Abstract
Aim
To administer repeated oral doses of netazepide to healthy subjects for the first time, to assess safety, tolerability, pharmacokinetics and effect on 24 h gastric pH and plasma gastrin.
Method
We did two randomized, double-blind, parallel group studies. The first compared netazepide 25 and 100 mg 12 hourly, omeprazole 20 mg once daily and placebo for 7 days. On day 7 only, we measured pH and assayed plasma gastrin. The second study compared netazepide 5, 10 and 25 mg and placebo once daily for 14 days. We measured pH on days 1, 7 and 14 and assayed plasma gastrin on days 1 and 14. We compared treatments by time gastric pH ≥ 4 during 0–4, 4–9, 9–13 and 13–24 h after the morning dose, and by plasma gastrin. P < 0.05 was significant.
Results
Netazepide was well tolerated. On day 7 of the first study, netazepide increased pH significantly only during 9–13 h after the 100 mg dose, whereas omeprazole raised pH significantly during all periods. Both netazepide and omeprazole increased plasma gastrin significantly. Netazepide had linear pharmacokinetics. In the second study, netazepide caused dose-dependent, sustained increases in pH on day 1, but as in the first study, netazepide had little effect on pH on days 7 and 14. Again, netazepide increased plasma gastrin significantly.
Conclusion
Although repeated doses of netazepide led to tolerance to its effect on pH, the accompanying increase in plasma gastrin is consistent with continued inhibition of acid secretion, via gastrin receptor antagonism and gene up-regulation.
Keywords: gastric pH, gastrin receptor antagonist, netazepide (YF476), plasma gastrin
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Non-clinical studies have shown that netazepide (YF476) is a potent, highly selective and orally active gastrin receptor antagonist. Activity, including suppression of gastric acid secretion, persists during repeated dosing.
In healthy subjects, single oral doses of netazepide cause dose-dependent increases in 24 h gastric pH. Onset of activity is as fast as ranitidine but much longer lasting.
WHAT THIS STUDY ADDS
A single oral dose of netazepide again caused dose-dependent, sustained increases in 24 h gastric pH in healthy subjects, but tolerance developed during repeated dosing, whereas the effect of omeprazole persisted.
Despite tolerance to the effect of netazepide on pH, netazepide increased plasma gastrin, as did omeprazole, which is consistent with persistent acid suppression by netazepide.
Time ≥ pH 4 is not the way to assess acid suppression by repeated doses of netazepide, which might still prove an effective treatment for acid-related conditions.
Introduction
The hormone gastrin [1] causes gastric acid secretion via stimulation of gastrin receptors (CCK-B or CCK-2 receptors) on enterochromaffin-like (ECL) gastric mucosal cells, which release histamine. That in turn stimulates histamine H2-receptors on parietal cells in the gastric mucosa [2] and thereby secretion of acid into the lumen of the stomach. Acid production is mediated by the proton pump on parietal cells, which also express gastrin receptors [3], but the role of those receptors in acid secretion is uncertain [4]. Gastrin also stimulates growth of ECL and parietal cells [1, 5, 6].
Non-clinical studies have shown that netazepide (YF476) is a potent, selective, competitive and orally active antagonist of gastrin receptors [7–9]. In our double-blind, placebo-controlled, crossover study of single doses of netazepide 5, 25 or 100 mg compared with ranitidine 150 mg in healthy subjects, netazepide was well tolerated and caused dose-dependent, long-lasting increases in basal and food-stimulated 24 h gastric pH, consistent with antagonism of gastrin receptors [10]. Onset of activity of netazepide and ranitidine was similarly rapid. However, activity of ranitidine lasted about 12 h, whereas that of netazepide exceeded 24 h. Compared with ranitidine 150 mg, netazepide 5 mg was as effective, and netazepide 25 and 100 mg were much more effective, over the 24 h after dosing. Median tmax and t1/2 after the 100 mg dose were about 1 and 7 h, respectively, and the pharmacokinetics were dose-proportional.
Those encouraging results justified further studies in healthy subjects, and we now report the first administration of repeated doses of netazepide. We did two parallel-group studies. The first compared netazepide 25 and 100 mg twice daily, omeprazole 20 mg once daily and placebo for 7 days. The aim was to assess the safety, tolerability and pharmacokinetics of netazepide, and to compare its effects on gastric pH and plasma gastrin with those of omeprazole. The second study compared netazepide 5, 10 and 25 mg and placebo once daily for 14 days, to find out whether lower doses of netazepide would give results similar to those of the first study.
We have presented these studies at meetings of the Clinical Section of the British Pharmacological Society [11, 12].
Methods
We did the studies at the Central Middlesex Hospital, London, England in accordance with the ICH Guideline for Good Clinical Practice and Declaration of Helsinki. Brent Ethics Committee approved the studies. Subjects gave written informed consent. We registered the studies under ClinicalTrials.gov NCT01599858 and NCT01597674.
Treatments
Yamanouchi Pharmaceutical Co, Japan, supplied netazepide 5 and 25 mg capsules and matching placebo. The hospital pharmacy: supplied encapsulated omeprazole 20 mg tablets (Losec, AstraZeneca) and matching placebo capsules, packed and labelled treatments and randomized subjects to treatments.
Study design
First study
This study was double-blind, double-dummy and parallel group in design, and required 48 healthy men or women. Women not using a reliable method of contraception were excluded. Subjects were randomized to one of four treatments by mouth for 7 days: netazepide 25 mg 12 hourly, netazepide 100 mg 12 hourly, omeprazole 20 mg once daily and placebo. They took the morning dose after fasting overnight. Subjects were resident for 8 nights and returned for follow-up 5–10 days after the last dose. On day 7, they fasted overnight and had standard meals and drinks at 4, 9, 13 and 22 h after dosing. Meals and drinks were at usual times on all other days. We recorded intragastric pH every 6 s for 24 h on day 7, using a nasogastric pH electrode [10]. We took blood samples before each morning dose on days 2–7 and at 0.25, 0.5, 0.75, 1.0, 1.5, 2, 3, 4, 5, 6, 8, 10 and 12 h after the second dose on day 7 for assay of plasma netazepide. We also took blood samples at 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 16, 18, 20, 22 and 24 h after the morning dose on day 7 for assay of plasma gastrin. We assessed safety and tolerability by adverse events, vital signs, ECG and safety tests of blood and urine.
Second study
This study was double-blind and parallel group in design, and required 48 men. Subjects were randomized to one of four treatments by mouth once daily for 14 days: netazepide 5, 10 and 25 mg, and placebo, which they took in the morning after fasting overnight. On days 1, 7 and 14, they ate standard meals at 4, 9, 13 and 22 h after dosing. On days 3–6 and 9–13, they did not eat until at least 30 min after dosing, but were allowed water as required. All other meals and drinks were at usual times on those days. Subjects were resident for 2 nights on each of three occasions: from the evening before until the morning after dosing on days 1, 7 and 14. On days 3–6 and 9–13, subjects attended each morning, for dosing only. On days 1, 7 and 14, we recorded ambulatory gastric pH every 6 s from 0.5 h before until 24 h after dosing, and we took frequent blood samples from 0 to 24 h after dosing for assay of plasma gastrin, as in the first study. We also assessed safety and tolerability as in the first study.
Gastric pH
We prepared subjects and measured 24 h gastric pH, as described previously [10].
Plasma gastrin
We put 4 ml blood into lithium-heparin tubes containing 0.2 ml aprotinin 10 000 units ml−1 (Trasylol; Bayer), and centrifuged them (4°C, 800 g for 10 min) within 15 min of collection. We stored plasma in polypropylene tubes at −20°C until assay by ASI, St George's Hospital, London, UK using a validated 125I-radioimmunoassay (GammaDab®, DiaSorin, Stillwater, Minnesota, USA). The calibration line was linear over the concentration range 40 to 1000 pg l−1. The sensitivity was 6 pg l−1 and the coefficient of variation was ∼4%.
Plasma netazepide
We assayed plasma netazepide by HPLC-MS [13], as described previously [10].
Statistics
24h gastric pH
From the results of our study of single doses of netazepide [10], we calculated 12 subjects per group to be sufficient for both studies to detect a 70% increase in area under the curve (AUC) for gastric pH vs. time, with at least 80% power, assuming a between-subject coefficient of variation of 0.46 and significance of 5%. We analyzed pH data in four intervals: 0–4, 4–9, 9–13 and 13–24 h after the morning dose. For each interval, we calculated AUC, using the trapezoidal rule, and time pH ≥4.
We tested for differences among treatments by analysis of variance (anova), after log-transformation of variables, where appropriate. All tests were two-sided, and the significance level was α = 0.05. If treatment effect was significant in the overall model, we made pair wise comparisons between each active treatment and placebo, using the Wilcoxon rank sum test. The primary response variable was time pH ≥4.
To illustrate pH results, for the first study we calculated median values for every 1 h from 0 to 14 h after dosing and every 2 h from 14 to 24 h after dosing, and for the second study we calculated median values for every 2 min from 0 to 24 h after dosing. We calculated median values because the data were not normally distributed.
Plasma gastrin
For both studies, we calculated plasma gastrin AUC(0,24 h) by the trapezoidal rule. In the first study, we compared treatments by anova. In the second study, we measured plasma gastrin only for the netazepide 5 and 25 mg groups on days 1 and 14. In a post hoc analysis, we used a Kruskal Wallis test to compare the results for AUC(0,24 h) of plasma gastrin for netazepide 5 and 25 mg with those of placebo from the first study. We used a Wilcoxon rank sum test to do pairwise comparisons between netazepide dose levels and placebo only if there was an overall significant difference among treatments.
Pharmacokinetics
We used WinNonlin to derive pharmacokinetic parameters for plasma netazepide concentrations after the second dose on day 7 of the first study: Cmax, tmax, AUC(0,t), AUC(0,∞) and t1/2, as described previously [10]. We used netazepide concentrations before dosing on days 2–7 to assess when steady-state had been reached and whether netazepide had accumulated after repeated doses.
Results
Subjects
Forty-nine subjects, 25 women and 24 men, entered the first study. Their mean age and body mass index (BMI) were 24.1 years (range 19–33 years) and 23.3 kg m−2 (range 19.5–29.6 kg m−2), respectively. All subjects were Europid. We withdrew one woman whose pH electrode failed. Four groups of 12 subjects completed the study.
Forty-nine men entered the second study. Their mean age and BMI were 24.9 years (range 18–40 years) and 23.86 kg m−2 (range 19.5–28.4 kg m−2), respectively. Forty-seven subjects were Europid, one was Negroid and one was Asian/Indian. We withdrew one subject after five doses of what proved to be netazepide 25 mg, because of nausea, abdominal discomfort and diarrhoea. Another subject had a high basal gastric pH and plasma gastrin, consistent with achlorhydria, so we excluded his results from the statistical analysis. Three groups of 12 subjects and one group of 11 (netazepide 5 mg) subjects completed the study.
Tolerability and safety
Netazepide was well tolerated in both studies. Subsequent re-challenge of the subject who had gastro-intestinal symptoms after five doses of netazepide 25 mg was uneventful. Otherwise, any adverse events were minor, transient and occurred across the treatments. There were no clinically relevant changes in any of the safety assessments.
Pharmacodynamics
First study
Median times gastric pH ≥ 4 are shown in Table 1 and median gastric pH values vs. time are illustrated in Figure 1. Median AUC(0,24 h) of plasma gastrin concentrations are shown in Table 1 and median plasma gastrin concentrations vs. time are illustrated in Figure 1B.
Table 1.
First study: median time (h) gastric pH ≥4 and median (range) AUC(0,24 h) of plasma gastrin (ng l−1 h) on day 7
| Placebo n = 12 | Netazepide 25 mg twice daily n = 12 | Netazepide 100 mg twice daily n = 12 | Omeprazole 20 mg once daily n = 12 | |
|---|---|---|---|---|
| Time pH ≥4 after morning dose | ||||
| 0–4 h | 0.0 (B) | 0.0 (B) | 0.0 (B) | 2.1 (A) |
| 4–9 h | 0.9 (B) | 0.6 (B) | 0.6 (B) | 2.6 (A) |
| 9–13 h | 0.5 (C) | 0.7 (BC) | 1.2 (AB) | 1.8 (A) |
| 13–24 h | 1.1 (B) | 0.2 (B) | 1.0 (B) | 2.9 (A) |
| Gastrin AUC(0,24 h) (ng l−1 h) | ||||
| Median | 985 | 1701* | 1556** | 2140*** |
| Range | 295–1620 | 390–3086 | 858–3743 | 1144–3211 |
In each time period, treatments with the same letter in parentheses are not significantly different from each other (P > 0.05). Compared with placebo:
P = 0.02
P = 0.01
P = 0.001.
Figure 1.
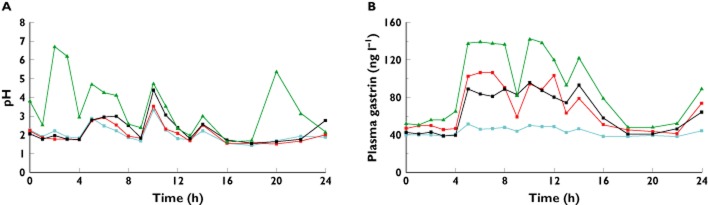
First study: median (n = 12 per group) (A) 24 h gastric pH and (B) 24 h plasma gastrin (ng l−1) on day 7.  placebo,
placebo,  omeprazole,
omeprazole,  netazepide 25 mg,
netazepide 25 mg,  netazepide 100 mg
netazepide 100 mg
There were characteristic and predictable variations in gastric pH during placebo treatment, corresponding to the times at which subjects ate and drank. Compared with placebo, gastric pH tended to be higher in the post-prandial periods after netazepide, but the time pH ≥4 was significant (P < 0.05) only for netazepide 100 mg during the period 9–13 h after the morning dose. Omeprazole increased the time gastric pH ≥ 4 significantly (P < 0.05) compared with either placebo, netazepide 25 or 100 mg, during all periods except 9–13 h after netazepide 100 mg.
Compared with placebo, netazepide 25 mg (P = 0.02), netazepide 100 mg (P = 0.01) and omeprazole (P = 0.001) all significantly increased AUC(0,24 h) of plasma gastrin. Gastrin concentrations after omeprazole were higher than those after netazepide 25 and 100 mg, especially after food, but the differences were not significant.
Second study
Median times gastric pH ≥ 4 are shown in Table 2 and median gastric pH values vs. time are illustrated in Figure 2. Median AUC(0,24 h) of plasma gastrin concentrations are shown in Table 3 and median plasma gastrin concentrations vs. time are illustrated in Figure 3. Again, there were characteristic and predictable variations in median gastric pH during placebo treatment, corresponding to the times at which the subjects ate and drank.
Table 2.
Second study: median time (h) gastric pH ≥ 4 on days 1, 7 and 14
| Day, and time interval (h) after morning dose | Netazepide | |||
|---|---|---|---|---|
| Placebo | 5 mg | 10 mg | 25 mg | |
| n = 12 | n = 11 | n = 12 | n = 12 | |
| Day 1 | ||||
| 0–4 | 0.0 | 1.5* | 1.7* | 2.3* |
| 4–9 | 0.1 | 1.0 | 1.2* | 3.6* |
| 9–13 | 0.3 | 0.5 | 1.2* | 1.5* |
| 13–24 | 0.3 | 0.4 | 1.0 | 2.0* |
| Day 7 | ||||
| 0–4 | 0.1 | 0.0 | 0.1 | 0.1 |
| 4–9 | 0.5 | 0.7 | 0.9 | 1.4 |
| 9–13 | 0.4 | 0.9 | 1.2* | 1.4* |
| 13–24 | 0.7 | 0.8 | 0.9 | 1.2 |
| Day 14 | ||||
| 0–4 | 0.0 | 0.0 | 0.0 | 0.0 |
| 4–9 | 0.6 | 0.7 | 0.6 | 1.0 |
| 9–13 | 0.8 | 0.6 | 0.9 | 1.5 |
| 13–24 | 1.1 | 0.9 | 0.3 | 1.1 |
Compared with placebo, P < 0.05.
Figure 2.
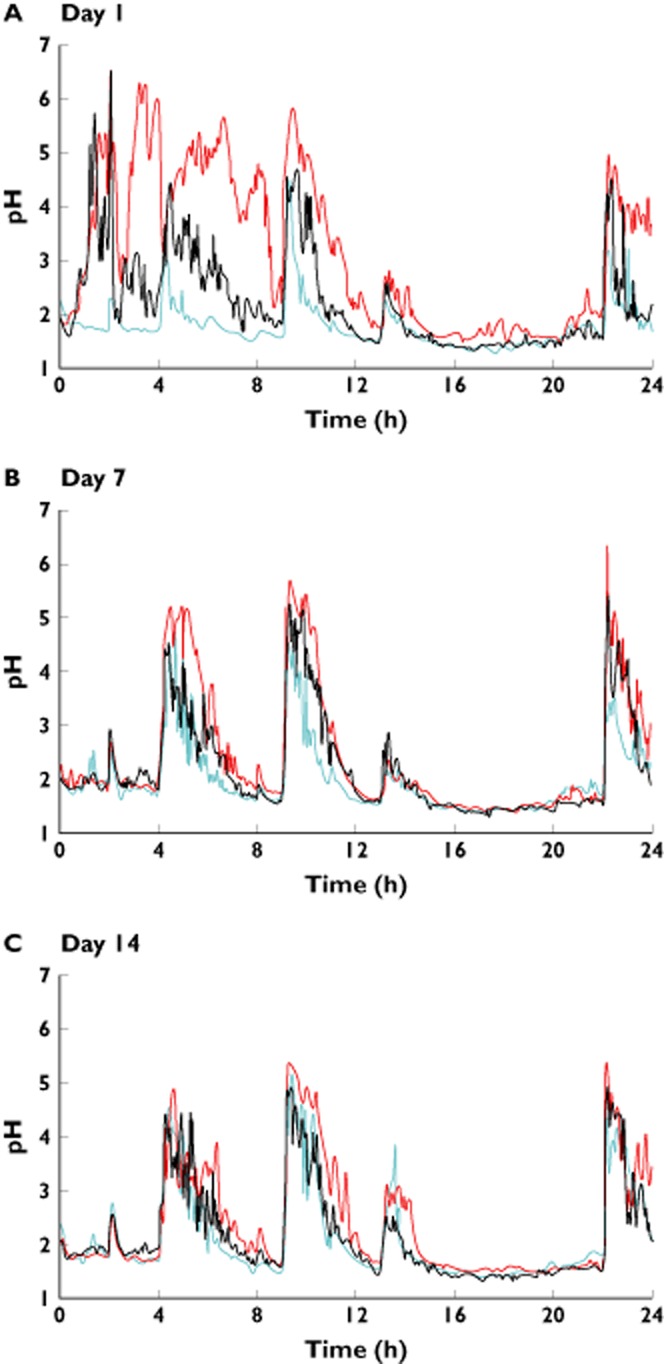
Second study: median gastric pH after on days A) 1, B) 7 and C) 14 of netazepide 5 mg ( ), netazepide 25 mg (
), netazepide 25 mg ( ) and placebo (
) and placebo ( ). n = 12 per group, except netazepide 5 mg where n = 11. Netazepide 10 mg omitted for clarity
). n = 12 per group, except netazepide 5 mg where n = 11. Netazepide 10 mg omitted for clarity
Table 3.
Second study: median AUC(0,24 h) of plasma gastrin concentrations (ng l−1 h)
| Gastrin AUC(0,24 h) (ng l−1 h) | ||||
|---|---|---|---|---|
| Placebo (n = 12) | Netazepide 5 mg (n = 11) | Netazepide 25 mg (n = 12) | ||
| Day 1 | Day 14 | Day 1 | Day 14 | |
| Median | ||||
| 985 | 1093 | 1093 | 1111 | 1315* |
| Range | ||||
| 295–1620 | 772–1998 | 738–2046 | 748–3304 | 981–5296 |
Placebo data from day 7 of study 1.
Compared with placebo, P = 0.01.
Figure 3.
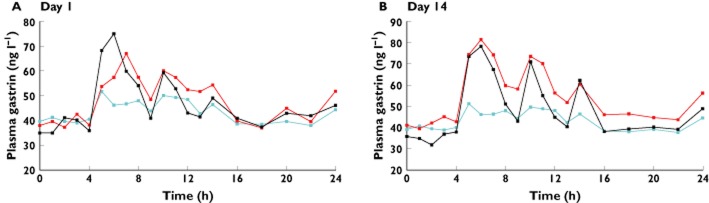
Second study: median plasma gastrin concentrations (ng l−1) on days A) 1 and B) 14 of netazepide 5 mg (n = 11) and 25 mg (n = 12) once daily. Placebo data (n = 12) from Day 7 of the first study. Netazepide 5 mg ( ), netazepide 25 mg (
), netazepide 25 mg ( ) and placebo (
) and placebo ( )
)
On day 1, gastric pH increased quickly after dosing with netazepide. After all meals, gastric pH fell more slowly after netazepide than after placebo. Even after breakfast, at 24 h after dosing, pH was higher after netazepide than after placebo. Trends were similar for all doses of netazepide. On day 1, compared with placebo, netazepide 5 mg significantly increased the time pH ≥ 4 only during the 0–4 h period, netazepide 10 mg significantly increased the time pH ≥ 4 during the 0–4, 4–9 and 9–13 h periods and netazepide 25 mg significantly increased the time pH ≥ 4 during all time periods up to 24 h after dosing. In contrast, on days 7 and 14, compared with placebo, gastric pH tended to be higher in the post-prandial periods after netazepide, but the time pH ≥4 was significantly higher only during 9–13 h after netazepide 10 or 25 mg.
Compared with placebo, plasma gastrin concentrations were higher after netazepide 5 and 25 mg on days 1 and 14. On day 1, there were no significant differences among netazepide 5 mg and 25 mg and placebo for AUC(0,24 h) of plasma gastrin (P = 0.26). However, there was a significant difference on day 14 (P = 0.04). Therefore, we used a Wilcoxon rank-sum test to do pairwise comparisons for day 14. There was no significant difference between netazepide 5 mg and placebo (P = 0.37), but there was a significant difference between netazepide 25 mg and placebo (P = 0.01). There was no significant difference among netazepide doses for AUC(0,24 h) of plasma gastrin on day 1 or on day 14.
Pharmacokinetics
Mean (range) pharmacokinetic parameters after the last dose of netazepide on day 7 were: Cmax 120 (76–188) ng ml−1, tmax 0.75 (0.5–4.0) h, AUC(0,t) 196 (160–300) ng ml−1 h and t1/2 3.4 (2.0–7.3) h for netazepide 25 mg and Cmax 569 (270–958) ng ml−1, tmax 1.0 (0.5–2.0) h, AUC(0,t) 933 (686–1379) ng ml−1 h and t1/2 4.1 (2.8–6.3) h for netazepide 100 mg. Trough concentrations of netazepide 25 mg and 10 mg showed that steady-state was reached by day 3 and that there was little or no accumulation by day 7. AUC(0,t) and Cmax increased in the ratio 4.8:1 and 4.7:1, respectively, while the dose increased in the ratio 4:1. Thus, the pharmacokinetics of netazepide were dose-proportional in the range studied.
Discussion
We were surprised by the trivial effect on 24 h gastric pH on day 7 of netazepide of 25 and 100 mg 12 hourly in the first study, given that single doses of 5, 25 and 100 mg had caused dose-dependent, sustained increases in 24 h gastric pH in our previous study in healthy subjects [10]. The discordant results cannot be attributed to deficiencies in our methods, because omeprazole increased gastric pH on day 7, as expected. Furthermore, netazepide 25 and 100 mg and omeprazole all increased 24 h plasma gastrin on day 7. Proton pump inhibitors (PPI) such as omeprazole increase circulating gastrin via inhibition of gastric acid production and up-regulation of the gastrin gene [14]. However, tolerance to the acid-suppressant effect of omeprazole does not develop after repeated doses despite the increase in gastrin, because PPIs act directly on the proton pump, the final stage in acid secretion. Histamine H2-receptor antagonists (H2RA), such as cimetidine and ranitidine, which are competitive antagonists, also increase circulating gastrin, via inhibition of acid secretion and up-regulation of histamine H2-receptors and adenylate cyclase in the parietal cell [15]. However, unlike the increase in circulating gastrin induced by PPI, the increase induced by H2RA does lead to tolerance [16, 17], because the increase in circulating gastrin by H2RA stimulates release of histamine from ECL cells, which reduces antagonism by the H2RA of histamine H2-receptors on the parietal cell. Indeed, lupitidine (SK&F 93479), a potent, long acting and irreversible H2RA, caused hypergastrinaemia profound enough to overcome suppression of gastric acid production [18, 19]. PPIs replaced H2RA as the preferred treatment for acid-related conditions when it was discovered that tolerance to H2RA after repeated dosing reduces their efficacy [20].
Initially, we wondered whether tolerance to netazepide in the first study might have been due to the observed increase in plasma gastrin, similar to the mechanism of tolerance to an H2RA. Therefore, we did a second study using netazepide 5, 10 and 25 mg once daily for 14 days, to assess whether lower and longer exposure to netazepide might prevent or reduce tolerance. We also improved the study design, by measuring 24 h gastric pH and collecting plasma samples for gastrin assay on days 1 and 14 as well as on day 7. On day 1, the first dose of netazepide 5, 10 and 25 mg caused dose-dependent, sustained increases in gastric pH, as did single doses of netazepide 5, 25 and 100 mg in our previous study [10]. However, the effect of netazepide on gastric pH was small on day 7, and even smaller on day 14. Because of these confirmatory results, the study sponsor decided to limit the number of plasma samples assayed for gastrin to those collected after netazepide 5 and 25 mg on days 1 and 14. Therefore, for comparison, we used the results for placebo from the first study. The comparison was valid because both studies were parallel group in design and used similar subjects and methods. Despite the limited data from the second study, netazepide clearly increased circulating gastrin, as in the first study.
Thus, overall, the results of these two repeated dose studies of netazepide were similar. Tolerance to the effect of netazepide on gastric pH occurred throughout the dose range studied.
Studies in rats subsequent to our studies in healthy subjects have also shown that netazepide increases circulating gastrin [14, 21–29]. The response is secondary to acid suppression by netazepide, which like omeprazole increases gastrin gene expression [14]. Furthermore, activity of netazepide persisted in rats dosed for up to 6 months, whether assessed by its ability to suppress gastric acid production or to prevent the growth promoting effects of hypergastrinaemia on ECL cells [14, 21–29]. In all the aforementioned studies in rats, gastric acid production was assessed by measurement of H+ secretion, not by pH. Studies of other gastrin receptor antagonists in rats have also shown that activity persists after chronic dosing [30, 31]. The increase in circulating gastrin induced by netazepide does not lead to tolerance to its ability to inhibit H+ secretion, because netazepide blocks gastrin receptors on ECL cells, thereby reducing or preventing release of histamine. Any reduction in gastric acid production, such as by PPI, H2RA, vagotomy [32, 33] or chronic atrophic gastritis [34], leads to an increase in circulating gastrin. Thus, the increase in circulating gastrin induced by repeated doses of netazepide in our healthy subjects is consistent with persistent suppression of gastric acid production. Indeed, we have since confirmed that netazepide does cause persistent suppression of gastric acid production, by showing that repeated doses of 100 mg daily inhibit the increase in pentagastrin-stimulated volume and H+ content of gastric aspirate, despite tolerance to the effect on pH 35.
Gastric pH is easy to measure continuously with a nasogastric electrode, and has been widely used in clinical trials as a surrogate measure of acid suppression by H2RA and PPIs [36]. A substantial increase in time pH ≥4 is regarded as essential to heal acid-related conditions. Because repeated doses of netazepide failed to achieve that goal, the development of netazepide for its original target disease of gastro-oesophageal reflux was abandoned for several years. pH is a logarithmic scale, so gastric pH may change little despite a large change in H+ secretion [4, 37] Furthermore, measurement of gastric pH alone ignores changes in volume of secretion. Therefore, the total amount of H+ collected per unit time would be a better test of acid suppression than pH. We discuss elsewhere the possible mechanisms for development of tolerance to the effect of netazepide on gastric pH after repeated doses 35.
Plasma concentrations of netazepide after repeated doses were similar to those after single doses in our previous study [10], which excludes a pharmacokinetic explanation for tolerance to the effect of netazepide on gastric pH. As in our single dose study, netazepide appeared to be eliminated biphasically, the terminal elimination phase beginning at 4–12 h after dosing. Since the interval between tmax and the last sampling time was only 11 h, and the last sample was at 12 h after dosing, the calculated t1/2 is unlikely to represent only the terminal elimination phase. The mean half-lives of about 2–4 h quoted for many subjects are probably influenced by the more rapid first phase of elimination, whereas the higher values are likely to represent a more accurate estimate of the true terminal elimination half-life. Also, the mean values of λz, t1/2 and AUC should be regarded only as estimates, because of the variability of the data and the short measurement period relative to the calculated terminal half-life.
Pre-dose plasma netazepide concentrations on days 2–7 suggested that little if any accumulation of netazepide occurred during repeated dosing. The trough values show that steady-state had been reached by day 3, 48 h after the first dose, which is consistent with an elimination half-life of netazepide of 10 h or less, based on the assumption that steady-state is typically reached within five half-lives after the start of dosing.
In conclusion, netazepide was well tolerated. Single doses caused dose-dependent, sustained increases in gastric pH, as in our previous study. Although tolerance to the effect on gastric pH developed during repeated doses of netazepide, the increase in circulating gastrin is consistent with persistent suppression of gastric acid, via gastrin receptor antagonism and up-regulation of the gastrin gene. Further studies are required to find out the mechanism for tolerance, and whether it might matter therapeutically.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author). Netazepide (YF476) came from research by Ferring, Chilworth, England. Yamanouchi, Japan, partnered Ferring in the early development of the compound (hence the code YF476). Ferring A/S, Indertoften 10, DK-2720 Vanlose, Denmark, funded the studies. Ferring has since licensed netazepide to Trio Medicines Ltd, a subsidiary of Hammersmith Medicines Research (HMR), a contract research organization. MB and SW are directors of HMR and Trio Medicines Ltd and MB owns both companies.
References
- 1.Dockray G, Varro A, Dimaline R, Wang T. The gastrins: their production and biological activities. Ann Rev Physiol. 2001;63:119–139. doi: 10.1146/annurev.physiol.63.1.119. [DOI] [PubMed] [Google Scholar]
- 2.Schubert ML, Peura DA. Control of gastric acid secretion in health and disease. Gastroenterology. 2008;134:1842–1860. doi: 10.1053/j.gastro.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 3.Schmitz F, Göke M, Otte J, Schrader H, Reimann B, Kruse M, Siegel E, Peters J, Herzig K, Fölsch U, Schmidt W. Cellular expression of CCK-A and CCK-B/gastrin receptors in human gastric mucosa. Regul Pept. 2001;102:101–110. doi: 10.1016/s0167-0115(01)00307-x. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Zhao C-M. Complexity of gastric acid secretion by targeted gene disruption in mice. Curr Pharm Des. 2010;16:1235–1240. doi: 10.2174/138161210790945904. [DOI] [PubMed] [Google Scholar]
- 5.Dimaline R, Varro A. Attack and defence in the gastric epithelium – a delicate balance. Exp Physiol. 2007;92:591–601. doi: 10.1113/expphysiol.2006.036483. [DOI] [PubMed] [Google Scholar]
- 6.Almeida-Vega S, Catlow K, Dimaline R, Varro A. Gastrin activates paracrine networks leading to induction of PAI-2 via MAZ and ASC-1. Am J Physiol. 2009;296:G414–G423. doi: 10.1152/ajpgi.90340.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Semple G, Ryder H, Rooker D, Batt A, Kendrick D, Szelke M, Ohta M, Satoh M, Nishida A, Akuzawa S, Miyata K. (3R)-N-(1-(tert-butylcarbonylmethyl)-2,3-dihydro-2-oxo-5-(2-pyridyl)-1H-1,4-benzodiazepin-3-yl)-N'-(3-(methylamino)phenyl)urea (YF476): a potent and orally active gastrin/CCK-B antagonist. J Med Chem. 1997;40:331–341. doi: 10.1021/jm960669+. [DOI] [PubMed] [Google Scholar]
- 8.Takinami Y, Yuki H, Nishida A, Akuzawa S, Uchida A, Takemoto Y, Ohta M, Satoh M, Semple G, Miyata K. YF476 is a new potent and selective gastrin/cholecystokinin-B receptor antagonist in vitro and in vivo. Aliment Pharmacol Ther. 1997;11:113–120. doi: 10.1046/j.1365-2036.1997.110281000.x. [DOI] [PubMed] [Google Scholar]
- 9.Takemoto Y, Yuki H, Nishida A, Ito H, Kobayashi-Uchida A, Takinami Y, Akuzawa S, Ohta M, Satoh M, Semple G, Miyata K. Effects of YF476, a potent and selective gastrin/CCK-B receptor antagonist, on gastric acid secretion in beagle dogs with gastric fistula. Arzneimittelforschung. 1998;48:403–407. [PubMed] [Google Scholar]
- 10.Boyce M, David O, Darwin K, Mitchell T, Johnston A, Warrington S. Single oral doses of netazepide (YF476), a gastrin receptor antagonist, cause dose-dependent, sustained increases in 24-h gastric pH compared with placebo and ranitidine in healthy subjects. Aliment Pharmacol Ther. 2012;36:181–189. doi: 10.1111/j.1365-2036.2012.05143.x. [DOI] [PubMed] [Google Scholar]
- 11.Boyce M, Warrington S, Johnston A, Harris A. Effect on gastric pH of repeated doses of YF476, a new gastrin antagonist, compared with omeprazole and placebo [abstract] Br J Clin Pharmacol. 2000;50:383P–384. [Google Scholar]
- 12.Boyce M, Warrington S, Lewis Y, Nentwich H, Harris A. Adaptation to the antisecretory effect of YF476, a new gastrin antagonist, in healthy men [abstract] Br J Clin Pharmacol. 2002;53:437P. [Google Scholar]
- 13.Redrup M, Leaf F, Miyashita A, Watanabe T, Higuchi S, Chasseaud L, Cheng K. Validation of a liquid chromatographic-tandem mass spectrometric method for the measurement of (R)-1-[2,3- dihydro-2-oxo-1-pivaloylmethyl-5-(2-pyridyl)-1H-1,4-benzodiazepin-3-yl]-3-(3methylaminophenyl)urea (YF476) in human plasma. J Chromatogr. 2002;772:317–325. doi: 10.1016/s1570-0232(02)00118-6. [DOI] [PubMed] [Google Scholar]
- 14.Takaishi S, Shibata W, Tomita H, Jin G, Yang X, Eriksen R, Dubeykovskaya Z, Asfaha S, Quante M, Betz K, Shulkes A, Wang T. In vivo analysis of mouse gastrin gene regulation in enhanced GFP-BAC transgenic mice. Am J Physiol Gastrointest Liver Physiol. 2011;300:G334–344. doi: 10.1152/ajpgi.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takeuchi K, Kajimura M, Kodaira M, Lin S, Hanai H, Kaneko E. Up-regulation of H2 receptor and adenylate cyclase in rabbit parietal cells during prolonged treatment with H2-receptor antagonists. Dig Dis Sci. 1999;44:1703–1709. doi: 10.1023/a:1026652119166. [DOI] [PubMed] [Google Scholar]
- 16.Smith J, Davey C, Nwekol C, Pounder R. Tolerance during 8 days of high-dose H2-blockade: placebo-controlled studies of 24-hour acidity and gastrin. Aliment Pharmacol Ther. 1990;4(Suppl. 1):47–63. [PubMed] [Google Scholar]
- 17.Qvigstad G, Arnestad JS, Brenna E, Waldum H. Treatment with proton pump inhibitors induces tolerance to histamine-2 receptor antagonists in Helicobacter pylori-negative patients. Scand J Gastroenterol. 1998;33:1244–1248. doi: 10.1080/00365529850172313. [DOI] [PubMed] [Google Scholar]
- 18.Betton G, Salmon G. Pathology of the forestomach in rats treated for 1 year with a new histamine H2-receptor antagonist, SK&F 93479 trihydrochloride. Scand J Gastroenterol. 1984;101(Suppl):103–108. [PubMed] [Google Scholar]
- 19.Betton G, Dormer C, Wells T, Pert P, Price C, Buckley P. Gastric EC. L-cell hyperplasia and carcinoids in rodents following chronic administration of H2-antagonists SK&F 93479 and oxmetidine and omeprazole. Toxicol Pathol. 1988;16:288–298. doi: 10.1177/019262338801600222. [DOI] [PubMed] [Google Scholar]
- 20.Garner A, Fadlallah H, Parsons M. 1976 and all that! 20 years of antisecretory therapy. Gut. 1996;39:784–786. doi: 10.1136/gut.39.6.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding X-Q, Lindström E, Håkanson R. Evaluation of three novel cholecystokinin-B/gastrin receptor antagonists: a study of their effects on rat stomach enterochromaffin-like cell activity. Pharmacol Toxicol. 1997;81:232–237. doi: 10.1111/j.1600-0773.1997.tb00052.x. [DOI] [PubMed] [Google Scholar]
- 22.Chen D, Zhao C-M, Norlén P, Björkgvist M, Ding X, Kitano M, Håkanson R. Effect of CCK-2 receptor blockade on rat stomach ECL cells. Cell Tissue Res. 2000;299:81–95. doi: 10.1007/s004419900136. [DOI] [PubMed] [Google Scholar]
- 23.Kitano M, Norlén P, Ding X-Q, Nakamura S, Håkanson R. Long-lasting CCK2 receptor blockade after a single subcutaneous injection of YF476 or YM022. Br J Pharmacol. 2000;130:699–705. doi: 10.1038/sj.bjp.0703342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Konagaya T, Bernsand M, Norlén P, Håkanson R. Mobilization of rat stomach ECL-cell histamine in response to short- or long-term treatment with omeprazole and/or YF 476 studied by gastric submucosal microdialysis in conscious rats. Br J Pharmacol. 2001;133:37–42. doi: 10.1038/sj.bjp.0704037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Björkqvist M, Dornonville de la Cour C, Zhao CM, Gagnemo-Persson R, Håkanson R, Norlén P. Role of gastrin in the development of gastric mucosa, ECL cells and A-like cells in newborn and young rats. Regul Pept. 2002;108:73–82. doi: 10.1016/s0167-0115(02)00111-8. [DOI] [PubMed] [Google Scholar]
- 26.Martinsen T, Kawase S, Håkanson R, Torp S, Fossmark R, Qvigstad G, Sandvik A, Waldum H. Spontaneous ECL cell carcinomas in cotton rats: natural course and prevention by a gastrin receptor antagonist. Carcinogenesis. 2003;24:1887–1896. doi: 10.1093/carcin/bgg156. [DOI] [PubMed] [Google Scholar]
- 27.Takaishi S, Cui G, Frederick D, Carlson J, Houghton J, Varro A, Dockray G, Ge Z, Whary M, Rogers A, Fox J, Wang T. Synergistic inhibitory effects of gastrin and histamine receptor antagonists on Helicobacter-induced gastric cancer. Gastroenterology. 2005;128:1965–1983. doi: 10.1053/j.gastro.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 28.Cui G, Takaishi S, Ai W, Betz K, Florholmen J, Koh T, Houghton J, Pritchard D, Wang T. Gastrin-induced apoptosis contributes to carcinogenesis in the stomach. Lab Invest. 2006;86:1037–1051. doi: 10.1038/labinvest.3700462. [DOI] [PubMed] [Google Scholar]
- 29.Kidd M, Siddique Z, Drozdov I, Gustafsson B, Camp R, Black J, Boyce M, Modlin I. The CCK(2) receptor antagonist, YF476, inhibits Mastomys ECL-cell hyperplasia and gastric carcinoid tumor development. Regul Pept. 2010;162:52–60. doi: 10.1016/j.regpep.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 30.Eissele R, Koop H, Bothe-Sandfort E, Arnold R. Effect of gastrin receptor antagonists on gastric acid secretion and gastrin and somatostatin release in the rat stomach. Digestion. 1992;53:179–188. doi: 10.1159/000200993. [DOI] [PubMed] [Google Scholar]
- 31.Nishida A, Kobayashi-Uchida A, Akuzawa S, Takinami Y, Shishido T, Kamato T, Ito H, Yamano M, Yuki H, Nagakura Y, Honda K, Miyata K. Gastrin receptor antagonist YM022 prevents hypersecretion after long term acid suppression. Am J Physiol. 1995;269:G699–G705. doi: 10.1152/ajpgi.1995.269.5.G699. [DOI] [PubMed] [Google Scholar]
- 32.Korman M, Hansky J, Scott P. Serum gastrin in duodenal ulcer. iii. Influence of vagotomy and pylorectomy. Gut. 1972;13:39–42. doi: 10.1136/gut.13.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollinshead J, Debas H, Yamada T, Elashoff J, Osadchey B, Walsh J. Hypergastrinaemia develops within 24 hours of truncal vagotomy in dogs. Gastroenterology. 1985;88:35–40. doi: 10.1016/s0016-5085(85)80129-3. [DOI] [PubMed] [Google Scholar]
- 34.Burkitt M, Pritchard D. Pathogenesis and management of gastric carcinoid tumours. Aliment Pharmacol Ther. 2006;24:1305–1320. doi: 10.1111/j.1365-2036.2006.03130.x. [DOI] [PubMed] [Google Scholar]
- 35.Boyce M, Warrington S, Black J. Netazepide, a gastrin receptor antagonist, causes dose-dependent, persistent inhibition of the responses to pentagastrin in healthy subjects. Br J Clin Pharmacol. in press. [DOI] [PMC free article] [PubMed]
- 36.van Herwaarden M, Samson M, Smout A. 24-h recording of intragastric pH: technical aspects and clinical relevance. Scand J Gastroenterol. 1999;230:9–16. [PubMed] [Google Scholar]
- 37.Johnston D, Wormsley K. Problems with the interpretation of pH measurements. Clin Investig. 1993;72:12–17. doi: 10.1007/BF00231110. [DOI] [PubMed] [Google Scholar]


