Abstract
Aims
Both rituximab and plasmapheresis can be associated in the treatment of immune-mediated kidney diseases. The real impact of plasmapheresis on rituximab pharmacokinetics is unknown. The aim of this study was to compare rituximab pharmacokinetics between patients requiring plasmapheresis and others without plasmapheresis.
Methods
The study included 20 patients receiving one or several infusions of rituximab. In 10 patients, plasmapheresis sessions were also performed (between two and six sessions per patient). Rituximab concentrations were measured in blood samples in all patients and in discarded plasma obtained by plasmapheresis using an enzyme-linked immunosorbent assay method. Data were analysed according to a population pharmacokinetic approach.
Results
The mean percentage of rituximab removed during the first plasmapheresis session ranged between 47 and 54% when plasmapheresis was performed between 24 and 72 h after rituximab infusion. Rituximab pharmacokinetics was adequately described by a two-compartment model with first-order elimination. Plasmapheresis had a significant impact on rituximab pharmacokinetics, with an increase of rituximab clearance by a factor of 261 (95% confidence interval 146–376), i.e. from 6.64 to 1733 ml h−1. Plasmapheresis performed 24 h after rituximab infusion decreased the rituximab area under the curve by 26%.
Conclusions
Plasmapheresis removed an important amount of rituximab when performed less than 3 days after infusion. The removal of rituximab led to a significant decrease of the area under the curve. This pharmacokinetic observation should be taken into account for rituximab dosing, e.g. an additional third rituximab infusion may be recommended when three plasmapheresis sessions are performed after the first rituximab infusion.
Keywords: pharmacokinetics, plasmapheresis, rituximab
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Some diseases treated by rituximab may require plasmapheresis.
Rituximab is a monoclonal antibody with a small volume of distribution and a long elimination half-life; thus, it is likely to be removed by plasmapheresis.
WHAT THIS STUDY ADDS
The proportion of the rituximab dose removed by plasmapheresis was quantified, as was the impact of the delay between drug administration and plasmapheresis.
By modelling the rituximab pharmacokinetics, dose adjustment was proposed in order to maintain overall plasma rituximab exposure in patients with plasmapheresis.
Introduction
Rituximab is a chimaeric monoclonal antibody approved for the treatment of non-Hodgkin's lymphoma, as monotherapy or in addition to chemotherapy [1], and rheumatoid arthritis [2]. It is also used for the treatment of chronic lymphocytic leukaemia [3], low-grade or follicular lymphoma and diffuse large B-cell lymphoma [4]. Given that rituximab leads to a rapid depletion of CD20-positive B cells in the peripheral blood, it has become an alternative therapeutic agent for several autoimmune diseases, such as thrombotic thrombocytopenic purpura (TTP) [5], cryoglobulinaemia or idiopathic membranous nephropathy [5, 6]. It has also gained interest in renal transplantation, as an induction therapy in anti-HLA-sensitized patients [7] or in ABO-incompatible kidney recipients [8], as well as for treatment of antibody-mediated acute renal allograft rejection [9].
In some conditions, such as cryoglobulinaemia, TTP or immunization in transplanted patients, plasmapheresis is used to remove circulating antibodies. The procedure consists of removal of blood, separation of blood cells from the plasma, and return of these blood cells to the body's circulation, diluted with fresh plasma or replacement physiological fluids, such as albumin solution. As all solutes in the plasma, including drugs [10, 11], can be removed by plasmapheresis, it is important to determine the effect of this procedure on the pharmacokinetics of rituximab. Given that this drug displays a small volume of distribution and a long elimination half-life [12] and shares the chemical properties of natural antibodies removed by plasmapheresis, a proportion of the dose administered, yet unknown, would inevitably be lost during the procedure. However, the literature on drug removal during plasmapheresis is sparse, with most publications consisting of case reports of overdoses, describing the effects of plasmapheresis on pharmaceutical agents [13]. Importantly, the impact of plasmapheresis on rituximab pharmacokinetics has not yet been reported.
The objective of this study was to compare pharmacokinetic parameters in patients treated with rituximab and multiple sessions of plasmapheresis with those of patients treated with rituximab but without any plasmapheresis sessions.
Methods
Patients, blood sampling and rituximab enzyme-linked immunosorbent assay
Between August 2008 and April 2009, a total of 20 patients hospitalized in two nephrology units of the Centre Hospitalier Universitaire of Toulouse were included in the study. The inclusion criteria were patients with an antibody-mediated disease requiring rituximab treatment. All patients gave written informed consent, and the study protocol was approved by the regional ethic committee (Comité de Protection des Personnes Sud Ouest I).
Ten patients were treated with concomitant rituximab and plasmapheresis, whereas the other patients (n = 10) received rituximab without plasmapheresis based on the clinician's decision. Rituximab was administered according to one of the following schedules: 375 mg m−2 weekly (from one to four infusions) or 1000 mg fixed dose on days 1 and 15. The first dose was administered throughout a 360 min intravenous infusion, while the others were given during a 90 min intravenous infusion. Blood samples were collected predose, at the end of infusion, and 24, 48, 72 and 168 h after the start of the first infusion, then predose and at end of subsequent infusions, and 14, 30, 60 and 90 days after the last infusion. For patients with plasmapheresis, additional samples were collected immediately before the beginning of plasmapheresis and 1 and 24 h after the end of the procedure. For each plasmapheresis session, the volume of removed plasma was measured, and an aliquot was stored at −80°C until analysis.
Rituximab concentrations in both circulating and removed plasma were measured using a previously published enzyme-linked immunosorbent assay method [14]. Briefly, the calibration range was 0.125–50 μg ml−1. The lower limit of quantification was 0.125 μg ml−1. The interday accuracy (% nominal) was between 14 and 16%, and the precision (coefficient of variation for replicate analysis) ranged from 5 to 13%.
Pharmacokinetic analyses
Removal of rituximab during plasmapheresis sessions
The amount of rituximab removed by plasmapheresis was determined from the volume of plasma discarded and the corresponding rituximab concentration. The percentage of rituximab extracted by plasmapheresis was determined relative to the dose administered.
Population pharmacokinetic model
A population pharmacokinetic approach using nonlinear mixed-effect modelling was used for data analysis. Rituximab plasma concentrations were analysed using the NONMEM program [15] (version VI, level 1.1; Icon Development Solutions, Ellicot City, MD, USA) with NM-TRAN and PPRED and the Compaq Visual Fortran compiler (version 5) using the first-order conditional estimation (FOCE) method with INTERACTION. A proportional model for interindividual variability and a combination model (i.e. proportional and additive) for residual variability were used. First, the best structural pharmacokinetic model was determined using the likelihood ratio test and based only on the data corresponding to patients without plasmapheresis. Then, analysis of the whole data set (i.e. data from patients without and with plasmapheresis, including the amount of rituximab recovered in removed plasma) was performed. Changes of rituximab clearance during the plasmapheresis procedure was modelled as follows: TVCL = θCL × θPPPP, where TVCL is the typical value of rituximab clearance, θCL the mean value of clearance and θPP the mean factor corresponding to the impact of plasmapheresis on rituximab clearance, with PP = 0 for no plasmapheresis and PP = 1 during plasmapheresis sessions. The final pharmacokinetic model was evaluated using bootstrap and visual predictive check methods. The 50th percentile concentration (as an estimate of the population-predicted concentration) and the 5th and 95th percentile concentrations were processed using R (RfN, version 2007a; The R foundation for Statistical Computing, Vienna, Austria) and then plotted with Stata v10 (StataCorp LP, College Station, TX, USA). Observed plasma rituximab concentrations were compared graphically with these predicted concentrations.
Influence of plasmapheresis on rituximab exposure
Individual pharmacokinetic parameters of each of the 20 analysed patients enabled the estimation of exposures based on the reference schedule of two weekly 750 mg infusions of rituximab. The impact of plasmapheresis on rituximab exposure was estimated by comparing the total area under the curve (AUC) if plasmapheresis was applied or not. Simulations were then performed to derive rituximab concentrations under various dosing regimens, with or without plasmapheresis. A first set of simulations were performed for two consecutive weekly 750 mg infusions, with a variable number of plasmapheresis sessions or a variable time between rituximab infusion and the first plasmapheresis session. The schedules simulated were either a unique plasmapheresis session at 24, 48 or 72 h after the first rituximab infusion or three consecutive sessions performed at 24, 48 and 72 h after the first rituximab infusion. Given that the number of rituximab infusions could vary in clinical practice, a second set of rituximab AUCs were also simulated after three and four weekly 750 mg infusions of rituximab, with or without plasmapheresis after the first infusion. In each case, the influence of plasmapheresis on rituximab exposure was assessed by comparing AUCs obtained with and without plasmapheresis.
Results
Patients
The characteristics of the 20 patients included are described in Table 1. Fifteen patients presented antibody-mediated acute renal allograft rejection, three had membranous glomerulonephritis, one cryoglobulinaemic vasculitis and one systemic lupus. Most of the patients received weekly infusions of rituximab at the dose of 375 mg m−2; 10 patients had two infusions, one had three infusions and eight had four infusions. One patient received two 1000 mg infusions on days 1 and 15. The weekly rituximab dose ranged between 600 and 1000 mg. A total of 33 plasmapheresis sessions were performed, most of them following the first administration of rituximab. One patient also underwent plasmapheresis following each of the first two infusions of rituximab, and another patient had plasmapheresis before each infusion of rituximab. These sessions occurred between 25 and 160 h (median 45 h) after rituximab administration. The mean volume of plasma removed was 3900 ml (ranging from 2600 to 5600 ml). A total of 244 rituximab plasma concentration values were available, ranging from eight to 58 per patient, corresponding to a median number of 12 or 29 in patients without or with plasmaspheresis, respectively. Thirty-three aliquots of plasma removed by plasmapheresis were also available.
Table 1.
Patient characteristics at baseline (n = 20)
| Patient characteristics | Patients without plasmapheresis | Patients with plasmapheresis | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years) | 51.5 | 13.2 | 46.0 | 13.2 |
| Weight (kg) | 69.4 | 9.9 | 72.6 | 24.3 |
| Body surface area (m2) | 1.83 | 0.13 | 1.83 | 0.27 |
| CD19-positive cells at baseline (mm−3) | 184 | 114 | 330 | 291 |
| Sex* | Number | Number | ||
|---|---|---|---|---|
| Male | 10 | 4 | ||
| Female | 0 | 6 |
| Diseases | ||||
|---|---|---|---|---|
| Antibody-mediated rejection of kidney graft | 6 | 9 | ||
| Membraneous glomerulonephritis | 3 | 0 | ||
| Cryoglobulinaemia | 0 | 1 | ||
| Lupus | 1 | 1 |
| Treatment | Median | Range | Median | Range |
|---|---|---|---|---|
| Number of rituximab infusions | 2 | 2–4 | 4 | 2–4 |
| Plasmapheresis sessions | – | – | 2 | 2–8 |
Significant difference (P < 0.05) between the two subgroups of patients.
Pharmacokinetics
Rituximab extraction during plasmapheresis
The median (range) percentage of rituximab dose recovered within the plasma removed by the first plasmapheresis was 49% (14–54%). The amount of rituximab removed was inversely correlated with the time interval between infusion of rituximab and the first plasmaphersesis (Figure 1). When considering only plasmapheresis that occurred between 25 and 66 h after infusion, the amount of rituximab removed was included in a narrow range (between 47 and 54%), but the correlation between the amount of rituximab removed and the time interval remained significant (r = 0.79). The amount of rituximab removed during each subsequent plasmapheresis session ranged from 9 to 29%. Whatever the plasmapheresis session, concentrations of rituximab in plasma removed were strongly correlated with the circulating plasma rituximab level at the time of plasmapheresis (r = 0.96).
Figure 1.
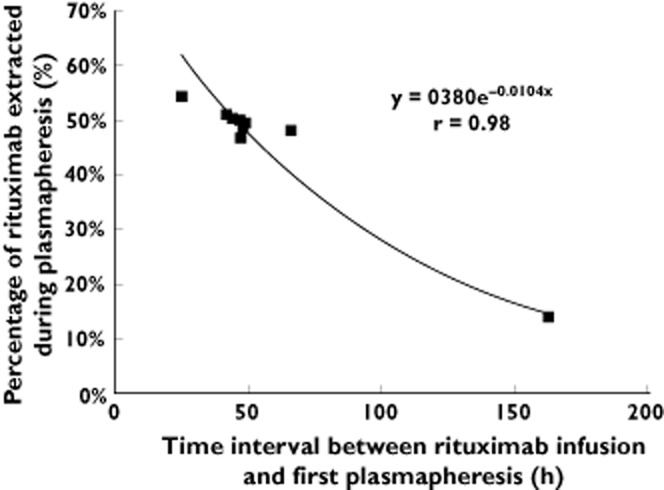
Percentage of rituximab extracted during the first plasmapheresis relative to the administered dose vs. time interval between rituximab infusion and plasmapheresis
Population pharmacokinetic model
Rituximab pharmacokinetics were adequately described by a two-compartment model with first-order elimination. Plasmapheresis had a significant impact on rituximab pharmacokinetics, with a 261-fold increase of rituximab clearance (95% confidence interval 146–376). Inclusion of interoccasion variability on the volume of the central compartment (V1) and clearance (CL; i.e. intrapatient change of V1 and CL between cycle 1 and cycle 2) significantly improved the fit. The quality of fit is illustrated by the visual predictive check plots shown in Figure 2. The mean pharmacokinetic parameters and factors corresponding to the impact of plasmapheresis are shown in Table 2. By considering the post hoc individual values, relationships between rituximab CL and sex (mean ± SD of 6.2 ± 1.7 vs. 7.4 ± 2.3 ml h−1 for females and males, respectively) and between CL and bodyweight [CL (ml h−1) = 0.063 × bodyweight (kg) + 2.6] corresponded to those expected [16], but were not statistically significant, probably owing to the limited number of patients.
Figure 2.

Visual predictive check plots for each group of patients (with and without plasmapheresis).  , observed values;
, observed values;  , median;
, median;  , 5th percentile;
, 5th percentile;  , 95th percentile
, 95th percentile
Table 2.
Rituximab pharmacokinetic parameters
| Model | Value (95% confidence interval) | Coefficient of variation (%) |
|---|---|---|
| Clearance (CL; ml h−1) = θ1 × θ2PP | ||
| Clearance (CL; ml h−1) | θ1 = 6.64 (3.29–9.99) | 35.6 |
| Plasmapheresis factor (PP) | θ2 = 261 (146–376) | – |
| Central volume (V; l) | θ3 = 2.48 (1.78–3.18) | 20.5 |
| Transfer constant K12 (h−1) | θ4 = 0.0158 (0.0078–0.0238) | – |
| Transfer constant K21 (h−1) | θ5 = 0.0154 (0.0116–0.0192) | 40.1 |
| Interoccasion variability on CL | – | 14.7 |
| Interoccasion variability on V | – | 14.8 |
Influence of plasmapheresis on rituximab exposure
Based on individual predicted concentration, the median (range) total AUC obtained after two weekly infusions of rituximab was 207 421 (132 737–482 375) mg l−1 h. When plasmapheresis was performed 24 h after the infusion of rituximab, AUC decreased by 26% (95% prediction interval 24–28%), while the decrease of AUC was 20% (95% prediction interval 19–21%) and 16% (16–18%) when plasmapheresis occurred at 48 and 72 h after the first infusion of rituximab, respectively. Subsequent plasmapheresis had a cumulative effect on AUC, with exposure decreasing by 36% [35–37%] when two additional plasmapheresis sessions were implemented at 48 and 72 h.
The simulations performed for various dosing regimens and plasmapheresis sessions showed that rituximab exposure obtained following three weekly infusions with three plasmapheresis sessions after the first infusion was slightly higher than that after two weekly infusions without plasmapheresis, by +13% (12–14%). Four weekly infusions with three plasmapheresis sessions after the first infusion were associated with a 62% (61.5–62.5%) increase of rituximab exposure in comparison to two weekly infusions without plasmapheresis.
Depletion of B cells (<10 CD19-positive cells mm−3) occurred in all patients between 7 and 30 days after the first rituximab infusion. After 3 months, only one patient (in the plasmapheresis group) had a reappearance of CD19-positive B cells (21 CD19-positive cells mm−3). After 6 months, two patients (one in each group) had a reappearance of CD19-positive B cells. Later lymphocyte counts could not be interpreted because patients received concomitant immunosuppressive treatments.
Discussion
Several reports support a link between rituximab plasma concentrations and efficacy, both in haematological [17, 18] and nonmalignant diseases [19]. Thus, interindividual pharmacokinetic variability is an important determinant of the clinical response. Some data obtained in haematological malignancies suggested that the amount of CD20 antigen (assessed either by the level of membrane CD20 expression or by tumour burden estimation) plays a role in rituximab pharmacokinetic variability, but these results are still controversial [20]. Some authors found that body surface area explained about 32% of the interindividual variability of clearance [21]. A recent study using population pharmacokinetic modelling, performed in 20 elderly patients with diffuse large B-cell lymphoma showed that rituximab clearance was significantly reduced (8.2 vs. 12.7 ml h−1) and elimination half-life significantly prolonged in women compared with men (t1/2β = 30.7 vs. 24.7 h) [16]. Overall, the pharmacokinetics of rituximab is related to a number of factors, including the dose administered, the frequency of administration, the inherent stability of the antibody, the specific and nonspecific clearance of the antibody [22], gender and bodyweight [16].
Plasmapheresis is used to remove harmful antibodies in various disorders. However, all antibodies are small enough to pass through the pores of the membrane, and plasmapheresis obviously has the potential to clear rituximab, too. The objective of our study was to evaluate the impact of plasmapheresis on rituximab pharmacokinetics, because only sparse data are available on the subject. Darabi and Berg [23] reported on two patients with autoimmune TTP who were given rituximab 24–36 h before plasma exchange, during which 1–1.5 times their plasma volume was removed. Based on their observations, the authors concluded that the regularly scheduled plasma exchanges did not interfere with the immunosuppressive effects of rituximab, owing to the rapid effects of rituximab on circulating CD19-positive/CD20-positive lymphocytes. McDonald et al. [24] showed that trough serum rituximab levels were lower in 30 patients treated for acute TTP by rituximab and plasmapheresis than in three patients treated by rituximab alone. These observations are consistent with the hypothesis of a removal of rituximab during plasmapheresis, but no study precisely described the pharmacokinetics of rituximab in this context. Thus, it is important to quantify the impact of plasmapheresis on rituximab pharmacokinetics and its plasma concentrations. In particular, guidelines based on rituximab pharmacokinetics are needed for a better adaptation of rituximab dosing or schedule of plasmapheresis.
In the present study, we assessed the pharmacokinetic behaviour of rituximab after heterogeneous schedules of administration of rituximab in 20 patients. We have demonstrated that this impact was dramatic, with a 261-fold increase of rituximab clearance during the plasmapheresis. Implementation of plasmapheresis following the first infusion of rituximab is accompanied by a large waste of rituximab, with almost half of the dose being removed if plasmapheresis is performed within the first 3 days following the infusion of rituximab.
Not surprisingly, the impact of plasmapheresis sessions was dependent on the time interval between rituximab administration and plasmapheresis sessions, the percentage extracted being inversely correlated with the time interval. However, there was only a slight variation in rituximab removal whether the plasmapheresis session occurred 1 or 3 days after the infusion, indicating that delay of the plasmapheresis session is a limited tool in order to diminish the decrease of rituximab concentration.
Simulations of rituximab plasma concentrations were used to assess how many supplemental doses of rituximab can compensate for the loss of rituximab during plasmapheresis. This was made possible by the modelling method we used (i.e. nonlinear mixed-effect method). The advantages of this method were as follows: (i) to analyse data presenting a certain level of heterogeneity in terms of rituximab dose and number of infusions; and (ii) to predict the individual rituximab plasma level according to individual pharmacokinetics parameters determined by the analysis of raw data. By this approach, the mean pharmacokinetics parameters observed were comparable to those described in other reports concerning autoimmune diseases [25, 26].
For a standard treatment of two weekly infusions of rituximab, we observed that one plasmapheresis session performed 1 day after the first infusion of rituximab would be associated with a 26% decrease in rituximab exposure. The decrease can be reduced to about 16% if the plasmapheresis session is delayed to 3 days after the infusion. The repetition of three plasmapheresis sessions after the first infusion of rituximab led to a 36% decrease in rituximab exposure. The AUC level obtained after three weekly infusions of rituximab while performing three plasmapheresis sessions after the first infusion would be close to the AUC level obtained after two weekly infusions of rituximab without plasmapheresis. This illustrates that even if plasmapheresis sessions are usually repeated after the first infusion of rituximab, the second infusion does not compensate for the loss of dose, contrary to a third supplemental dose. In contrast, a fourth infusion does not seem necessary.
Since Cravedi et al. [27] demonstrated that a rapid and lasting decrease of B cells can be obtained with one or two weekly infusions of rituximab in patients with idiopathic membranous nephropathy, the repetition of infusions is driven by the number of circulating B cells. A reduced number of infusions also seems effective in renal transplant patients treated by rituximab and receiving induction therapy or antirejection therapy [28].
Although a rapid decrease of circulating B cells can be obtained with a limited rituximab treatment, an early reappearance of B cells can be favoured by low concentrations of rituximab [29]. In our study, the correlation between duration of B-cell depletion and exposure of rituximab could not be assessed, because most patients received various associated immunosuppressive therapies that can influence the duration of B-cell depletion [28]. The correlation between rituximab concentration and B-cell depletion should be assessed by a prospective study.
Considering the substantial impact of plasmapheresis on rituximab exposure demonstrated in our study, we conclude that removal of rituximab during plasmapheresis should be taken into account to adapt the number of rituximab infusions. Reducing the number of infusions as described by Cravedi et al. is cost-effective [27]. Thus, it can be considered that two consecutive weekly infusions is a standard of care. However, the quantification of the influence of plasmapheresis on rituximab exposure allowed us to recommend an additional third infusion in order to compensate for the plasmapheresis elimination.
Acknowledgments
This study was supported by grants from Hoffman Laroche and from Association pour la Recherche en Transfusion (ART) and Association Midi-Pyrénées Santé.
The authors acknowledge Céline Roumiguié (Pharmacie, Centre Hospitalier Universitaire Toulouse, France), Marie Elise Llau (Direction de la Recherche et de l'Innovation du CHU de Toulouse, France) and all nurses (Département de néphrologie et transplantation d'organe CHU Rangueil, Toulouse, France) for their participation.
Competing Interests
All authors have completed the Unified Competing Interest form and declare: F. Puisset had support from Hofmann Laroche and Association Midi Pyrénées Santé, J. Pourrat had support from Association pour la Recherche en Transfusion for the submitted work; no financial relationships with any organization that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, Heyman MR, Bence-Bruckler I, White CA, Cabanillas F, Jain V, Ho AD, Lister J, Wey K, Shen D, Dallaire BK. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 2.Edwards JC, Leandro MJ, Cambridge G. B lymphocyte depletion therapy with rituximab in rheumatoid arthritis. Rheum Dis Clin North Am. 2004;30:393–403. doi: 10.1016/j.rdc.2004.01.006. viii. [DOI] [PubMed] [Google Scholar]
- 3.Tedeschi A, Vismara E, Ricci F, Morra E, Montillo M. The spectrum of use of rituximab in chronic lymphocytic leukemia. Onco Targets Ther. 2010;3:227–246. doi: 10.2147/OTT.S8151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keating GM. Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma. Drugs. 2010;70:1445–1476. doi: 10.2165/11201110-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 5.George JN, Woodson RD, Kiss JE, Kojouri K, Vesely SK. Rituximab therapy for thrombotic thrombocytopenic purpura: a proposed study of the Transfusion Medicine/Hemostasis Clinical Trials Network with a systematic review of rituximab therapy for immune-mediated disorders. J Clin Apher. 2006;21:49–56. doi: 10.1002/jca.20091. [DOI] [PubMed] [Google Scholar]
- 6.Kazkaz H, Isenberg D. Anti B cell therapy (rituximab) in the treatment of autoimmune diseases. Curr Opin Pharmacol. 2004;4:398–402. doi: 10.1016/j.coph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, Warren DS, Simpkins CE, Dagher NN, Singer AL, Zachary AA, Segev DL. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365:318–326. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 8.Genberg H, Kumlien G, Wennberg L, Berg U, Tyden G. ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: a 3-year follow-up. Transplantation. 2008;85:1745–1754. doi: 10.1097/TP.0b013e3181726849. [DOI] [PubMed] [Google Scholar]
- 9.Becker YT, Becker BN, Pirsch JD, Sollinger HW. Rituximab as treatment for refractory kidney transplant rejection. Am J Transplant. 2004;4:996–1001. doi: 10.1111/j.1600-6143.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- 10.Fauvelle F, Petitjean O, Tod M, Guillevin L. Clinical pharmacokinetics during plasma exchange. Therapie. 2000;55:269–275. [PubMed] [Google Scholar]
- 11.Okechukwu CN, Meier-Kriesche HU, Armstrong D, Campbell D, Gerbeau C, Kaplan B. Removal of basiliximab by plasmapheresis. Am J Kidney Dis. 2001;37:E11. [PubMed] [Google Scholar]
- 12.Onrust SV, Lamb HM, Balfour JA. Rituximab. Drugs. 1999;58:79–88. doi: 10.2165/00003495-199958010-00009. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim RB, Liu C, Cronin SM, Murphy BC, Cha R, Swerdlow P, Edwards DJ. Drug removal by plasmapheresis: an evidence-based review. Pharmacotherapy. 2007;27:1529–1549. doi: 10.1592/phco.27.11.1529. [DOI] [PubMed] [Google Scholar]
- 14.Blasco H, Lalmanach G, Godat E, Maurel MC, Canepa S, Belghazi M, Paintaud G, Degenne D, Chatelut E, Cartron G, Le GC. Evaluation of a peptide ELISA for the detection of rituximab in serum. J Immunol Methods. 2007;325:127–139. doi: 10.1016/j.jim.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 15.Beal SL, Sheiner LB. Estimating population kinetics. Crit Rev Biomed Eng. 1982;8:195–222. [PubMed] [Google Scholar]
- 16.Muller C, Murawski N, Wiesen MH, Held G, Poeschel V, Zeynalova S, Wenger M, Nickenig C, Peter N, Lengfelder E, Metzner B, Rixecker T, Zwick C, Pfreundschuh M, Reiser M. The role of sex and weight on rituximab clearance and serum elimination half-life in elderly patients with DLBCL. Blood. 2012;119:3276–3284. doi: 10.1182/blood-2011-09-380949. [DOI] [PubMed] [Google Scholar]
- 17.Berinstein NL, Grillo-Lopez AJ, White CA, Bence-Bruckler I, Maloney D, Czuczman M, Green D, Rosenberg J, McLaughlin P, Shen D. Association of serum Rituximab (IDEC-C2B8) concentration and anti-tumor response in the treatment of recurrent low-grade or follicular non-Hodgkin's lymphoma. Ann Oncol. 1998;9:995–1001. doi: 10.1023/A:1008416911099. [DOI] [PubMed] [Google Scholar]
- 18.Tobinai K, Igarashi T, Itoh K, Kobayashi Y, Taniwaki M, Ogura M, Kinoshita T, Hotta T, Aikawa K, Tsushita K, Hiraoka A, Matsuno Y, Nakamura S, Mori S, Ohashi Y. Japanese multicenter phase II and pharmacokinetic study of rituximab in relapsed or refractory patients with aggressive B-cell lymphoma. Ann Oncol. 2004;15:821–830. doi: 10.1093/annonc/mdh176. [DOI] [PubMed] [Google Scholar]
- 19.Looney RJ, Anolik JH, Campbell D, Felgar RE, Young F, Arend LJ, Sloand JA, Rosenblatt J, Sanz I. B cell depletion as a novel treatment for systemic lupus erythematosus: a phase I/II dose-escalation trial of rituximab. Arthritis Rheum. 2004;50:2580–2589. doi: 10.1002/art.20430. [DOI] [PubMed] [Google Scholar]
- 20.Cartron G, Blasco H, Paintaud G, Watier H, Le GC. Pharmacokinetics of rituximab and its clinical use: thought for the best use? Crit Rev Oncol Hematol. 2007;62:43–52. doi: 10.1016/j.critrevonc.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 21.Ng CM, Bruno R, Combs D, Davies B. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol. 2005;45:792–801. doi: 10.1177/0091270005277075. [DOI] [PubMed] [Google Scholar]
- 22.Mangel J, Buckstein R, Imrie K, Spaner D, Franssen E, Pavlin P, Boudreau A, Pennell N, Combs D, Berinstein NL. Pharmacokinetic study of patients with follicular or mantle cell lymphoma treated with rituximab as ‘in vivo purge’ and consolidative immunotherapy following autologous stem cell transplantation. Ann Oncol. 2003;14:758–765. doi: 10.1093/annonc/mdg201. [DOI] [PubMed] [Google Scholar]
- 23.Darabi K, Berg AH. Rituximab can be combined with daily plasma exchange to achieve effective B-cell depletion and clinical improvement in acute autoimmune TTP. Am J Clin Pathol. 2006;125:592–597. doi: 10.1309/RLNM-J01W-BJRN-LH03. [DOI] [PubMed] [Google Scholar]
- 24.McDonald V, Manns K, Mackie IJ, Machin SJ, Scully MA. Rituximab pharmacokinetics during the management of acute idiopathic thrombotic thrombocytopenic purpura. J Thromb Haemost. 2010;8:1201–1208. doi: 10.1111/j.1538-7836.2010.03818.x. [DOI] [PubMed] [Google Scholar]
- 25.Regazzi MB, Iacona I, Avanzini MA, Arcaini L, Merlini G, Perfetti V, Zaja F, Montagna M, Morra E, Lazzarino M. Pharmacokinetic behavior of rituximab: a study of different schedules of administration for heterogeneous clinical settings. Ther Drug Monit. 2005;27:785–792. doi: 10.1097/01.ftd.0000184162.60197.c1. [DOI] [PubMed] [Google Scholar]
- 26.Iacona I, Lazzarino M, Avanzini MA, Rupolo M, Arcaini L, Astori C, Lunghi F, Orlandi E, Morra E, Zagonel V, Regazzi MB. Rituximab (IDEC-C2B8): validation of a sensitive enzyme-linked immunoassay applied to a clinical pharmacokinetic study. Ther Drug Monit. 2000;22:295–301. doi: 10.1097/00007691-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Cravedi P, Ruggenenti P, Sghirlanzoni MC, Remuzzi G. Titrating rituximab to circulating B cells to optimize lymphocytolytic therapy in idiopathic membranous nephropathy. Clin J Am Soc Nephrol. 2007;2:932–937. doi: 10.2215/CJN.01180307. [DOI] [PubMed] [Google Scholar]
- 28.Genberg H, Hansson A, Wernerson A, Wennberg L, Tyden G. Pharmacodynamics of rituximab in kidney allotransplantation. Am J Transplant. 2006;6:2418–2428. doi: 10.1111/j.1600-6143.2006.01497.x. [DOI] [PubMed] [Google Scholar]
- 29.Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG, Hogan MC, Dillon JJ, Hickson LJ, Li X, Cattran DC. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol. 2010;5:2188–2198. doi: 10.2215/CJN.05080610. [DOI] [PMC free article] [PubMed] [Google Scholar]


