Abstract
Aims
To evaluate the comparative efficacy (50% reduction in seizure frequency) and tolerability (premature withdrawal due to adverse events) of anti-epileptic drugs (AEDs) for refractory epilepsy.
Methods
We searched Cochrane Central Register of Controlled Trials (Cochrane Library 2009, issue 2) including Epilepsy Group's specialized register, MEDLINE (1950 to March 2009), EMBASE (1980 to March 2009), and Current Contents Connect (1998 to March 2009) to conduct a systematic review of published studies, developed a treatment network and undertook a network meta-analysis.
Results
Forty-three eligible trials with 6346 patients and 12 interventions, including placebo, contributed to the analysis. Only three direct drug comparator trials were identified, the remaining 40 trials being placebo-controlled. Conventional random-effects meta-analysis indicated all drugs were superior in efficacy to placebo (overall odds ratio (OR] 3.78, 95% CI 3.14, 4.55) but did not permit firm distinction between drugs on the basis of the efficacy or tolerability. A Bayesian network meta-analysis prioritized oxcarbazepine, topiramate and pregabalin on the basis of short term efficacy. However, sodium valproate, levetiracetam, gabapentin and vigabatrin were prioritized on the basis of short-term efficacy and tolerability, with the caveat that vigabatrin is recognized as being associated with serious visual disturbance with chronic use.
Conclusion
Of the wide range of AEDs licensed for the treatment of refractory epilepsy, sodium valproate, levetiracetam and gabapentin demonstrated the best balance of efficacy and tolerability. Until regulators mandate greater use of active comparator trials with longer term follow-up, network meta-analysis provides the only available means to quantify these clinically important parameters.
Keywords: anticonvulsants/therapeutic use, comparative study, epilepsy/drug therapy, meta-analysis, review, treatment outcome
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
About one-third of patients with epilepsy have refractory disease with much of the prescribing occurring in primary care.
Anti-epileptic drugs (AEDs) prescribed as adjunctive treatment for refractory epilepsy are all more effective than placebo at reducing seizure rate but there are limited data on the comparative efficacy and tolerability of these drugs.
WHAT THIS STUDY ADDS
We used systematic review and network meta-analysis to identify and compare the efficacy and tolerability profile of 11 AEDs to assist clinicians in prescribing decisions.
Introduction
Epilepsy, the occurrence of recurrent, unprovoked seizures [1] has an incidence of 50 to 100 cases per 100 000 population per year and a prevalence of approximately 5 to 8 cases per 1000 population [2, 3]. It is the most prevalent serious neurologic condition and results in considerable morbidity. Approximately one-third of patients with partial onset seizures develop chronic refractory ‘drug resistant’ epilepsy, the inability to derive sustained seizure freedom following a trial of two anti-epileptic drugs (AEDs) [4] thus requiring treatment with a combination of agents [5, 6]. Although diagnosis and initiation of therapy should initially be conducted within specialist departments in secondary/tertiary care centres, it is usually general practitioners in primary care who are tasked with this and involved in the long term management of this condition.
In Europe and the USA in the last two decades, 14 AEDs have been granted a marketing authorization for adjunctive treatment of partial seizures with or without secondary generalization in adults. However, based on the guidelines for clinical evaluation of AEDs from the International League Against Epilepsy (ILAE) [7], drug regulatory authorities only require new AEDs to demonstrate efficacy over placebo when added to baseline therapy. The current failure to obligate trials of the comparative effectiveness of emerging AEDs means that guideline developers and prescribers have difficulty making an informed choice among a range of available treatment options [8]. Moreover, in the absence of an established treatment hierarchy, it becomes progressively more difficult to establish the place in therapy for any new AED with the result that relevant guidance from the UK National Institute of Health and Clinical Excellence (NICE) lists a range of first and second line options [9]. The practical importance of the current (placebo-controlled) model used to approve AEDs has been highlighted as being questionable, offering little help in clinical decision making [10].
Comparative effectiveness (CE) research has the aim of defining which of a range of available treatments are the most effective, safe, or least costly when a number of options are available [11–14], helping clinicians use existing treatments more effectively. Where direct treatment comparisons have not been made, network meta-analysis can provide an objective way of comparing alternative treatments [15–17]. The approach respects the randomized allocation of treatments and permits the estimation of relative effectiveness of the different preparations with the aim of constructing a rational treatment hierarchy. We conducted a systematic review, constructed a treatment network and performed a network meta-analysis of all published studies of AEDs licensed for adjunctive treatment of chronic refractory partial epilepsy (with or without secondary generalization) to obtain indirect comparisons on their relative efficacy and tolerability.
Methods
Literature search
We searched for relevant randomized trials in the Cochrane Central Register of Controlled Trials (Cochrane Library 2009, issue 2), which contains the Epilepsy Group's specialized register, MEDLINE (via PubMED) (1950 to March 2009), EMBASE (1980 to March 2009) and Current Contents Connect databases (part of ISI Web of Knowledge) (1998 to MARCH 2009), adopting PRISMA (Preferred Reporting Items for Systematic reviews and Meta-analysis) recommendations [18, 19]. The search terms and limits are provided in the Supporting Information (Tables S1 and S2). We supplemented the database search by a hand-search of the reference lists of the identified studies (Figure 1).
Figure 1.
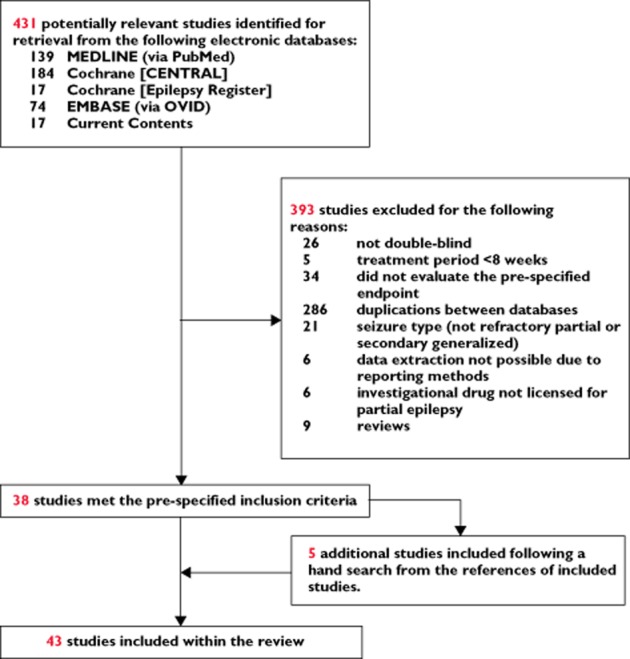
Study selection for inclusion in network meta-analysis
Study selection
We included trials if they were randomized, double-blind, placebo-controlled or active-controlled add-on design investigating acetazolamide, carbamazepine, clobazam, clonazepam, ethosuximide, gabapentin, lacosamide, lamotrigine, levetiracetam, oxcarbazepine, phenobarbital, phenytoin, pregabalin, primidone, sodium valproate, tiagabine, topiramate, valproic acid, vigabatrin or zonisamide; recruited adult patients (>18 years) with simple/complex partial seizure with or without secondary generalized tonic clonic seizures, were parallel or crossover design, of ≥8 weeks duration and reported seizure frequency and/or adverse effects as an outcome.
We excluded trials with a pre-randomization run-in response-conditional design where patients were allocated treatment only if they showed a predetermined response or if randomization was preceded by an open-label period in order to minimize the inclusion of data from an enriched population. Additional exclusion criteria included trials which incorporated a surgical intervention, use of other therapies which may affect seizure frequency, trials of open-label design, observational studies, conference proceedings and publications available only in abstract form to permit extraction of data from the most robustly conducted studies of similar design. Studies published in non-English language were also excluded. Eligible studies identified were cross-checked against previous systematic reviews.
Outcome measures
The efficacy endpoint, specified a priori, was responder rate, defined as a 50% reduction in seizure rate from baseline, as recommended by the European Medicines Agency (EMA). For studies that did not report their results in this manner, we calculated the proportion of patients with a 50% reduction in seizure rate through analysis of seizure diary rate at end of study minus baseline seizure rate. The tolerability endpoint, specified a priori, was the incidence of premature withdrawal from treatment due to drug-related adverse events. Where trials included groups randomized to different doses of the same drug, we selected the group receiving the dose closest to that in established use based on our clinical experience.
Quality assessment and data collection
Two investigators (PNB and AMG) reviewed abstracts and full text articles retrieved by the search and selected potentially relevant publications against the pre-specified inclusion and exclusion criteria. To ensure consistency of data abstraction for each study a structured form was used. Important clinical and methodological study characteristics were tabulated, including: (i) characteristics of trial participants (including age, prior therapy, and seizure type), (ii) type of intervention (including type, dose, and duration) and (iii) type of outcome measure. Any discrepancies or lack of agreement between the two reviewers were referred to a third independent investigator (ADH) for arbitration. All analyses were directly reported or recalculated as intention to treat.
Quantitative data analysis
We performed two analyses. First, we conducted a standard random-effects meta-analysis [20] of placebo-controlled trials of AEDs for refractory epilepsy to derive a pooled odds ratio (OR) and 95% confidence interval (CI) for both the efficacy and tolerability endpoints. To evaluate heterogeneity of the effect estimates we used the Cochran Q (Chi-squared), Higgins I-squared and tau-squared statistics [21]. The Cochran Q is calculated as the weighted sum of squared differences between individual study effects and the pooled effect across studies, with the weights being those used in the pooling method which forms part of the DerSimonian-Laird random-effects model. The I-squared statistic is a more intuitive, simpler expression which describes the proportion of variation across studies that is due to heterogeneity rather than chance. The magnitude of heterogeneity can be quantified by calculating a point estimate of the among-study variance of true effects; tau-squared [22]. To explore potential inconsistency and small study bias further we used L'Abbe and Funnel plots, respectively. The L'Abbe plot [23] visually expresses within group variations in observed results through a plot of the event rate in the treatment group on the vertical axis and the control group on the horizontal axis. The funnel plot [24, 25] helps visualize evidence for small study bias of which publication bias is a potential cause. The Egger test was also used to assess evidence for small study bias. We used Review Manager (RevMan) version 5.0 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008) to generate the Forest plots and Funnel plots, and used StatsDirect® 2.7.7 (Altrincham, Cheshire, UK) to construct the L'Abbe plots.
Next, we analyzed the relative effectiveness and tolerability profile of each AED using an extension of the multivariate Bayesian hierarchical random-effects model for mixed multiple treatment comparisons with minimally informative prior distributions [17, 26–28]. This form of logistic regression analysis combines both direct and indirect data without breaking randomization within a Markov chain Monte Carlo (MCMC) framework. Indirect estimates can be combined in large samples if there is no interaction between the treatment effects and the populations or major subgroups in a trial [29]. The model included random effects at the level of trials (see Supporting Information Figures S1 and S2) which allowed the estimation of the variance of treatment effects between trials. The treatment network was synthesized using WinBUGS (Windows based Bayesian inference Using Gibbs Sampling) (MRC Biostatistics Unit, Cambridge, UK) [30]. We adapted a WinBUGS program from the University of Bristol (http://www.bristol.ac.uk/social-community-medicine/projects/mpes/mtc/). MCMC simulation, using Gibbs sampling, is an algorithm used to generate a sequence of samples from a joint probability distribution of two or more random variables and is particularly well adapted to sampling the treatment effects (posterior distribution) of a Bayesian network. We used this approach to generate the posterior distribution for each OR of interest. We took the median of the posterior distribution as our point estimate and the 2.5th and 97.5th centiles provided the 95% credible interval (CrI). In general Bayesian CrI and frequentist CI are non-interchangeable as CrI incorporate problem-specific contextual information from the prior distribution whereas CI are based solely on the on the data [31]. The interpretation of Bayesian CrI is that the posterior probability that the parameter lies within the CrI is 95%. We selected a non-informative prior for the log OR which was distributed normally with a mean of zero and variance of 10 000. This implies that we did not have strong beliefs about the value of each effect size in advance of the analysis, and allowed more direct comparison with the standard (frequentist meta-analysis). Twenty thousand iterations were used for each chain in the Bayesian analysis following a burn-in of 20 000. Convergence was assessed as this is a condition for reliable inferences from MCMC simulation approaches. This was done via visual assessment of trace plots and time series plots where convergence was deemed to be achieved in the presence of a relatively constant mean and minimal variance. The visual assessment was corroborated through assessment of the ratio of the Monte Carlo error (MCe) with its standard deviation (SD) being sufficiently small (MCe/SD <0.05). The control event rate (CER) used within the model was 0.16 for efficacy and 0.05 for tolerability. These were calculated as the mean across the studies which met the inclusion criteria as a means of determining the placebo-corrected response across the treatment network.
As number needed to treat (NNT) and number needed to harm (NNH) have increasingly been noted as being more clinically useful parameters in highlighting treatment effects, we calculated these from the relative risk (RR) estimates generated by WinBUGS using one of the following equations: 1/(1 − RR) × CER if the RR was <1 or 1/(RR − 1) × CER if the RR was >1 [32]. We estimated the 95% CrI values for NNT and NNH using the 95% CrI estimated for the RR using the same formulae above. We also developed a treatment hierarchy based on estimating the (posterior) probability that each treatment is the best and the 95% CrI for the relative rank of each AED.
Finally, we tested for consistency of any available direct and indirect treatment comparison by testing the null hypothesis that the indirect evidence was equal to the direct evidence on the log odds scale using the normal distribution [29]. Convergence of Markov chains was deemed to be achieved if plots of the Gelman–Rubin statistics indicated that the widths of pooled runs and individual runs stabilized around the same value and their ratio around 1 [33]. We also assessed the goodness of fit of the model through analysis of the posterior mean of the sum of the residual deviance contributions of each data point [34, 35]. A good fit would give a result that approximates the number of data points included within the analysis, a result which could also only occur when the posterior distribution is approximately multivariate normal or the total population included within the analysis (aggregate of all studies) follows a normal distribution.
Results
Out of 431 potentially eligible reports, a total of 43 studies, describing 11 AEDs and including 8546 patients with refractory epilepsy, met the inclusion criteria and were included within the network meta-analysis (see Figure 1) [36–77]. All studies were published as full journal articles.
Study characteristics
The main characteristics of the studies included are given in Table 1(additional details available in Supporting Information Table S4). Of the 43 studies, 40 were placebo-controlled, two were active-controlled (sodium valproate vs. vigabatrin, and gabapentin vs. vigabatrin) [76, 77] and one was a three-arm study (pregabalin vs. lamotrigine vs. placebo) [78]. Figure 2 displays the treatment network graphically. The mean number of adjunctive baseline medications was between 1 and 3. The duration of maintenance therapy ranged from 8 to 24 weeks. None of the studies investigating acetazolamide, carbamazepine, clobazam, clonazepam, ethosuximide, phenobarbital, phenytoin or primidone fulfilled the inclusion criteria. Reasons for exclusion were a lack of double-blinding or trial duration of less than 8 weeks. Twenty of the 43 studies (6212 patients) investigated the AED against placebo at more than one dose. Of 65 possible pair-wise comparisons between the available interventions, including placebo, only 13 were actually reported and in all but three the comparator was placebo.
Table 1.
Characteristics of studies included within the network meta-analysis
| Study | Study design (weeks of treatment*) | Daily treatment (number of subjects allocated) | Male : Female | Age range (years) | Type of epilepsy | Number of concomitant AEDs (specified where stated) |
|---|---|---|---|---|---|---|
| Placebo-controlled studies | ||||||
| Gabapentin | ||||||
| UK Gabapentin Study Group (1990) [36] | Parallel (14) | GBP 1200 mg (61), placebo (66) | 53:74 | 14–73 | SPS/CPS ± GTCS | ≤2 |
| Sivenius et al. (1991) [37] | Parallel (12) | GBP 900 mg (18), GBP 1200 mg (9), placebo (18) | 20:23 | 16–59 | SPS/CPS ± GTCS | ≤2 (CBZ, CLZ, VPA, PHT) |
| Anhut et al. (1994) [38] | Parallel (12) | GBP 900 mg (111), GBP 1200 mg (52), placebo (109) | 154:120 | 12–67 | SPS/CPS ± GTCS | ≤2 |
| Yamauchi et al. (2006) [39] | Parallel (12) | GBP 1200 mg (86), GBP 1800 mg (41), placebo (82) | 101:108 | ≥16 | SPS/CPS | ≤2 |
| Lacosamide | ||||||
| Ben-Menachem et al. (2007) [40] | Parallel (12) | LCS 600 mg (106), LCS 400 mg (108), LCS 200 mg (107), placebo (97) | 192:226 | 18–68 | SPS/CPS ± GTCS | ≤2 |
| Halász et al. (2009) [41] | Parallel (12) | LCS 400 mg (159), LCS 200 mg (163), placebo (163) | 250:235 | 16–70 | SPS/CPS ± GTCS | ≥2 |
| Lamotrigine | ||||||
| Loiseau et al. (1990) [42] | Crossover (2 × 8) | LTG (23), placebo (23) [different doses] | 12:11 | 21–54 | SPS/CPS ± GTCS | ≤2 |
| Matsuo et al. (1993) [43] | Crossover (2 × 12) | LTG (126), placebo (67) [different doses] | 60:133 | 18–63 | SPS/CPS ± GTCS | ≤3 |
| Messenheimer et al. (1994) [44] | Crossover (2 × 12) | LTG (88), placebo (98) [different doses] | 46:52 | 18–64 | SPS/CPS ± GTCS | ≤3 |
| Biton et al. (2005) [45] | Parallel (12) | LTG (58), placebo (59) [different doses] | 62:55 | 2–55 | GTCS | ≤2 (PHT, PHB, CBZ, PRM, VPA) |
| Naritoku et al. (2007) [46] | Parallel (12) | LTG (118), placebo (121) [different doses] | 117:119 | ≥12 | SPS/CPS ± GTCS | ≤2 |
| Baulac et al. (2010) [77] | Parallel (17) | LTG (141), placebo (141) [different doses] | 132:149 | 16–67 | SPS/CPS | ≤3 |
| Levetiracetamne | ||||||
| Cereghino et al. (2000) [47] | Parallel (14) | LEV 1000 mg (98), LEV 3000 mg (101), placebo (95) | 178:116 | 16–75 | SPS/CPS ± GTCS | ≥2 |
| Shorvon et al. (2000) [48] | Parallel (12) | LEV 1000 mg (106), LEV 2000 mg (106), placebo (112) | 157:167 | 16–65 | SPS/CPS ± GTCS | ≤2 |
| Betts et al. (2000) [49] | Parallel (24) | LEV 2000 mg (42), LEV 4000 mg (38), placebo (39) | 73:46 | 16–70 | SPS/CPS ± GTCS | ≤3 |
| Ben-Menachem et al. (2000) [50] | Parallel (12) | LEV 3000 mg (181), placebo (105) | 137:149 | 16–70 | CPS | 1 |
| Boon et al. (2002) [51] | Crossover (2 × 12) | LEV 2000 mg (202), placebo (200) | 157:167 | 16–65 | SPS/CPS ± GTCS | ≥3 |
| Tsai et al. (2006) [52] | Parallel (12) | LEV 2000 mg (47), placebo (47) | 42:52 | 16–60 | SPS/CPS ± GTCS | ≤3 |
| Peltola et al. (2009) [53] | Parallel (12) | LEV 1000 mg (79), placebo (79) | 99:59 | 12–68 | SPS/CPS ± GTCS | ≤3 |
| Zhou et al. (2008) [54] | Parallel (12) | LEV 3000 mg (13), placebo (11) | 13:11 | 16–70 | SPS/CPS ± GTCS | ≤2 |
| Xiao et al. (2009) [55] | Parallel (12) | LEV 3000 mg (28), placebo (28) | 24:36 | 17–60 | SPS/CPS ± GTCS | ≤2 (PHT, CBZ, PHB, PRM, VPA, TPM, GBP, LMG) |
| Oxcarbazepine | ||||||
| Barcs et al. (2000) [56] | Parallel (24) | OXC 600 mg (168), OXC 1200 mg (177), OXC 2400 mg (174), placebo (173) | 341:351 | 15–65 | SPS/CPS ± GTCS | ≤3 |
| Pregabalin | ||||||
| French et al. (2003) [57] | Parallel (12) | PRB 50 mg (88), PRB 150 mg (86), PRB 300 mg (90), PRB 600 mg (89), placebo (100) | 218:235 | 12–75 | SPS/CPS ± GTCS | ≤3 |
| Arroya et al. (2004) [58] | Parallel (12) | PRB 150 mg (99), PRB 600 mg (92), placebo (96) | 145:142 | 17–73 | SPS/CPS ± GTCS | ≤3 |
| Beydoun et al. (2005) [59] | Parallel (12) | PRB 200 mg TDS (111), PRB 300 mg BD (104), placebo (98) | 156:156 | 17–82 | SPS/CPS ± GTCS | ≤4 |
| Lee et al. (2009) [60] | Parallel (12) | PRB 600 mg (119), placebo (59) | 86:92 | ≥18 | SPS/CPS ± GTCS | ≤4 (CBZ, VPA, TPM, LTG, PHB, OXC) |
| Baulac et al. (2010) [78] | Parallel (17) | PRB 600 mg (152), placebo (141) | 133:159 | 16–82 | SPS/CPS | ≤3 |
| Tiagabine | ||||||
| Uthman et al. (1998) [61] | Parallel (20) | TGB 16 mg (61), TGB 32 mg (88), TGB 56 mg (57), placebo (91) | 58:42 | 12–77 | SPS/CPS ± GTCS | ≤3 (PHT, CBZ, PHB, PRM) |
| Kalviainen et al. (1998) [62] | Parallel (22) | TGB 30 mg (77), placebo (77) | 90:64 | 16–75 | SPS/CPS ± GTCS | ≤6 (CBZ, CLZ, PHT, VPA, VGB) |
| Topiramate | ||||||
| Tassinari et al. (1996) [63] | Parallel (12) | TPM 600 mg (30), placebo (30) | 24:6 | 18–65 | SPS/CPS ± GTCS | ≤2 |
| Ben-Menachem et al. (1996) [64] | Parallel (13) | TPM 800 mg (28), placebo (28) | 23:5 | 19–63 | SPS/CPS ± GTCS | ≤2 |
| Faught et al. (1996) [65] | Parallel (16) | TPM 200 mg (45), TPM 400 mg (45), TPM 600 mg (46), placebo (45) | 143:38 | 19–68 | SPS/CPS ± GTCS | ≤2 (CBZ, PHT) |
| Privitera et al. (1996) [66] | Parallel (18) | TPM 600 mg (48), TPM 800 mg (48), TPM 1000 mg (47), placebo (47) | 152:38 | 18–68 | SPS/CPS ± GTCS | ≤2 |
| Sharief et al. (1996) [67] | Parallel (11) | TPM 400 mg (23), placebo (24) | 40:7 | 18–65 | SPS/CPS ± GTCS | ≤2 (CBZ, PHT, VPA, PHB, PRD) |
| Yen et al. (2000) [68] | Parallel (14) | TPM 300 mg (23), placebo (23) | 19:27 | 18–54 | SPS/CPS | ≥4 (CBZ, VPA, LTG, PHT) |
| Vigabatrin | ||||||
| French et al. (1996) [69] | Parallel (12) | VGB 3000 mg (93), placebo (90) | 80:102 | 18–60 | CPS ± GTCS | ≤2 (CBZ, PHT) |
| Bruni et al. (2000) [70] | Parallel (36) | VGB 3000 mg [mean] (58), placebo (53) | 61:50 | 18–50 | CPS ± GTCS | ≤2 |
| Zonisamide | ||||||
| Schmidt et al. (1993) [71] | Parallel (12) | ZNS 500 mg [mean] (71), placebo (68) | 81:58 | 18–59 | CPS | ≤3 (CBZ, PHT, VPA, PHB, PRM) |
| Faught et al. (2001) [72] | Crossover (20) | ZNS 100 mg, 200 mg, 400 mg (118), placebo (85) | 104:99 | 13–68 | SPS/CPS ± GTCS | ≤2 (CBZ, PHT, VPA, PHB, PRM) |
| Brodie (2004) [73] | Parallel (12) | ZNS 400 mg (73), placebo (71) | 85:59 | 18–59 | SPS/CPS ± GTCS | ≤2 (CBZ, PHT, VPA, PHB, PRM) |
| Sackarelles et al. (2004) [74] | Parallel (12) | ZNS 500 mg [mean] (78), placebo (74) | 101:51 | 17–68 | CPS ± GTCS | ≤2 (CBZ, PHT, PHB, PRM) |
| Brodie et al. (2005) [75] | Parallel (24) | ZNS 100 mg (56), ZNS 300 mg (55), ZNS 500 mg (118), placebo (120) | 232:171 | 12–77 | SPS/CPS ± GTCS | ≤4 (CBZ, CLB, GBP, LTG, PHB, PHT, TPM, VPA) |
| Active-controlled studies | ||||||
| Vigabatrin | ||||||
| Brodie et al. (1999) [76] | Parallel (12) | VGB 2000 mg to 400 mg (108) | 106:109 | 12–78 | SPS/CPS ± GTCS | CBZ |
| VPA 1000 mg to 2000 mg (107) | ||||||
| Lindberger et al. (2000) [77] | Parallel (8) | GBP 2400 mg to 3600 mg (50) | 51:51 | 13–68 | SPS/CPS | ≤2 |
| VGB 2000 mg – 4000 mg (52) | ||||||
| Baulac et al. (2010) [78] | Parallel (17) | LTG 300 mg to 400 mg (141) | 155:138 | 18–82 | SPS/CPS | ≤3 |
| PRB 300 mg to 600 mg (152) |
For those studies where more than one dose of the active agent was compared against placebo, the dose underlined reflects the dose for which data were extracted from the trial to permit comparison. CPS, complex partial onset seizure; GBP, gabapentin; GTCS, secondary generalized tonic-clonic seizure; LCS, lacosamide; LEV, levetiracetam; LMG, lamotrigine; OXC, oxcarbazepine; PRB, pregabalin; SPS, simple partial onset seizure; TGB, tiagabine; TPM, topiramate; VGB, vigabatrin; VPA, sodium valproate; ZNS, zonisamide.
Weeks of treatment refers to the double-blind period consisting of both the titration to target and target stabilization phases.
Figure 2.
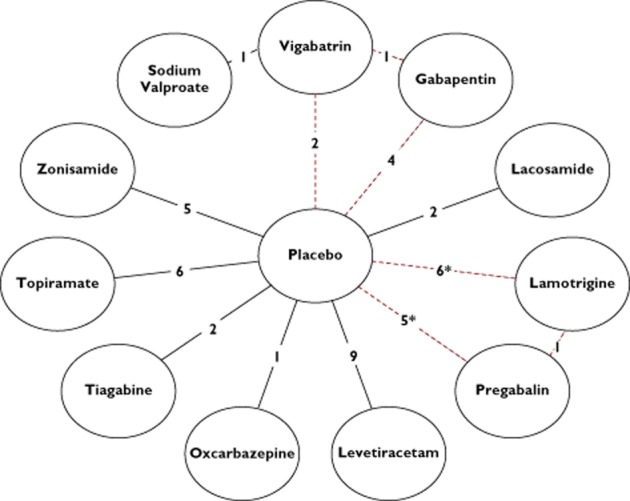
AEDs included within the network meta-analysis. Each AED represents a node within the star-shaped network. The links between the nodes represent direct comparative data, where the number along the line indicates the number of studies for that particular link within the network. The red (dotted) line represents a loop of direct comparative data, which allows mixed treatment comparison (* includes the 3-arm study)
Comparison of effect sizes
Standard meta-analysis: efficacy and tolerability
The results of the standard meta-analysis of placebo-controlled trials (stratified by drug) demonstrated that each AED was more efficacious than placebo in reducing seizure events by >50% from baseline (Figure 3A) with an overall OR 3.78 (95% CI 3.14, 4.55) (Figure 3C). For the efficacy endpoint, there was small to moderate evidence of heterogeneity (tau-squared = 0.15; I-squared = 46%) which was attributable to specific AED drug class (levetiracetam, pregabalin, tiagabine and zonisamide). Similarly, meta-analysis of tolerability indicated a greater overall odds of premature withdrawal due to the development of adverse effects for all AEDs vs. placebo (OR 3.27, 95% CI 2.37, 4.52) (Figure 3B,D), with moderate evidence of heterogeneity (tau-squared = 0.45; I-squared = 55%), again attributable to specific AED class (tiagabine, topiramate and zonisamide). Based on these data there was no strong evidence favouring any one particular AED over another on the basis of efficacy, although oxcarbazepine appeared to be the least well tolerated.
Figure 3.
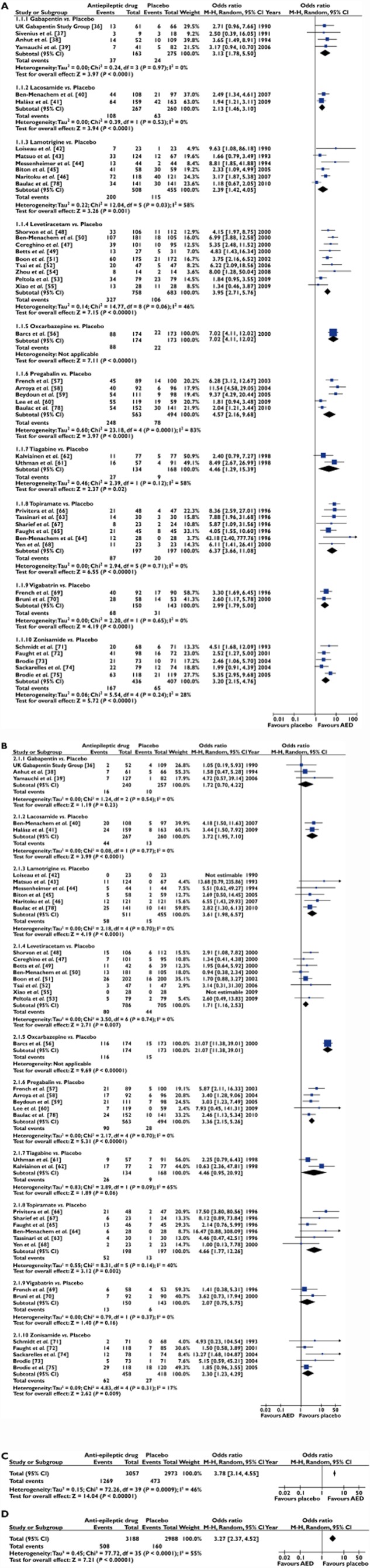
(A) Forest plot (RevMan v5.0) of the odds ratios for efficacy (50% responder rate) of randomized controlled trials comparing an AED vs. placebo as add-on treatment for refractory epilepsy, respectively. The black squares represent the odds ratio for individual studies of AED vs. placebo and the horizontal line represents the 95% confidence interval of the odds ratio. The black diamond represents the random-effects pooled odds ratio for studies reporting on the same AED where its width represents the 95% confidence intervals. Estimates to the right of the vertical line (i.e. odds ratio >1) are indicative of a statistically significant increase in efficacy, relative to placebo, in patients randomized to the active intervention. (B) Forest plot (RevMan v5.0) of the odds ratios for tolerability (withdrawal from treatment due to an intolerable adverse event) of randomized controlled trials comparing an AED vs. placebo as add-on treatment for refractory epilepsy, respectively. The black squares represent the odds ratio for individual studies of AED vs. placebo and the horizontal line represents the 95% confidence interval of the odds ratio. The black diamond represents the random-effects pooled odds ratio for studies reporting on the same AED where its width represents the 95% confidence intervals. Estimates to the right of the vertical line (i.e. odds ratio >1) are indicative of a statistically significant increase in withdrawal rate, relative to placebo, in patients randomized to the active intervention. (C) Summary Forest plot (RevMan v5.0) of the odds ratio for efficacy (50% responder rate) of randomized controlled trials comparing an AED vs. placebo as add-on treatment for refractory epilepsy. (D) Summary Forest plot (RevMan v5.0) of the odds ratio for tolerability (withdrawal from treatment due to an intolerable adverse event) of randomized controlled trials comparing an AED vs. placebo as add-on treatment for refractory epilepsy
We detected no evidence of significant heterogeneity in efficacy or tolerability (P < 0.05) in different trials of the same drug following a review of the L'Abbe plots with the possible exception of pregabalin (efficacy analysis Cochran Q = 15.36, P = 0.002; Figure 3A). A review of the Funnel plots did not reveal concerns regarding publication bias (see Supporting Information Figure S3).
Network meta-analysis: efficacy, tolerability, and prescribing hierarchy
The median OR estimates, for the efficacy and tolerability endpoints, generated by the Bayesian random-effects network meta-analysis are given in Table 2. As a non-informative prior was used, these closely resemble those generated from the frequentist random-effects meta-analysis (see Supporting Information Tables S6 and S7). We detected no evidence of significant inconsistency (Bucher's test) between directly observed and inferred treatment effects within the loop identified in Figure 2 for either efficacy (P = 0.26) or tolerability (P = 0.22). For both outcomes, the model showed reasonable goodness of fit to the data (number of data points) as determined by the posterior mean of the residual deviance [efficacy = 86.0 (84), 95% CrI 62.8, 115.9, P = 0.419; tolerability = 77.6 (80), 95% CrI 57.9, 100.5, P = 0.555]. A visual assessment of the trace plots (history) and time series (density) plots also did not reveal cause for concern regarding inconsistency (data available on request).
Table 2.
Comparative efficacy and tolerability of the 11 anti-epileptic drugs (AED)
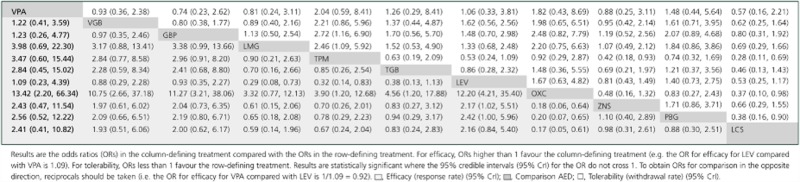 |
In contrast to the standard meta-analysis, network meta-analysis allowed the ordering of AEDs according to efficacy and tolerability. There was an approximately two-fold difference in short term efficacy (based on the 50% responder rate), lacosamide being the least and topiramate the most efficacious at the doses evaluated (Figure 4A). There was an approximately five-fold difference in short term tolerability with valproate being the best and oxcarbazepine being the least well tolerated at the doses evaluated (Figure 4B). Because treatment decisions for refractory partial epilepsy may be based on a balance between efficacy and tolerability, we estimated the NNT and NNH for each drug. Four drugs (valproate, levetiracetam, gabapentin and vigabatrin) demonstrated the best combination of short term efficacy and tolerability (Figure 5). Though similarly effective, oxcarbazepine was less well tolerated than all other agents. The remaining agents (topiramate, pregabalin, tiagabine, zonisamide, lamotrigine and lacosamide) demonstrated intermediate short term efficacy and tolerability.
Figure 4.
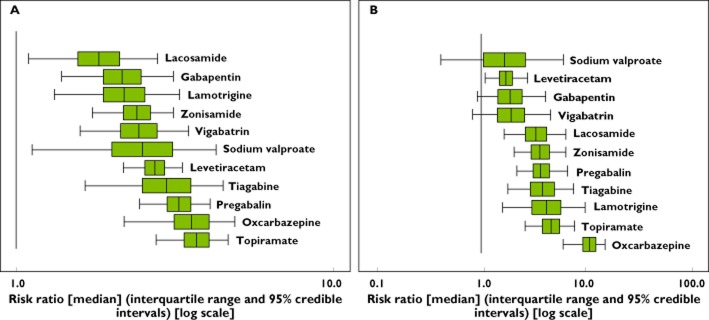
(A) Box plots (WinBUGS) of the risk ratios of AEDs vs. placebo as estimated by the network meta-analysis regarding efficacy (50% responder rate). The individual plots represent the 95% credible interval (horizontal black line either side of the green box) and the interquartile range (green box, representing where 25% to 75% of the data lies); the vertical black line reflects the median risk ratio (RR). A larger RR is indicative of a greater proportion of patients achieving a 50% reduction in seizure frequency, relative to placebo. Where the 95% credible interval crosses the line of unity, this is indicative of a non-statistically significant difference to placebo. AEDs are listed in descending rank order. (B) Box plots (WinBUGS) of the risk ratios of AEDs vs. placebo as estimated by the network meta-analysis regarding tolerability (premature discontinuation). The individual plots represent the 95% credible interval (horizontal black line either side of the green box) and the interquartile range (green box, representing where 25% to 75% of the data lies); the vertical black line reflects the median risk ratio (RR). A larger RR is indicative of a greater proportion of patients withdrawing from treatment prematurely, relative to placebo. Where the 95% credible interval crosses the line of unity, this is indicative of a non-statistically significant difference to placebo. AEDs are listed in descending rank order
Figure 5.
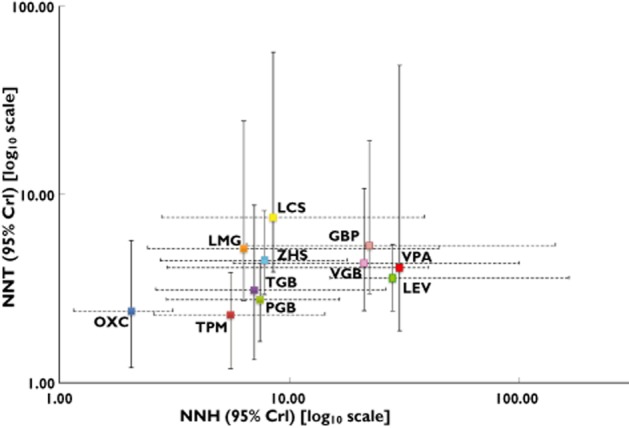
A comparison of the number needed to treat vs. number needed to harm (with 95% credible intervals) for efficacy (50% responder rate) and tolerability (withdrawal rate), respectively. Drugs in the lower right of the plot (i.e. low NNT and high NNH) are highly efficacious and well tolerated; conversely, drugs in the upper left of the plot (i.e. high NNT and low NNH) are less effective and poorly tolerated. Note that equal weighting of the efficacy and tolerability endpoints has been applied (i.e. the same emphasis is placed on achieving a 50% reduction in seizure rate in one patient as for incurring a premature withdrawal due to an intolerable adverse event in another).  , gabapentin (GBP);
, gabapentin (GBP);  , lacosamide (LCS);
, lacosamide (LCS);  , lamotrigine (LMG);
, lamotrigine (LMG);  , levetiracetam (LEV);
, levetiracetam (LEV);  , oxcarbazepine (OXC);
, oxcarbazepine (OXC);  , pregabalin (PGB);
, pregabalin (PGB);  , sodium valproate (VPA);
, sodium valproate (VPA);  , tiagabine (TGB);
, tiagabine (TGB);  , topiramate (TPM);
, topiramate (TPM);  , vigabatrin (VGB);
, vigabatrin (VGB);  , zonisamide (ZNS)
, zonisamide (ZNS)
Discussion
Principal findings
This network meta-analysis provides the most comprehensive and explicit assessment of the short term comparative efficacy and tolerability of AEDs licensed for the adjunctive management of chronic refractory partial epilepsy with or without secondary generalization. The standard meta-analysis demonstrated that all AEDs were superior to placebo in preventing seizures. However, there was a dearth of published, randomized clinical trials that directly compared one AED with another. Using a Bayesian hierarchical model to conduct a mixed-treatment network meta-analysis, levetiracetam, vigabatrin, sodium valproate and gabapentin emerged as agents with the best combination of short term efficacy and tolerability with the caveat that vigabatrin is recognized as being associated with serious visual disturbance with chronic use.
Relation to previous studies
Five meta-analyses and a narrative review exist related to this question which have produced conflicting results, partly due to differences in methodology and inclusion criteria. Marson et al. [79, 80] included 13 published and 15 unpublished randomized controlled trials, comparing six AEDs against placebo (gabapentin, lamotrigine, tiagabine, topiramate, vigabatrin, and zonisamide), concluding that there was a lack of conclusive evidence to determine a prescribing hierarchy accounting for differences in efficacy or tolerability. Shorvon et al. [81] subsequently included 36 published articles (including randomized controlled trials in full and abstract form), comparing eight AEDs against placebo, again reaching the same conclusion. Otoul et al. [82] later updated the meta-analysis conducted by Marson et al. by comparing the six AEDs indirectly against levetiracetam. No restrictions were placed regarding age of patients. Unlike the previous two reviews, the authors here found levetiracetam to demonstrate significant efficacy compared with gabapentin and lamotrigine. No significant differences regarding tolerability profile were noted between the seven AEDs evaluated. In contrast, the present analysis using a treatment network did not demonstrate levetiracetam to possess a significantly more effective profile although it was one of four AEDs which showed a trend towards better efficacy and tolerability. Beyenburg et al. [83] conducted a meta-analysis of modern AEDs to determine the true effect of each agent in reducing seizure frequency not attributable to other factors, principally placebo response. Although the search period was similar to that of the present study, the inclusion of both adults and children resulted in a larger number of eligible studies. The key finding of this paper was the estimation of a placebo-corrected difference of 21% (95% CI 19, 24; P < 0.0001). This is akin to our calculated value of 16%, where the difference is likely to be the result of differences in the inclusion criteria. Rheims et al. [84] more recently conducted a meta-analysis in adult patients investigating the different parameters which may determine response to treatment. The number of publications and AEDs included were again greater than the present study as a result of the inclusion criteria permitting AEDs currently under investigation, or not indicated for refractory focal epilepsy. The authors concluded that although responder rate increased over the years for the placebo arm, a parallel increase was also observed in the active arm. Further, the use of last observation carried forward (LOCF) data overestimated the responder rate and analysis of efficacy at the level of doses for each AED did not reveal statistically significant differences. Lastly, Devinsky & Cramer commended the use of meta-analysis as the best available technique in the absence of comparative trial data [85]. However, this predated the use of network meta-analysis. As such, the present analysis utilized a common placebo-corrected value, calculation of intention-to-treat dataset, and assessment of each AED at the most clinically relevant dose incorporating a Bayesian paradigm, an approach which is recommended for its clinical relevance [86], to determine a treatment hierarchy. Prior overviews have supported the validity of network meta-analysis for indirect treatment comparisons provided certain conditions are met [87], with emerging examples of its application in several disease areas [88–92].
Strengths and weaknesses
We identified the relevant trials by explicit systematic review and the analysis conformed to PRISMA recommendations [18, 93]. The efficacy outcome we selected is universally accepted as an informative outcome measure concerning AED efficacy (CHMP/EWP/566/98 Rev. 2) [94]. All studies included in the present analysis were fully published unlike one of the previously published meta-analyses where over 50% (15/29) of included studies were unpublished [79] thus minimizing the risk of heterogeneity as full trial methodology was known. Furthermore, our analysis only included agents which are currently licensed in the UK for the adjunctive treatment of refractory epilepsy [84]. Lastly, we utilized a multiple treatment comparison design which allowed both direct and indirect comparisons via the construction of a treatment network unlike standard meta-analysis [95].
Multiple treatment options exist in epilepsy but, in common with many other therapeutic areas treatment comparisons may be missing, and/or there may be preferences towards certain comparators (including placebo) [96]. These were features of the current analysis where of the 65 possible comparisons between the 11 AEDs (and placebo), only 13 were conducted and all but three were placebo-controlled trials. A major reason for the lack of direct active comparator trials is that regulatory authorities such as the Committee for Medicinal Products for Human Use (on behalf of the EMA), do not require such trials to be conducted as a condition of the marketing authorization of a new drug. A recently published guideline on clinical investigation of medicinal products in the treatment of epileptic disorders continues to recommend that add-on studies (the addition of a new AED to existing therapies) should be of randomized, double-blind, placebo-controlled, parallel group study design, although the guideline also recommends that as more AEDs are approved for the add-on indication, comparative trials may be considered and that an evaluation of efficacy of such agents may be conducted through meta-analysis [94]. Despite the above recommendation, AEDs continue to be trialled and receive regulatory approval on the basis of placebo-controlled studies, as highlighted by recent approval of eslicarbazepine and retigabine which continue to compound the situation. In the absence of prior active-comparator trial, guidance from the National Institute for Clinical Excellence (NICE) in the UK, for example, does not make recommendations about the selection or sequence of AED therapy with regard to specific drugs within the categories of older and newer AEDs [9]. An additional and crucial problem which arises from the absence of a prevailing treatment hierarchy in the presence of multiple therapeutic options is that the number of legitimate active comparator trials for any new AED becomes very large indeed, in itself providing an additional obstacle to comparator trials of new agents. Thus indirect comparisons, through network meta-analysis such as the one conducted here, might not only help develop rational treatment hierarchies based on existing therapies but also inform on the choice of high priority comparator agents for future trials of new AEDs.
Nevertheless, it is also important to take note of several important limitations. First, although individual trials only provide information over a short period of time (typically 8 to 16 weeks), this is the duration of follow-up required to meet regulatory criteria. The findings should be extrapolated to longer term use with caution, particularly as some drugs, for example vigabatrin, are recognized as being associated with the development of adverse effects with long term use. Second, the trials included in this analysis spanned 19 years during which time the available services and the management of epilepsy has changed. Importantly, the patient population recruited to more recent trials may have more refractory or ‘drug resistant’ epilepsy than patients recruited to earlier studies who were exposed to fewer previously licensed therapies. However, statistical tests for heterogeneity (tau-squared and I-squared) considered that the patient populations were sufficiently similar to generate overall estimates, values noted as mild-moderate and moderate, respectively for the efficacy and tolerability endpoints. This point has been investigated in detail by Beyenburg et al. [83] who recently conducted a meta-analysis of modern AEDs, concluding that meta-regression showed no difference in effects between studies published before 2001 vs. 2001 and later for 50% reduction in seizure (Z = −0.50, P = 0.62) or seizure freedom (Z = −0.52, P = 0.60). The year 2001 was chosen to reflect the comparison of first vs. second generation AEDs. Beyenburg et al. also sought to explore whether or not factors such as number of past AEDs trialled or highest dose studied vs. all available doses had an influence on seizure-free outcome. However they concluded that insufficient data were available from the published manuscripts to enable such analyses. Nonetheless, the inclusion of multiple doses for each AED, specifically those at the higher end, may potentially skew the overall results with regards to increasing the incidence of premature discontinuation due to treatment-related adverse events without a corresponding increase in responder rate. Such heterogeneity would appear on Forest plot analyses as wider confidence/credible intervals and may reduce the validity of the results and limit generalization of the findings. On this basis, despite a reduction in overall patient numbers within the meta-analysis, it was decided to include data only for the dose closest to that prescribed in clinical practice for each AED. Third, although the 50% responder rate is an endpoint which is endorsed by regulatory authorities such as the EMA, it does not take into account the duration and/or severity of seizures. Fourth, although it was our intention to restrict the analysis to the adult population, a number of studies actually included a mixed population of adults and children (≥10 years) without sub-grouping their results. Nonetheless, as the majority of data relates to investigation in adults the conclusions drawn should remain relevant to the use of AEDs in the adult population only. Fifth, this analysis only provides a non-specific estimate for withdrawal rates due to an intolerable adverse effect rather than firm conclusions on the severity of a specific adverse effect, such as ataxia, or the incidence of serious/rare adverse effects such as Steven-Johnson syndrome or suicidal tendency. Furthermore, although the inclusion criteria specified a double-blind design to promote robustness of the findings, it is appreciated that patients randomized to active therapy may become aware that they are receiving active treatment due the emergence of treatment-related adverse effects, such as sedation. Sixth, the studies included may be limited in their ability to report on adverse effects as the studies were primarily designed and powered to address the effectiveness of the AED under investigation and had a maximum duration of 24 weeks [97]. Seventh, in specifying such strict inclusion criteria to ensure higher quality trials were included in the quantitative analysis we had to exclude trials investigating AEDs used in routine clinical practice today, such as carbamazepine. Finally, data within this analysis only relate to the use of AEDs for the treatment of patients with chronic refractory partial epilepsy and should not be extrapolated to patients with other forms of epilepsy, such as primary generalized epilepsy. As for any medication, prescribing decisions should take into account specific contra-indications and advice on prescribing in special groups such as women of childbearing age.
The present study included data from publications in the English language only. The decision to not include data from publications in languages other than English (LOE) was made based on the conclusions reached by the Canadian Agency for Drug and Technologies in Health (CADTH) and the UK National Institute of Health Research (NIHR) (review of impact of language restrictions on systematic reviews). First, the CADTH performed a systematic review of meta-analyses of conventional medicines, comparing those that did and those that did not include publications in LOE concluding that they could find no evidence of a systematic bias from the use of language restrictions [98]. Second, a Health Technology Appraisal (on behalf of the NIHR) also found that language restrictions did not bias the results of systematic reviews of conventional medicines, even after sensitivity analyses were conducted, noting that the results do not appear to be influenced by statistical heterogeneity or publication bias [99].
Finally, as carbamazepine is widely recommended and accepted as being the first-line option for patients with partial-onset seizures [9, 100], we believe that the non-inclusion of this particular AED within this analysis should not limit its findings.
Implications for clinical practice
This review is focused on the most common form of epilepsy seen in clinical practice, partial onset seizure with or without secondary generalization in the refractory setting. The stimulus for this stems from the lack of a guideline which provides explicit detail on how each agent licensed for this indication should be prescribed relative to one another.
Although it is widely accepted that the best model of providing answers with the highest clinical relevance would be from a large scale multiple comparison head-to-head trial, the costs associated with such a model renders the chances of this transpiring to be very small. The present study attempts to provide a response to one of the limitations of current trial methodology as specified within the discussion of the recent publication by Beyenburg et al. [83] – ‘given the absence of double-blind, placebo-controlled comparative AED trials, we could not compare the efficacy among individual AEDs’ – by generating a treatment network utilizing a Bayesian framework. In order to generate a robust and simplified network from which to permit translation into a treatment hierarchy a single dose per AED was extracted for the analysis. This strategy follows the results of Rheims et al. [84] where an analysis of dose selection resulted in mostly non-significant difference for the primary endpoint (50% reduction in seizure frequency). In light of these data, such analyses were not repeated and instead data reporting for the dose investigated which most closely reflects that in current clinical practice were extracted for each AED. Until conduct of such studies, we have reported a methodologically and statistically rigorous analysis of AEDs currently available in the UK to assist clinical decision-making.
Lastly, an important implication of our findings is that although it clear that the modern AEDs are more effective than placebo when used as adjunctive therapy for refractory focal epilepsy, they are of limited efficacy (in comparison with older agents) and are correlated with an increase in treatment-related intolerable adverse effects. The current strategies for developing, investigating and licensing of AEDs thus require re-evaluation.
Conclusion
This systematic review and Bayesian network meta-analysis highlights the paucity of long term prospective randomized active comparator trials of AEDs currently licensed for refractory epilepsy. In comparison with the five previously published meta-analyses reporting on this topic and building on the recommendations issued by NICE, the use of indirect treatment comparisons indicated that levetiracetam, vigabatrin, gabapentin, and sodium valproate demonstrated the best combination of short term efficacy and tolerability, whereas oxcarbazepine, while equally effective, was the least well tolerated in the short term. As the use of carbamazepine is widely recommended and accepted as being an effective first line option for patients with partial onset focal seizures, despite there being no trials investigating this agent within the present analysis, clinical practice has dictated this agent to demonstrate a good balance of efficacy and tolerability in both the short and long term. With the exception of vigabatrin which is associated with visual field defects in long term use, in the absence of evidence definitively indicating the clinical superiority or tolerability of one AED over another, we suggest that other factors such as acquisition cost, dosing regimen, licensing indications and contra-indications be the differential in selecting among these AEDs. The logical next step for future trials would be the commissioning of multiple active comparisons, similar to the SANAD study in primary generalized epilepsy and partial epilepsy [101, 102]. Until regulators mandate greater use of such trials, network meta-analyses incorporating mixed treatment models may provide useful information on comparative efficacy and safety of treatments for epilepsy and other common diseases.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work and no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; AG is a limited company director. The limited company receives, or likely to receive, income from all pharmaceutical companies. LS has undertaken consultancy work for GSK on an area unrelated to the submitted work, and has received numerous research council and charity grants, on behalf of his institution, for areas unrelated to the submitted work. AH has received numerous research council and charity grants, on behalf of his institution, for areas unrelated to the submitted work. All other authors have no financial relationship with any organization that could appear to have influenced the submitted work.
Author contribution
All co-authors listed contributed to the final manuscript in the following manner: ADH conceived the study; ADH, PNB, AMG and SD participated in its design; PNB and AMG participated in the systematic review and data extraction; RS and DW assisted PNB in the statistical analysis for conventional and Bayesian meta-analysis, respectively; JPC, RJM and ADH assisted in the draft of the initial manuscript including analysis of results and translation of trial data to clinical practice and all authors provided revisions and approved the final manuscript submitted. This manuscript is not under consideration or review by any other journal.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Figure S1
WinBUGS model for the network meta-analysis (efficacy)
Figure S2
WinBUGS model for the network meta-analysis (tolerability)
Figure S3
Funnel plots (Revman v5.0) of randomized controlled trials comparing each AED vs. placebo as add-on treatment for refractory epilepsy for (A) efficacy (50% responder rate) and (B) tolerability [withdrawal from treatment due to intolerable adverse event(s)]
Table S1
Search strategy – MEDLINE (via PubMed)
Table S2
Limits applied within each database searched
Table S3
Criterion used within the systematic review
Table S4
Raw data extracted from clinical trials for the meta-analysis
Table S5
Year of introduction of AEDs to the European/US market (included within this review)
Table S6
Estimates of the summary relative risk (from the frequentist and Bayesian network meta-analysis) relating to responder rate and calculated NNT (from risk ratio) relative to placebo
Table S7
Estimates of the summary relative risk (from the frequentist and Bayesian network meta-analysis) relating to premature discontinuation rate and calculated NNH (from risk ratio) relative to placebo
Table S8
(A) Ranking of AEDs on the basis of efficacy (network meta-analysis estimates); (B) Ranking of AEDs on the basis of tolerability (network meta-analysis estimates); (c) Cost of 1 month's supply of each AED at the treatment dose used within the analysis (pound sterling, excluding VAT) (British National Formulary: Edition 63)
References
- 1.Blume WT. Glossary of descriptive terminology for ictal semiology: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:1212–1218. doi: 10.1046/j.1528-1157.2001.22001.x. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. 2009. Epilepsy. Fact Sheet number 999.
- 3.Shorvon SD. The epidemiology and treatment of chronic and refractory epilepsy. Epilepsia. 1996;37:S1–S3. doi: 10.1111/j.1528-1157.1996.tb06027.x. [DOI] [PubMed] [Google Scholar]
- 4.Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Hauser WA, Mathern G, Moshe SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- 5.Cockerell OC, Sander JWAS, Hart YM, Shorvon SD, Johnson AL. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet. 1995;346:140–144. doi: 10.1016/s0140-6736(95)91208-8. [DOI] [PubMed] [Google Scholar]
- 6.Devinsky O. Patients with refractory seizures. N Engl J Med. 1999;340:1565–1570. doi: 10.1056/NEJM199905203402008. [DOI] [PubMed] [Google Scholar]
- 7.Guidelines for clinical evaluation of antiepileptic drugs. Epilepsia. 1989;30:400–408. [PubMed] [Google Scholar]
- 8.Lathyris DN, Patsopoulos NA, Salanti G, Ioannidis JPA. Industry sponsorship and selection of comparators in randomized clinical trials. Eur J Clin Invest. 2010;40:172–182. doi: 10.1111/j.1365-2362.2009.02240.x. [DOI] [PubMed] [Google Scholar]
- 9.National Institute for Clinical Excellence. The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary care and secondary care. Clinical Guideline 137.2012. Accessed 18-1-2012.
- 10.The mad world of epilepsy drugs. Drug Ther Bull. 2009;47:49. doi: 10.1136/dtb.2009.04.0013. [DOI] [PubMed] [Google Scholar]
- 11.Institute of Medicine. Roundtable on evidence-based medicine. Learning what works best: the nation's need for evidence on comparative effectiveness in health care. 2008. 22-4-2010.
- 12.Institute of Medicine. Initial National Priorities for Comparative Effectiveness Research. Washington, DC: The National Academies Press; 2009. [Google Scholar]
- 13.Sox HC, Greenfield S. Comparative effectiveness research: a report from the Institute of Medicine. Ann Intern Med. 2009;151:203–205. doi: 10.7326/0003-4819-151-3-200908040-00125. [DOI] [PubMed] [Google Scholar]
- 14.US Department of Health and Human Services. Federal Coordinating Council for Comparative Effectiveness Research: Report to the President and the Congress. 2009.
- 15.Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21:2313–2324. doi: 10.1002/sim.1201. [DOI] [PubMed] [Google Scholar]
- 16.Salanti G, Higgins JP, Ades AE, Ioannidis JP. Evaluation of networks of randomized trials. Stat Methods Med Res. 2008;17:279–301. doi: 10.1177/0962280207080643. [DOI] [PubMed] [Google Scholar]
- 17.Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med. 2004;23:3105–3124. doi: 10.1002/sim.1875. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG for the PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Cook DJ, Eastwood S, Olkin I, Rennie D, Stroup DF. Improving the quality of reports of meta-analyses of randomised controlled trials: the QUOROM statement. Lancet. 1999;354:1896–1900. doi: 10.1016/s0140-6736(99)04149-5. [DOI] [PubMed] [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Higgins JPT. Commentary: heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol. 2008;37:1158–1160. doi: 10.1093/ije/dyn204. [DOI] [PubMed] [Google Scholar]
- 23.L'Abbe KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Ann Intern Med. 1987;107:224–233. doi: 10.7326/0003-4819-107-2-224. [DOI] [PubMed] [Google Scholar]
- 24.Sterne JAC, Egger M, Smith GD. Investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–105. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sterne JAC, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 26.Whitehead A. Meta-Analysis of Controlled Clinical Trials. Chichester: John Wiley & Sons; 2002. [Google Scholar]
- 27.Spiegelhalter DJ, Abrams KR, Myles JP. Bayesian Approaches to Clinical Trials and Health-Care Evaluation. Chichester: John Wiley & Sons; 2004. [Google Scholar]
- 28.Sutton AJ, Abrams KR. Bayesian methods in meta-analysis and evidence synthesis. Stat Methods Med Res. 2001;10:277–303. doi: 10.1177/096228020101000404. [DOI] [PubMed] [Google Scholar]
- 29.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–691. doi: 10.1016/s0895-4356(97)00049-8. [DOI] [PubMed] [Google Scholar]
- 30.Lunn DJ, Thomas A, Best N, Spiegelhalter DJ. WinBUGS – a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput. 2000;10:325–337. [Google Scholar]
- 31.Lee PM. Bayesian Statistics: An Introduction. 4th edn. West Sussex, UK: Wiley; 2012. [Google Scholar]
- 32.Sackett DL, Strauss SE, Richardson WS, Rosenberg W, Haynes RB, editors. Evidence Based Medicine: How to Practice and Teach EBM. Edinburgh: Harcourt; 2000. [Google Scholar]
- 33.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–455. [Google Scholar]
- 34.Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Ser B Stat Methodol. 2002;64:583–639. [Google Scholar]
- 35.Dias S, Welton NJ, Sutton AJ, Ades AE. 2011. NICE DSU Technical Support Document 2: A Generalised Linear Modelling Framework for Pairwise and Network Meta-Analysis of Randomised Controlled Trials.
- 36.UK Gabapentin Study Group. Gabapentin in partial epilepsy. Lancet. 1990;335:1114–1117. [PubMed] [Google Scholar]
- 37.Sivenius J, Kalviainen R, Ylinen A, Riekkinen PJ. Double-blind study of gabapentin in the treatment of partial seizures. Epilepsia. 1991;32:539–542. doi: 10.1111/j.1528-1157.1991.tb04689.x. [DOI] [PubMed] [Google Scholar]
- 38.Anhut H, Ashman P, Feuerstein TJ, Sauermann W, Saunders M, Schmidt B. Gabapentin (neurontin) as add-on therapy in patients with partial seizures: a double-blind, placebo-controlled study. Epilepsia. 1994;35:795–801. doi: 10.1111/j.1528-1157.1994.tb02513.x. [DOI] [PubMed] [Google Scholar]
- 39.Yamauchi T, Kaneko S, Yagi K, Sase S. Treatment of partial seizures with gabapentin: double-blind, placebo-controlled, parallel-group study. Psychiatry Clin Neurosci. 2006;60:507–515. doi: 10.1111/j.1440-1819.2006.01553.x. [DOI] [PubMed] [Google Scholar]
- 40.Ben-Menachem E, Biton V, Jatuzis D, Abous D, Rudd D. Efficacy and safety of oral lacosamide as adjunctive therapy in adults with partial-onset seizures. Epilepsia. 2007;48:1308–1317. doi: 10.1111/j.1528-1167.2007.01188.x. [DOI] [PubMed] [Google Scholar]
- 41.Halász P, Kälviäinen R, Mazurkiewicz-Beldzińska M, Rosenow F, Doty P, Hebert D, Sullivan T, (on behalf of the SP755 Study Group) Adjunctive lacosamide for partial-onset seizures: efficacy and safety results from a randomized controlled trial. Epilepsia. 2009;50:443–453. doi: 10.1111/j.1528-1167.2008.01951.x. [DOI] [PubMed] [Google Scholar]
- 42.Loiseau P, Yuen AW, Duche B, Menager T, Arne-Bes MC. A randomised double-blind placebo-controlled crossover add-on trial of lamotrigine in patients with treatment-resistant partial seizures. Epilepsy Res. 1990;7:136–145. doi: 10.1016/0920-1211(90)90099-h. [DOI] [PubMed] [Google Scholar]
- 43.Matsuo F, Bergen D, Faught E, Messenheimer JA, Dren AT, Rudd GD, Lineberry CG. Placebo-controlled study of the efficacy and safety of lamotrigine in patients with partial seizures. U.S. Lamotrigine Protocol 0.5 Clinical Trial Group. Neurology. 1993;43:2284–2291. doi: 10.1212/wnl.43.11.2284. [DOI] [PubMed] [Google Scholar]
- 44.Messenheimer J, Ramsay RE, Willmore LJ, Leroy RF, Zielinski JJ, Mattson R, Pellock JM, Valakas AM, Womble G, Risner M. Lamotrigine therapy for partial seizures: a multicenter, placebo-controlled, double-blind, cross-over trial. Epilepsia. 1994;35:113–121. doi: 10.1111/j.1528-1157.1994.tb02920.x. [DOI] [PubMed] [Google Scholar]
- 45.Biton V, Sackellares JC, Vuong A, Hammer AE, Barrett PS, Messenheimer JA. Double-blind, placebo-controlled study of lamotrigine in primary generalized tonic-clonic seizures. Neurology. 2005;65:1737–1743. doi: 10.1212/01.wnl.0000187118.19221.e4. [DOI] [PubMed] [Google Scholar]
- 46.Naritoku DK, Warnock CR, Messenheimer JA, Borgohain R, Evers S, Guekht AB, Karlov VA, Lee BL, Rios Pohl L. Lamotrigine extended-release as adjunctive therapy for partial seizures. Neurology. 2007;69:1610–1618. doi: 10.1212/01.wnl.0000277698.33743.8b. [DOI] [PubMed] [Google Scholar]
- 47.Cereghino JJ, Biton V, Bou-Khalil B, Dreifuss F, Gauer LJ, Leppik I. Levetiracetam for partial seizures: results of a double-blind, randomized clinical trial. Neurology. 2000;55:236–242. doi: 10.1212/wnl.55.2.236. [DOI] [PubMed] [Google Scholar]
- 48.Shorvon SD, Lowenthal A, Janz D, Bielen E, Loiseau P. Multicenter double-blind, randomized, placebo-controlled trial of levetiracetam as add-on therapy in patients with refractory partial seizures. Epilepsia. 2000;41:1179–1186. doi: 10.1111/j.1528-1157.2000.tb00323.x. [DOI] [PubMed] [Google Scholar]
- 49.Betts T, Waegemans T, Crawford P. A multicentre, double-blind, randomized, parallel group study to evaluate the tolerability and efficacy of two oral doses of levetiracetam, 2000 mg daily and 4000 mg daily, without titration in patients with refractory epilepsy. Seizure. 2000;9:80–87. doi: 10.1053/seiz.2000.0380. [DOI] [PubMed] [Google Scholar]
- 50.Ben ME, Falter U. Efficacy and tolerability of levetiracetam 3000 mg/day in patients with refractory partial seizures: a multicenter, double-blind, responder-selected study evaluating monotherapy. Epilepsia. 2000;41:1276–1283. doi: 10.1111/j.1528-1157.2000.tb04605.x. [DOI] [PubMed] [Google Scholar]
- 51.Boon P, Chauvel P, Pohlmann EB, Otoul C, Wroe S. Dose-response effect of levetiracetam 1000 and 2000 mg/day in partial epilepsy. Epilepsy Res. 2002;48:77–89. doi: 10.1016/s0920-1211(01)00323-0. [DOI] [PubMed] [Google Scholar]
- 52.Tsai JJ, Yen DJ, Hsih MS, Chen SS, Hiersemenzel R, Edrich P, Lai CW. Efficacy and safety of levetiracetam (up to 2000 mg/day) in Taiwanese patients with refractory partial seizures: a multicenter, randomized, double-blind, placebo-controlled study. Epilepsia. 2006;47:72–81. doi: 10.1111/j.1528-1167.2006.00372.x. [DOI] [PubMed] [Google Scholar]
- 53.Peltola J, Coetzee C, Jiménez F, Litovchenko T, Ramaratnam S, Zaslavaskly L, Lu Z, Sykes DM. Once-daily extended-release levetiracetam as adjunctive treatment of partial-onset seizures in patients with epilepsy: a double-blind, randomized, placebo-controlled trial. Epilepsia. 2009;50:406–414. doi: 10.1111/j.1528-1167.2008.01817.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhou B, Zhang Q, Tian L, Xiao J, Stefan H, Zhou D. Effects of levetiracetam as an add-on therapy on cognitive function and quality of life in patients with refractory partial seizures. Epilepsy Behav. 2008;12:305–310. doi: 10.1016/j.yebeh.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 55.Xiao Z, Li JM, Wang XF, Xiao F, Xi ZQ, Ly Y, Sun HB. Efficacy and safety of levetiracetam (3,000 mg/day) as an adjunctive therapy in Chinese patients with refractory partial seizures. Eur Neurol. 2009;61:233–239. doi: 10.1159/000197109. [DOI] [PubMed] [Google Scholar]
- 56.Barcs G, Walker EB, Elger CE, Scaramelli A, Stefan H, Sturm Y, Moore A, Flesch G, Kramer L, D'Souza J. Oxcarbazepine placebo-controlled, dose-ranging trial in refractory partial epilepsy. Epilepsia. 2000;41:1597–1607. doi: 10.1111/j.1499-1654.2000.001597.x. [DOI] [PubMed] [Google Scholar]
- 57.French JA, Kugler AR, Robbins JL, Knapp LE, Garofalo EA. Dose-response trial of pregabalin adjunctive therapy in patients with partial seizures. Neurology. 2003;60:1631–1637. doi: 10.1212/01.wnl.0000068024.20285.65. [DOI] [PubMed] [Google Scholar]
- 58.Arroyo S, Anhut H, Kugler AR, Lee CM, Knapp LE, Garofalo EA, Messmer S. Pregabalin add-on treatment: a randomized, double-blind, placebo-controlled, dose response study in adults with partial seizures. Epilepsia. 2004;45:20–27. doi: 10.1111/j.0013-9580.2004.31203.x. [DOI] [PubMed] [Google Scholar]
- 59.Beydoun A, Uthman BM, Kugler AR, Greiner MJ, Knapp LE, Garofalo EA. Safety and efficacy of two pregabalin regimens for add-on treatment of partial epilepsy. Neurology. 2005;64:475–480. doi: 10.1212/01.WNL.0000150932.48688.BE. [DOI] [PubMed] [Google Scholar]
- 60.Lee BI, Yi S, Hong SB, Kim MK, Lee SA, Lee SK, Shin DJ, Kim JM, Song HK, Heo K, Lowe W, Leon T. Pregabalin add-on therapy using a flexible, optimized dose schedule in refractory partial epilepsies: a double-blind, randomized, placebo-controlled, multicenter trial. Epilepsia. 2009;50:464–474. doi: 10.1111/j.1528-1167.2008.01954.x. [DOI] [PubMed] [Google Scholar]
- 61.Uthman BM, Rowan AJ, Ahmann PA, Leppik IE, Schachter SC, Sommerville KW, Shu V. Tiagabine for complex partial seizures: a randomized, add-on, dose-response trial. Arch Neurol. 1998;55:56–62. doi: 10.1001/archneur.55.1.56. [DOI] [PubMed] [Google Scholar]
- 62.Kalviainen R, Brodie MJ, Duncan J, Chadwick D, Edwards D, Lyby K. A double-blind, placebo-controlled trial of tiagabine given three-times daily as add-on therapy for refractory partial seizures. Epilepsy Res. 1998;30:31–40. doi: 10.1016/s0920-1211(97)00082-x. [DOI] [PubMed] [Google Scholar]
- 63.Tassinari CA, Michelucci R, Chauvel P, Chodkiewicz J, Shorvon S, Henriksen O, Dam M, Reife R, Pledger G, Karim R. Double-blind, placebo-controlled trial of topiramate (600 mg daily) for the treatment of refractory partial epilepsy. Epilepsia. 1996;37:763–768. doi: 10.1111/j.1528-1157.1996.tb00649.x. [DOI] [PubMed] [Google Scholar]
- 64.Ben-Menachem E, Henriksen O, Dam M, Mikkelsen M, Schmidt D, Reid S, Reife R, Kramer L, Pledger G, Karim R. Double-blind, placebo-controlled trial of topiramate as add-on therapy in patients with refractory partial seizures. Epilepsia. 1996;37:539–543. doi: 10.1111/j.1528-1157.1996.tb00606.x. [DOI] [PubMed] [Google Scholar]
- 65.Faught E, Wilder BJ, Ramsay RE, Reife RA, Kramer LD, Pledger GW, Karim RM. Topiramate placebo-controlled dose-ranging trial in refractory partial epilepsy using 200-, 400-, and 600-mg daily dosages. Topiramate YD Study Group. Neurology. 1996;46:1684–1690. doi: 10.1212/wnl.46.6.1684. [DOI] [PubMed] [Google Scholar]
- 66.Privitera M, Fincham R, Penry J, Reife R, Kramer L, Pledger G, Karim R. Topiramate placebo-controlled dose-ranging trial in refractory partial epilepsy using 600-, 800-, and 1,000-mg daily dosages. Topiramate YE Study Group. Neurology. 1996;46:1678–1683. doi: 10.1212/wnl.46.6.1678. [DOI] [PubMed] [Google Scholar]
- 67.Sharief M, Viteri C, Ben-Menachem E, Weber M, Reife R, Pledger G, Karim R. Double-blind, placebo-controlled study of topiramate in patients with refractory partial epilepsy. Epilepsy Res. 1996;25:217–224. doi: 10.1016/s0920-1211(96)00029-0. [DOI] [PubMed] [Google Scholar]
- 68.Yen DJ, Yu HY, Guo YC, Chen C, Yiu CH, Su MS. A double-blind, placebo-controlled study of topiramate in adult patients with refractory partial epilepsy. Epilepsia. 2000;41:1162–1166. doi: 10.1111/j.1528-1157.2000.tb00321.x. [DOI] [PubMed] [Google Scholar]
- 69.French JA, Mosier M, Walker S, Sommerville K, Sussman N. A double-blind, placebo-controlled study of vigabatrin three g/day in patients with uncontrolled complex partial seizures. Neurology. 1996;46:54–61. doi: 10.1212/wnl.46.1.54. [DOI] [PubMed] [Google Scholar]
- 70.Bruni J, Guberman A, Vachon L, Desforges C. Vigabatrin as add-on therapy for adult complex partial seizures: a double-blind, placebo-controlled multicentre study. The Canadian Vigabatrin Study Group. Seizure. 2000;9:224–232. doi: 10.1053/seiz.2000.0381. [DOI] [PubMed] [Google Scholar]
- 71.Schmidt D, Jacob R, Loiseau P, Deisenhammer E, Klinger D, Despland A, Egli M, Bauer G, Stenzel E, Blankenhorn V. Zonisamide for add-on treatment of refractory partial epilepsy: a European double-blind trial. Epilepsy Res. 1993;15:67–73. doi: 10.1016/0920-1211(93)90011-u. [DOI] [PubMed] [Google Scholar]
- 72.Faught E, Ayala R, Montouris GG, Leppik IE. Randomized controlled trial of zonisamide for the treatment of refractory partial-onset seizures. Neurology. 2001;57:1774–1779. doi: 10.1212/wnl.57.10.1774. [DOI] [PubMed] [Google Scholar]
- 73.Brodie MJ. Zonisamide clinical trials: European experience. Seizure. 2004;13(Suppl. 1):S66–S70. doi: 10.1016/j.seizure.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 74.Sackellares JC, Ramsay RE, Wilder BJ, Browne TR, III, Shellenberger MK. Randomized, controlled clinical trial of zonisamide as adjunctive treatment for refractory partial seizures. Epilepsia. 2004;45:610–617. doi: 10.1111/j.0013-9580.2004.11403.x. [DOI] [PubMed] [Google Scholar]
- 75.Brodie MJ, Duncan R, Vespignani H, Solyom A, Bitenskyy V, Lucas C. Dose-dependent safety and efficacy of zonisamide: a randomized, double-blind, placebo-controlled study in patients with refractory partial seizures. Epilepsia. 2005;46:31–41. doi: 10.1111/j.0013-9580.2005.14704.x. [DOI] [PubMed] [Google Scholar]
- 76.Brodie MJ, Mumford JP. Double-blind substitution of vigabatrin and valproate in carbamazepine-resistant partial epilepsy. 012 Study group. Epilepsy Res. 1999;34:199–205. doi: 10.1016/s0920-1211(98)00110-7. [DOI] [PubMed] [Google Scholar]
- 77.Lindberger M, Alenius M, Frisen L, Johannessen SI, Larsson S, Malmgren K, Torason T. Gabapentin versus vigabatrin as first add-on for patients with partial seizures that failed to respond to monotherapy: a randomized, double-blind, dose titration study. GREAT Study Investigators Group. Gabapentin in refractory epilepsy add-on treatment. Epilepsia. 2000;41:1289–1295. doi: 10.1111/j.1528-1157.2000.tb04607.x. [DOI] [PubMed] [Google Scholar]
- 78.Baulac M, Leon T, O'Brien TJ, Whalen E, Barrett J. A comparison of pregabalin, lamotrigine, and placebo as adjunctive therapy in patients with refractory partial-onset epilepsy. Epil Res. 2010;91:10–19. doi: 10.1016/j.eplepsyres.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 79.Marson AG, Kadir ZA, Hutton JL, Chadwick DW. The new antiepileptic drugs: a systematic review of their efficacy and tolerability. Epilepsia. 1997;38:859–880. doi: 10.1111/j.1528-1157.1997.tb01251.x. [DOI] [PubMed] [Google Scholar]
- 80.Marson AG, Kadir ZA, Chadwick DW. New antiepileptic drugs: a systematic review of their efficacy and tolerability. BMJ. 1996;313:1169–1174. doi: 10.1136/bmj.313.7066.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shorvon SD. The choice of drugs and approach to drug treatments in partial epilepsy. In: Shorvon SD, Perucca E, Fish D, Dodson E, editors. The Treatment of Epilepsy. 2nd edn. Oxford: Blackwell Science Ltd; 2004. pp. 317–333. [Google Scholar]
- 82.Otoul C, Arrigo C, Rijckevorsel KV, French JA. Meta-analysis and indirect comparisons of levetiracetam with other second-generation antiepileptic drugs in partial epilepsy. Clin Neuropharmacol. 2005;28:72–78. doi: 10.1097/01.wnf.0000159956.87511.67. [DOI] [PubMed] [Google Scholar]
- 83.Beyenburg S, Stavem K, Schmidt D. Placebo-corrected efficacy of modern antiepileptic drugs for refractory epilepsy: systematic review and meta-analysis. Epilepsia. 2010;51:7–26. doi: 10.1111/j.1528-1167.2009.02299.x. [DOI] [PubMed] [Google Scholar]
- 84.Rheims S, Perucca E, Cucherat M, Ryvlin P. Factors determining response to antiepileptic drugs in randomized controlled trials. A systematic review and meta-analysis. Epilepsia. 2011;52:219–233. doi: 10.1111/j.1528-1167.2010.02915.x. [DOI] [PubMed] [Google Scholar]
- 85.Devinsky O, Cramer J. Safety and efficacy of standard and new antiepileptic drugs. Neurology. 2000;55:S5–10. [PubMed] [Google Scholar]
- 86.Diamond GA, Kaul S. Prior convictions: Bayesian approaches to the analysis and interpretation of clinical megatrials. J Am Coll Cardiol. 2004;43:1929–1939. doi: 10.1016/j.jacc.2004.01.035. [DOI] [PubMed] [Google Scholar]
- 87.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Psaty BM, Lumley T, Furberg CD, Schellenbaum G, Pahor M, Alderman MH, Weiss NS. Health outcomes associated with various antihypertensive therapies used as first-line agents: a network meta-analysis. JAMA. 2003;289:2534–2544. doi: 10.1001/jama.289.19.2534. [DOI] [PubMed] [Google Scholar]
- 89.Singh JA, Christensen R, Wells GA, Suarez-Almazor ME, Buchbinder R, Lopex-Olivo MA, Ghogoma ET, Tugwell P. A network meta-analysis of randomized controlled trials of biologics for rheumatoid arthritis: a Cochrane overview. CMAJ. 2009;181:787–796. doi: 10.1503/cmaj.091391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Thijs V, Lemmens R, Fieuws S. Network meta-analysis: simultaneous meta-analysis of common antiplatelet regimens after transient ischaemic attack or stroke. Eur Heart J. 2008;29:1086–1092. doi: 10.1093/eurheartj/ehn106. [DOI] [PubMed] [Google Scholar]
- 91.Mills E, Perri D, Cooper C, Nachega JB, Wu P, Tleyjeh I, Phillips P. Antifungal treatment for invasive Candida infections: a mixed treatment comparison meta-analysis. Ann Clin Microbiol Antimicrob. 2009;8:23. doi: 10.1186/1476-0711-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huynh T, Perron S, O'Loughlin J, Joseph L, Labracque M, Tu JV, Theroux P. Comparison of primary percutaneous coronary intervention and fibrinolytic therapy in ST-segment-elevation myocardial infarction: Bayesian hierarchical meta-analyses of randomized controlled trials and observational studies. Circulation. 2009;119:3101–3109. doi: 10.1161/CIRCULATIONAHA.108.793745. [DOI] [PubMed] [Google Scholar]
- 93.Egger M, Jüni P, Bartlett C, Holenstein F, Sterne J. How important are comprehensive literature searches and the assessment of trial quality in systematic reviews? Empirical study. Health Technol Assess. 2003;7(1) [PubMed] [Google Scholar]
- 94.European Medicines Agency: Committee for Medicinal Products for Human Use (CHMP) Guideline on Clinical Investigation of Medicinal Products in the Treatment of Epileptic Disorders [Draft]. CHMP/EWP/566/98 Rev. 2. 2009. accessed 22-4-2010. [DOI] [PubMed]
- 95.Ioannidis JPA. Integration of evidence from multiple meta-analyses: a primer on umbrella reviews, treatment networks and multiple treatments meta-analyses. CMAJ. 2009;181:488–493. doi: 10.1503/cmaj.081086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Salanti G, Kavvoura FK, Ioannidis JPA. Exploring the geometry of treatment networks. Ann Intern Med. 2008;148:544–553. doi: 10.7326/0003-4819-148-7-200804010-00011. [DOI] [PubMed] [Google Scholar]
- 97.Ioannidis JPA, Lau J. Completeness of safety reporting in randomized trials: an evaluation of 7 medical areas. JAMA. 2001;285:437–443. doi: 10.1001/jama.285.4.437. [DOI] [PubMed] [Google Scholar]
- 98.Morrison A, Moulton K, Clark M, Polisena J, Fiander M, Mierzwinski-Urban M, Mensinkai S, Clifford T, Hutton B. English-Language Restriction When Conducting Systematic Review-Based Meta-Analyses: Systematic Review of Published Studies. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009. [Google Scholar]
- 99.Moher D, Pham B, Lawson ML, Klassen TP. The inclusion of reports of randomised trials published in languages other than English in systematic reviews. Health Technol Assess. 2003;7(41) doi: 10.3310/hta7410. [DOI] [PubMed] [Google Scholar]
- 100.French JA, Kanner AM, Bautista J, Abou-Khalil B, Browne T, Harden CL, Theodore WH, Bazil C, Stern J, Schachter SC, Bergen D, Hirtz D, Montouris GD, Nespeca M, Gidal B, Marks WJ, Turk WJ, Fischer JH, Bourgeois B, Wilner A, Faught RE, Sachdeo RC, Beydoun A, Glauser TA. Efficacy and tolerability of the new antiepileptic drugs I: treatment of new onset epilepsy: report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62:1252–1260. doi: 10.1212/01.wnl.0000123693.82339.fc. [DOI] [PubMed] [Google Scholar]
- 101.Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, Cramp C, Cockerell OC, Cooper PN, Doughty J, Eaton B, Gamble C, Goulding PJ, Howell SJL, Hughes A, Jackson M, Jacoby A, Kellett M, Lawson GR, Leach JP, Nicolaides P, Roberts R, Shackley P, Shen J, Smith DF, Smith PEM, Tudur-Smith C, Vanoli A, Williamson PR. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000–1015. doi: 10.1016/S0140-6736(07)60460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Marson AG, Al-Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, Cramp C, Cockerell OC, Cooper PN, Doughty J, Eaton B, Gamble C, Goulding PJ, Howell SJL, Hughes A, Jackson M, Jacoby A, Kellett M, Lawson GR, Leach JP, Nicolaides P, Roberts R, Shackley P, Shen J, Smith DF, Smith PEM, Tudur-Smith C, Vanoli A, Williamson PR. The SANAD study of effectiveness of valproate, lamotrigine, or topiramate for generalised and unclassifiable epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1016–1026. doi: 10.1016/S0140-6736(07)60461-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


