-up in view of the horizontal position of the patient, and long acquisition time with risk of facial movement.
Whilst other MRI sequences are often more traditionally used for delineating bony anatomy, such as short tau inversion recovery and proton density weighted sequences, Cotton et al28 studied T1 and T2 weighted spin echo sequences, concluding that these sequences permitted visualization of the cephalometric points, thus providing the best available comparators for assessing “Black Bone” MRI.
It has been demonstrated that there are some inherent difficulties when attempting to compare sagittal MRI images with the gold standard lateral cephalogram. Some of the cephalometric landmarks incorporated into traditional analysis are based on projection of bony landmarks rather than representing true anatomy, which resulted in fewer landmarks being available in the true mid-sagittal plane for comparison. The central incisor teeth are poorly defined on a single mid-sagittal MRI image, making measurements here inaccurate. Overall, discrepancies between the MRI and the lateral cephalogram measurements were in the range of 1–4 mm, compounded by difficulties associated with performing cephalometric measurements on the lateral cephalogram itself. Frequent difficulties with the lateral cephalogram included identification of the PNS in the presence of unerrupted wisdom teeth, a problem not encountered on mid-sagittal MRI images. In addition, the lateral cephalogram has inherent projection inaccuracies both because of its 2D nature and the requirement for interpolation, in cases where there is even the most minor of facial skeletal asymmetry. The discrepancies found between the “Black Bone” and the lateral cephalogram results may therefore reflect the problems associated with using the lateral cephalogram as a gold standard. It is noted that in total, only eight data sets from patients and volunteers were included in this aspect of the study, with review by one assessor, and a larger study would be required to fully assess the significance between results.
It was not possible with the available software to undertake measurements across multiple MRI image slices. Whilst landmark identification on 3D reconstructed images has become possible, it is not currently possible to produce such 3D reconstructed images of the craniofacial skeleton from “Black Bone” data sets with the software used in this study. The “Black Bone” sequence is acquired as a 3D volume-making reconstruction in any plane as a post-processing option possible. Because many of the traditional landmarks used in lateral cephalometry are based on projection rather than definable landmarks on the skull, additional reliable landmarks that can be identified on both orthogonal and 3D imaging are required. Of the midline structures, it was found that the nasion, menton, pogonion and anterior nasal spine were most reliably identified.
Methods to create 3D images of the craniofacial skeleton from “Black Bone” MRI have been developed, but these currently remain time-intensive processes. Furthermore, cephalometry on 3D reconstructed images requires the definition of landmarks on complex curving structures.5,31 Cavalcanti et al32 examined the precision and accuracy of anthropometric measurements using 3D volume rendered CT. Using 13 cadaver heads, they reviewed ten linear measurements based on eight craniometric anatomical landmarks, which were identified both on the 2D and 3D images, and, subsequently, directly measured on the cadavers. The standard error was between 0.45% and 1.44%, with no statistically significant difference between imaging and physical measurements.
The volume acquisition of “Black Bone” data sets like CT lends itself to landmark identification in orthogonal planes, making it a feasible contender in future cephalometric work. The image acquisition time is short, and there are no ethical issues in completing MRI examinations in healthy volunteers to obtain the necessary database of normative values. The main limitation of the technique is that, as with all MRI sequences, imaging is degraded by the presence of metallic artefacts, making cephalometric analysis in the presence of fixed orthodontic appliances particularly challenging.
In conclusion, “Black Bone” MRI has been demonstrated to offer a potential non-ionizing alternative to CT and CBCT for 3D cephalometry.
Funding
Supported by the AO Research Fund of the AO Foundation (Dübendorf, Switzerland), project number C-09-1-W.
References
- 1.Broadbent BH. A new x-ray technique and its application to orthodontia. Angle Orthod 1931; 1: 45–66 [Google Scholar]
- 2.Palomo JM, Yang CY, Hans MG. Clinical application of three-dimensional craniofacial imaging in orthodontics. J Med Sci 2005; 25: 269–278 [Google Scholar]
- 3.Moyers RE, Bookstein FL. The inappropriateness of 5conventional cephalometrics. Am J Orthod 1979; 75: 599–617 [DOI] [PubMed] [Google Scholar]
- 4.Geelen W, Wenzel A, Gotfredsen E, Kruger M, Hansson LG. Reproducibility of cephalometric landmarks on conventional film, hardcopy, and monitor-displayed images obtained by the storage phosphor technique. Eur J Orthodon 1998; 20: 331–340 [DOI] [PubMed] [Google Scholar]
- 5.El H, Palomo JM. Measuring the airway in 3 dimensions: a reliability and accuracy study. Am J Orthod Dentofacial Orthop 2010; 137(Suppl. 4):e1–e9 10.1016/j.ajodo.2010.01.014 [DOI] [PubMed] [Google Scholar]
- 6.Baumrind S, Frantz RC. The reliability of head film measurements. 2. Conventional angular and linear measures. Am J Orthod 1971; 60: 505–517 [DOI] [PubMed] [Google Scholar]
- 7.Tsang KH, Cooke MS. Comparison of cephalometric analysis using a non-radiographic sonic digitizer (DigiGraph Workstation) with conventional radiography. Eur J Orthod 1999; 21: 1–13 [DOI] [PubMed] [Google Scholar]
- 8.Baumrind S, Frantz RC. The reliability of head film measurements. Am J Orthod 1971; 60: 111–127 [DOI] [PubMed] [Google Scholar]
- 9.Papadopoulos MA, Jannowitz C, Boettcher P, Henke J, Stolla R, Zeilhofer H, et al. Three-dimensional fetal cephalometry: an evaluation of the reliability of cephalometric measurements based on three-dimensional CT reconstructions and on dry skulls of sheep fetuses. J Craniomaxillofac Surg 2005; 33: 229–237 [DOI] [PubMed] [Google Scholar]
- 10.Kragskov J, Bosch C, Gyldensted C, Sindet-Pedersen S. Comparison of the reliability of craniofacial anatomic landmarks based on cephalometric radiographs and three-dimensional CT scans. Cleft Palate Craniofac J 1997; 34: 111–116 [DOI] [PubMed] [Google Scholar]
- 11.American Academy of Oral and Maxillofacial Radiology Clinical recommendations regarding use of cone beam computed tomography in orthodontics. Position statement by the American Academy of Oral and Maxillofacial Radiology. Oral Surg Oral Med Oral Path Oral Rad 2013; 116: 238–257 10.1016/j.oooo.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 12.Eley KA, McIntyre A, Watt-Smith SR, Golding SJ. “Black Bone” MRI: a partial flip angle technique for radiation reduction in craniofacial imaging. Br J Radiol 2012; 85: 272–278 10.1259/bjr/95110289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eley KA, Watt-Smith SR, Golding SJ. “Black bone” MRI: a potential alternative to CT when imaging the head and neck: report of eight clinical cases and review of the Oxford experience. Br J Radiol 2012; 85: 1457–1464 10.1259/bjr/16830245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayome M, Park JH, Kook YA. New three-dimensional cephalometric analyses among adults with a skeletal class I pattern and normal occlusion. Korean J Orthod 2013; 43: 62–73 10.4041/kjod.2013.43.2.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frongia G, Piancino MG, Bracco P. Cone-beam computed tomography: accuracy of three-dimensional cephalometry analysis and influence of patient scanning position. J Craniofac Surg 2012; 23: 1038–1043 10.1097/SCS.0b013e318252d5e1 [DOI] [PubMed] [Google Scholar]
- 16.Periago DR, Scarfe WC, Moshiri M, Scheetz JP, Silveira AM, Farman AG. Linear accuracy and reliability of cone beam CT derived 3-dimensional images constructed using an orthodontic volumetric rendering program. Angle Orthod 2008; 78: 387–395 10.2319/122106-52.1 [DOI] [PubMed] [Google Scholar]
- 17.Miracle AC, Mukherji SK. Conebeam CT of the head and neck, part 1: physical principles. AJNR Am J Neuroradiol 2009; 30: 1088–1095 10.3174/ajnr.A1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pauwels R, Beinsberger J, Collaert B, Theodorakou C, Rogers J, Walker A, et al. Effective dose range for dental cone beam computed tomography scanners. Eur J Radiol 2012; 81: 267–271 10.1016/j.ejrad.2010.11.028 [DOI] [PubMed] [Google Scholar]
- 19.Roberts JA, Drage NA, Davies J, Thomas DW. Effective dose from cone beam CT examinations in dentistry. Br J Radiol 2009; 82: 35–40 10.1259/bjr/31419627 [DOI] [PubMed] [Google Scholar]
- 20.Kumar V, Ludlow J, Cevidanes LH, Mol A. In vivo comparison of conventional and cone beam CT synthesized cephalograms. Angle Orthod 2008; 78: 873–879 10.2319/082907-399.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waitzman AA, Posnick JC, Armstrong DC, Pron GE. Craniofacial skeletal measurements based on computed tomography: part I. accuracy and reproducibility. Cleft Palate Craniofac J 1992; 29: 112–117 [DOI] [PubMed] [Google Scholar]
- 22.Cavalcanti MG, Haller JW, Vannier MW. Three-dimensional computed tomography landmark measurement in craniofacial surgical planning: experimental validation in vitro. J Oral Maxillofac Surg 1999; 57: 690–694 [DOI] [PubMed] [Google Scholar]
- 23.Citardi MJ, Herrmann B, Hollenbeak CS, Stack BC, Cooper M, Bucholz RD. Comparison of scientific calipers and computer-enabled CT review for the measurement of skull base and craniomaxillofacial dimensions. Skull Base 2001; 11: 5–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopes PM, Moreira CR, Perrella A, Antunes JL, Cavalcanti MG. 3-D volume rendering maxillofacial analysis of angular measurements by multislice CT. Oral Surg Oral Med Oral Path Oral Radiol Endod 2008; 105: 224–230 10.1016/j.tripleo.2007.08.036 [DOI] [PubMed] [Google Scholar]
- 25.Bharadwaj R, Ravikumar A, Krishnaswamy NR. Evaluation of craniofacial morphology in patients with obstructive sleep apnea using lateral cephalometry and dynamic MRI. Indian J Dent Res 2011; 22: 739–748 10.4103/0970-9290.94566 [DOI] [PubMed] [Google Scholar]
- 26.Pirila-Parkkinen K, Lopponen H, Nieminen P, Tolonen U, Paakko E, Pirttiniemi P. Validity of upper airway assessment in children: a clinical, cephalometric, and MRI study. Angle Orthod 2011; 81: 433–439 10.2319/063010-362.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee RW, Sutherland K, Chan AS, Zeng B, Grunstein RR, Darendeliler MA, et al. Relationship between surface facial dimensions and upper airway structures in obstructive sleep apnea. Sleep 2010; 33: 1249–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cotton F, Rozzi F, Vallee B, Pachai C, Hermier M, Guihard-Costa A, et al. Cranial sutures and craniometric points detected on MRI. Surg Radiol Anat 2005; 27: 64–70 10.1007/s00276-004-0283-6 [DOI] [PubMed] [Google Scholar]
- 29.Cevidanes LH, Busby B, Cheek C, Proffit WR, Gerig G, Franco AA, et al. Precision of 3D craniofacial landmark data using magnetic resonance scans. International Association for Dental Research, Conference Abstracts 2002 (Abstr 3128)
- 30.Plooij JM, Maal TJJ, Haers P, Borstlap WA, Kuijpers-Jagtman AM, Berge SJ. Digital three-dimensional image fusion processes for planning and evaluating orthodontics and orthognathic surgery. A systematic review. Int J Oral Maxillofac Surg 2011; 40: 341–352 10.1016/j.ijom.2010.10.013 [DOI] [PubMed] [Google Scholar]
- 31.deOliveira AE, Cevidanes LH, Phillips C, Motta A, Burke B, Tyndall D. Observer reliability of three-dimensional cephalometric landmark identification on cone-beam computerized tomography. Oral Surg Oral Med Oral Path Oral Radiol Endod 2009; 107: 256–265 10.1016/j.tripleo.2008.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavalcanti MG, Rocha SS, Vannier MW. Craniofacial measurements based on 3D-CT volume rendering: implications for clinical applications. Dentomaxillofac Radiol 2004; 33: 170–176 10.1259/dmfr/13603271 [DOI] [PubMed] [Google Scholar]