Abstract
Direct predation upon parasites has the potential to reduce infection in host populations. For example, the fungal parasite of amphibians, Batrachochytrium dendrobatidis (Bd), is commonly transmitted through a free-swimming zoospore stage that may be vulnerable to predation. Potential predators of Bd include freshwater zooplankton that graze on organisms in the water column. We tested the ability of two species of freshwater crustacean (Daphnia magna and D. dentifera) to consume Bd and to reduce Bd density in water and infection in tadpoles. In a series of laboratory experiments, we allowed Daphnia to graze in water containing Bd while manipulating Daphnia densities, Daphnia species identity, grazing periods and concentrations of suspended algae (Ankistrodesmus falcatus). We then exposed tadpoles to the grazed water. We found that high densities of D. magna reduced the amount of Bd detected in water, leading to a reduction in the proportion of tadpoles that became infected. Daphnia dentifera, a smaller species of Daphnia, also reduced Bd in water samples, but did not have an effect on tadpole infection. We also found that algae affected Bd in complex ways. When Daphnia were absent, less Bd was detected in water and tadpole samples when concentrations of algae were higher, indicating a direct negative effect of algae on Bd. When Daphnia were present, however, the amount of Bd detected in water samples showed the opposite trend, with less Bd when densities of algae were lower. Our results indicate that Daphnia can reduce Bd levels in water and infection in tadpoles, but these effects vary with species, algal concentration, and Daphnia density. Therefore, the ability of predators to consume parasites and reduce infection is likely to vary depending on ecological context.
Keywords: Batrachochytrium dendrobatidis, chytridiomycosis, eutrophication, Lithobates sphenocephalus, parasites, trophic interactions
Introduction
Host–parasite interactions, while often studied in isolation, are affected by a multitude of direct and indirect effects from other members of the community (Omacini et al. 2001; Lafferty 2004; Lafferty et al. 2006). Predators are one notable community member that can strongly impact infectious diseases. In some cases, predators can increase infection in their prey (e.g., through impacts on host immune function or host traits; Ramirez and Snyder 2009; Duffy et al. 2011). In other cases, predators can decrease disease risk in their prey (e.g., by decreasing prey population sizes or directly consuming infected hosts; Packer et al. 2003; Keesing et al. 2006; Duffy et al. 2005). Predators can also impact disease risk in non-prey species via consumption of disease vectors or free-living stages of parasites (Grutter 1996; Nelson and Jackson 2006; Orlofske et al. 2012). Therefore, predators have the potential to alter rates of infection in both prey and nonprey species through a variety of routes.
The potential role of predation in reducing infectious disease risk is of particular interest when applied to medicine and conservation. Indeed, manipulation of predator densities has been suggested as a potential conservation measure (Packer et al. 2003). In some cases, the focus is on the potential for predators to reduce density of vectors. For example, augmentation of populations of mosquito predators has been suggested as a way to control mosquito-borne parasites such as malaria (Nelson and Jackson 2006; Howard et al. 2007). In other cases, the focus has been on the potential for predators to directly prey upon the parasites. It is this latter scenario that is the focus of the experiments reported here. We studied the potential for predation upon the fungus Batrachochytrium dendrobatidis (Bd), which has caused population declines and extirpations of amphibians around the globe (Bosch et al. 2001; Lips et al. 2006; Skerratt et al. 2007; Wake and Vredenburg 2008). Predation by zooplankton on free-swimming Bd zoospores has been suggested as a possible method for biocontrol of this fungus (Buck et al. 2011). Our study further evaluates this possibility.
There are reasons to expect that free-swimming Bd might be vulnerable to predation. First, Bd is generally transmitted through an aquatic zoospore stage that swims through water to infect new hosts (Longcore et al. 1999). The length of time that zoospores can remain infectious is context-dependent; Piotrowski et al. (2004) found that 95% of zoospores stop moving after just 24 h in distilled water, while Johnson and Speare (2003) reported motile zoospores in lake water after 7 weeks. Given the potential for a long free-swimming stage, Bd zoospores may be at risk of direct predation during this infectious period. Second, many bodies of water contain numerous microcrustaceans that have the potential to consume Bd zoospores. For example, Daphnia are generalist grazers of algae, bacteria, cyanobacteria, protozoans, fungi, and detritus. One species of Daphnia (D. galeata hyalina) has been shown to consume zoospores of a pathogenic chytrid of diatoms, reducing infection in the hosts (Kagami et al. 2004). Bd zoospores are generally 3−5 μm in diameter (Longcore et al. 1999), which is within the preferred range of food size for many Daphnia (Burns 1968; Geller and Muller 1981). Therefore, Daphnia are good candidates for predators of Bd.
Three previous studies have directly investigated the potential for Daphnia to impact Bd. In a laboratory experiment, Buck et al. (2011) demonstrated that Daphnia can consume Bd zoospores. However, the ability of Daphnia to digest those zoospores was not tested, and previous studies have shown that passage through a Daphnia gut can actually increase growth of some organisms (Porter 1976). Therefore, it is possible that Bd zoospores can be ingested by Daphnia but not digested. Two additional laboratory studies demonstrated that Daphnia reduce the number of zoospores detected in water samples (Woodhams et al. 2011; Hamilton et al. 2012), but a mesocosm experiment did not find changes in infection rates in tadpoles (Hamilton et al. 2012). Each of these studies only investigated one species of Daphnia, but Daphnia species vary in body size, which can influence feeding preferences and rates (Burns 1968; Hall et al. 2007). Additionally, the laboratory studies testing for Daphnia predation upon Bd (Woodhams et al. 2011; Hamilton et al. 2012) combined Daphnia and Bd zoospores in clean water without the presence of other food sources for Daphnia. Other studies have demonstrated that gut passage time and food assimilation efficiency in Daphnia change with food concentration (DeMott et al. 2010). Therefore, the presence of alternative food resources (as is the case in natural communities containing Bd and Daphnia) may alter the consumption and digestion rates of Daphnia on Bd zoospores.
In this study, we tested the ability of Daphnia to consume Bd zoospores and reduce both environmental levels of Bd and infection in tadpoles. In a series of laboratory experiments, we varied density of two species of Daphnia (D. magna and D. dentifera) to compare the effectiveness of each species at consuming Bd. Additionally, as algal levels can vary greatly between water bodies, we manipulated the density of suspended algae (food for Daphnia) to determine its effects on Bd consumption by D. dentifera. Our goal was to understand the impact of zooplankton predation on Bd levels in the environment and hosts.
Methods
Study organisms
To ensure that amphibians used in the experiment were not previously infected with Bd, we collected southern leopard frogs (Lithobates sphenocephalus; formerly Rana sphenocephala) as eggs from Fall Line Sandhills Wildlife Management Area near Butler, Georgia, USA. We collected partial clutches of 11 egg masses and immediately brought them to the laboratory to be reared in 37.8 L aquaria filled with aged tap water treated with tap water conditioner (API). The laboratory was maintained at ∼20°C with a 12:12 light:dark photoperiod.
We used D. magna isolated from Kaimes Pond in Scotland, UK, and D. dentifera from Midland Lake in Indiana, USA. We chose these species because they vary in body size; D. magna is a large Eurasian species and can grow up to ∼5 mm in length (Bottrell et al. 1976); D. dentifera is a smaller North American species that grows to ∼2 mm (Hall et al. 2007; Fig. 1). We used one isofemale line for each species to reduce variation among individual Daphnia. Daphnia were 11−14 days old at the beginning of each experiment. We used Bd Strain SR-810, which was originally isolated from a Lithobates catesbeianus tadpole from South Carolina (Schloegel et al. 2009). For use in the experiment, we cultured Bd onto 1% tryptone agar petri dishes and allowed them to grow for 4−7 days.
Figure 1.
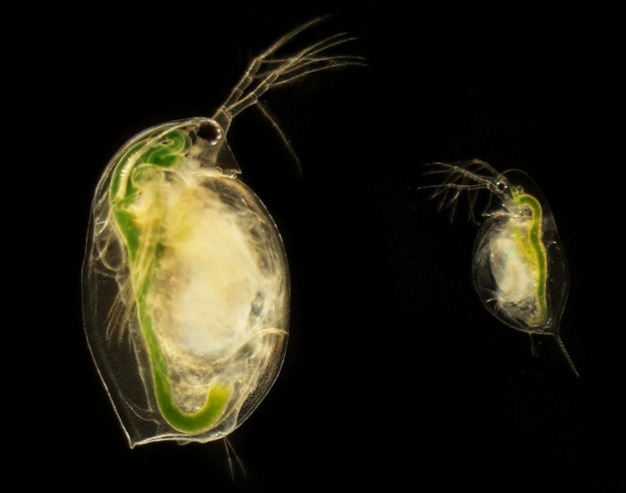
Photograph of Daphnia species used in this experiment. The individual on the left is a D. magna adult female, and the individual on the right is a D. dentifera adult female.
This study was conducted in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The Institutional Animal Care and Use Committee (IACUC) at Georgia Institute of Technology was informed of this study, but did not require animal care protocols for the early stage tadpoles used in these experiments.
Experimental design
We performed three experiments manipulating D. magna density, D. dentifera density, grazing period, and algal density. Each experiment had a similar protocol. For all experiments, we placed Daphnia into 400 mL glass beakers filled with 250 mL of media (50% filtered lake water and 50% artificial Daphnia medium; Kluttgen et al. 1994). We then flooded Bd-inoculated petri dishes with 15 mL media for 30 min. This method allows for zoospores to release into the water without dislodging sporangia that are attached to the agar (Boyle et al. 2004; Searle et al. 2011). We pooled inoculum from these dishes and quantified zoospore densities using a hemocytometer. We then added ∼2.0 × 104 Bd zoospores to each beaker to create a concentration of 80 zoospores per mL in the beakers. Beakers were only inoculated with Bd once, at the beginning of the experiment. We fed the green alga Ankistrodesmus falcatus to Daphnia immediately after addition of Bd zoospores at densities described below. Additionally, we established three beakers that were treated in the same manner as the others (with 0, 1, and 5 Daphnia per beaker) except Bd inoculum was not added to the water. The samples from unexposed beakers served as negative experimental controls for infection analysis (described below). Daphnia grazed for 5, 24, or 72 h (see below). We chose these times because previous studies have demonstrated that Daphnia can reduce Bd densities in water after 5 or 72 h (5 h: Hamilton et al. 2012; 72 h: Woodhams et al. 2011). We fed A. falcatus to Daphnia in the 5- or 24- h grazing period once immediately after addition of Bd zoospores, and we fed A. falcatus to Daphnia in the 72-h treatments three times; immediately after addition of Bd zoospores, then every 24 h. Under the conditions in this experiment, individual D. dentifera can filter over 10 mL of water per day (Hall et al. 2010), while D. magna can filter even greater amounts of water due to their larger body size. Thus, in the treatments with 25 Daphnia per beaker, it is highly likely that the entire contents of a beaker would have filtered at least once during a 24-h period.
After the grazing period, we removed all Daphnia (including any offspring born during the experiment) from the beakers using a glass Pasteur pipette. For the 72-h grazing period, we also removed offspring every 24 h to reduce variation among replicates. After removal of all Daphnia, we stirred each beaker vigorously using a glass stir rod and removed 1.5 mL water from 3.5 cm below the water's surface and placed it into a microcentrifuge tube. After taking water samples, we added one L. sphenocephalus tadpole to each beaker where they remained for 24 h. We then euthanized each tadpole in an individual container containing an overdose of buffered MS-222. The MS-222 solution also acted as a wash to remove any zoospores that may have been in the tadpole's water or on the surface of the tadpole, but not infecting them. By euthanizing tadpoles immediately after the 24-h exposure, we were able to focus on the effects of Daphnia on Bd transmission (rather than the progression of infection); additional rounds of infection were unlikely to develop in this time period, because the life cycle of Bd takes 5 days under optimal conditions (Johnson and Speare 2003). We preserved tadpoles individually in 95% ethanol. Tadpole mass among experiments is compared in Figure S2 and Data S1.
Experiments 1 and 2 manipulated the density of D. magna and D. dentifera with two grazing periods, 5 h or 72 h (3 days). For experiment 1, we used six density treatments with 0, 1, 5, 10, or 25 D. magna per beaker and one treatment with 25 D. dentifera per beaker. This is equivalent to 0, 4, 20, 40, and 100 D. magna per L and 100 D. dentifera per L. Each treatment was replicated 10 times. Experiment 2 had the same design as experiment 1, but focusing only on D. dentifera at densities of 0, 1, 5, 10, and 25 D. dentifera per beaker (0, 4, 20, 40, and 100 D. dentifera per L). For experiments 1 and 2, we added 2.5 × 106 cells of A. falcatus to each beaker to create a concentration of 1 × 104 cells/mL.
For experiment 3, we manipulated the density of Daphnia and the density of suspended algae. We used two densities of D. dentifera (0 or 25 individuals per beaker; 0 or 100 D. dentifera per L) and two densities of A. falcatus (1 × 104 cells/mL [“high food”] and 80 cells/mL [“low food”]). The density of A. falcatus in the high-food treatment was the same as in experiments 1 and 2, while the low-food treatment had a density of algal cells equal to the concentration of Bd zoospores in the water. We allowed Daphnia to graze for 24 h in experiment 3, as an intermediate time period of the grazing times from experiments 1 and 2.
Zoospore quantification
To quantify Bd concentrations in water and tadpoles, we performed quantitative PCR (qPCR) on water samples and tadpole mouthparts. Extractions on water samples followed Hamilton et al. (2012) with modifications. Briefly, we centrifuged water samples for 10 min at 16k and removed all but 50 μL supernatant. We then added 150 μL PrepMan Ultra (Applied Biosystems, Foster City, CA) and 40 mg silica/zirconium beads (Biospec Products, Bartlesvill, OK) and homogenized tubes for 45 s on a Vortex-Genie 2 vortex (MO BIO Laboratories Inc., Carlsbad, CA) then centrifuged at 13k for 30 s. We repeated homogenizing and centrifugation then heated samples to 100°C for 10 min. After cooling for 5 min, we centrifuged samples for 3 min at 13k, collected supernatant, and diluted it to a 10% solution with nuclease-free water. We extracted tadpole samples according to Boyle et al. (2004) except using 60 μL Prepman Ultra instead of 40 μL. We performed qPCR according to Boyle et al. (2004) on a Mastercycler ep realplex (Eppendorf) and analyzed each sample in triplicate. We included a no-template control (nanopure water instead of amphibian sample) in each qPCR plate and never observed amplification in these controls. If a sample tested positive for Bd in only one replicate, we reanalyzed the sample. We considered a sample positive for Bd if we detected Bd in 2 of 3 replicates (run once) or 3 of 6 replicates (run twice). We performed qPCR on all water and tadpole samples from each experiment including the three samples from each experiment that were not exposed to Bd. All of the unexposed samples were negative for the presence of Bd.
Statistical analyses
We performed all statistical analyses in R version 2.15.1 (R Core Development Team 2012). Our infection data were in the form of genome equivalents per sample and contained a large number of zeros. We therefore fit zero-inflated negative binomial models to our infection data using the “pscl” package. This allowed us to model both the presence of Bd in a sample and the amount of Bd detected (Zuur et al. 2009). For each experiment, we fit a separate model for water and tadpole samples. For experiments 1 and 2, our initial models included D. magna or D. dentifera density (respectively), grazing period (5 vs. 72 h), and the interaction between these two predictors. For experiment 3, our initial models included food level, Daphnia density, and the interaction term. We then dropped predictors from the models based on likelihood ratio tests (package “lmtest” Zuur et al. 2009).
To compare the effects of D. magna versus D. dentifera density in experiment 1, we performed generalized linear models (GLMs) on the proportion of samples positive for Bd (binomial GLM with a logit link) in three treatments: 0 Daphnia, 25 D. magna, and 25 D. dentifera. For significant effects, we then performed GLMs comparing two treatments at a time and corrected for multiple comparisons with a Bonferroni correction. For the samples that were positive for infection, we also performed an ANOVA on the log amount of Bd detected for water and tadpole samples in each experiment using the same predictors. When ANOVA revealed a significant effect, we followed with a Tukey's HSD test to directly compare treatments.
Results
Experiment 1
Daphnia magna density affected both the amount of Bd detected in the water (X2 = 7.15, P = 0.008; Table 1, Fig. 2C) and the proportion of tadpole samples positive for Bd (X2 = 6.53, P = 0.011; Table 1, Fig. 2B). Grazing period affected the proportion of water and tadpole samples positive for Bd and the amount of Bd detected in water samples (P < 0.001 for all comparisons; Table 1, Fig. 2). Likelihood ratio tests suggested that inclusion of the grazing period × Daphnia density interaction did not significantly improve model fit. Therefore, this term was removed from both models during model selection following the procedure in Zuur et al. (2009).
Table 1.
Summary of statistical findings from the reduced zero-inflated negative binomial models
| Experiment | Sample type | Infection measurement | Predictor | Test statistic | df | P |
|---|---|---|---|---|---|---|
| 1: Daphnia magna at five densities grazing 5 or 72 h1 | Water | Proportion | Daphnia density | X2 = 2.32 | 1 | 0.128 |
| Grazing period | X2 = 77.06 | 1 | <0.001 | |||
| Amount | Daphnia density | X2 = 7.15 | 1 | 0.008 | ||
| Grazing period | X2 = 43.45 | 1 | <0.001 | |||
| Tadpole | Proportion | Daphnia density | X2 = 6.53 | 1 | 0.011 | |
| Grazing period | X2 = 12.01 | 1 | 0.001 | |||
| Amount | Grazing period | X2 = 2.50 | 1 | 0.114 | ||
| 2: Daphnia dentifera at five densities grazing 5 or 72 h1 | Water | Proportion | Daphnia density | X2 = 13.76 | 1 | <0.001 |
| Grazing period | X2 = 64.16 | 1 | <0.001 | |||
| Amount | Grazing period | X2 = 50.82 | 1 | <0.001 | ||
| Tadpole | Proportion | Grazing period | X2 = 69.46 | 1 | <0.001 | |
| Amount | Daphnia density | X2 = 3.00 | 1 | 0.083 | ||
| Grazing period | X2 = 6.29 | 1 | 0.012 | |||
| 3: Presence/absence of D. dentifera varying food density2 | Water | Amount | Daphnia density | X2 = 8.17 | 1 | 0.004 |
| Food density | X2 = 4.53 | 1 | 0.033 | |||
| Daphnia density x Food density | X2 = 6.21 | 1 | 0.013 | |||
| Tadpole | Proportion | Daphnia density | X2 = 7.70 | 1 | 0.005 | |
| Amount3 | Daphnia density | X2 = 10.80 | 1 | 0.001 | ||
| Daphnia density x Food density | X2 = 9.28 | 1 | 0.002 |
Initial models for experiments 1 and 2 included D. magna or D. dentifera density (respectively), grazing period, and the interaction term.
Initial model for experiment 3 included food level, D. dentifera density, and the interaction term.
The results for amount of Bd detected in tadpole samples in experiment 3 were driven by a single sample. When we removed this sample, there were no significant predictors for this response.
Figure 2.
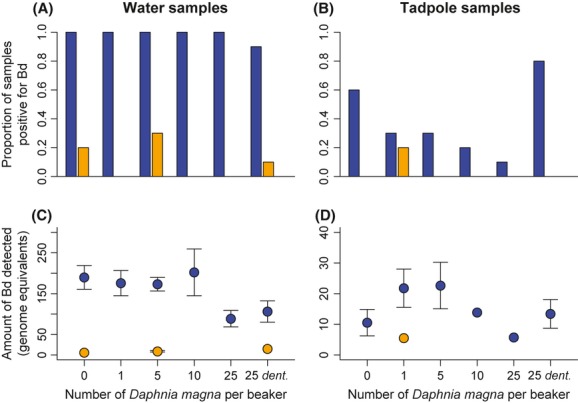
Results from experiment 1. Infection prevalence and amount of Batrachochytrium dendrobatidis (Bd) detected in samples from experiment 1. Numbers on the x-axis indicated the number of Daphnia magna per beaker while “25 dent.” indicates the treatment with 25 D. dentifera per beaker. Dark (blue) bars and points represent the 5-h grazing period and light (orange) bars and points represent the 72-h grazing period. After the grazing period, we exposed all tadpoles to grazed water for 24 h. The proportion of samples positive for Bd is shown for both (A) water and (B) tadpole samples (n = 10 for each bar). The average amount of Bd detected per treatment in Bd-positive samples for (C) water and (D) tadpoles is shown with error bars (±SE) for treatments with three or more positive samples.
Across species (comparing 0 Daphnia, 25 D. magna, and 25 D. dentifera), we found that treatments with 25 D. magna or 25 D. dentifera both reduce the amount of Bd detected in water samples (F2,26 = 4.39, P = 0.023; Table 2, Fig. 2C). A post hoc test showed no difference between 25 D. magna and 25 D. dentifera in terms of the amount of Bd detected in water (P = 086; Fig. 2C). Treatments differed significantly in the proportion of tadpoles that became infected (X2 = 11.62, P = 0.003, Table 2, Fig. 2B). A post hoc test revealed that D. magna reduced the proportion of positive tadpole samples compared with the control treatments, while D. dentifera did not (Bonferroni corrected α = 0.0167; comparing 0 Daphnia with 25 D. magna: X2 = 5.94, P = 0.015; comparing 0 Daphnia with 25 D. dentifera: X2 = 0.966, P = 0.326; Fig. 2B). The proportion of water samples that were positive for Bd and the amount of Bd detected in tadpoles did not differ among these three treatments (see Table 2).
Table 2.
Comparisons between treatments in experiment 1 containing 0 Daphnia, 25 D. magna, or 25 D. dentifera
| Sample type | Infection measurement | Test statistic | df | P |
|---|---|---|---|---|
| Water | Proportion | X2 = 2.27 | 2 | 0.322 |
| Amount | F = 4.39 | 2,26 | 0.0231 | |
| Tadpole | Proportion | X2 = 11.62 | 2 | 0.0032 |
| Amount | F = 0.09 | 2,12 | 0.914 |
A Tukey's HSD test revealed no difference between the treatments with 25 D. magna and those with 25 D. dentifera. However, both treatments with Daphnia had less Bd than the 0 Daphnia treatment.
Post hoc tests revealed that treatments with 25 D. dentifera did not differ from the 0 Daphnia treatment. However, treatments with 25 D. magna had a smaller proportion of samples testing positive for Bd compared with the 0 Daphnia treatment and the treatment with 25 D. dentifera.
Experiment 2
Daphnia dentifera density influenced the proportion of water samples positive for Bd (X2 = 13.76, P < 0.001; Table 1, Fig. 3A), but did not significantly influence the proportion of tadpoles positive for Bd, or the amount of Bd detected in water or tadpoles (see Table 1, Fig. 3B−D). The proportion of samples positive for Bd and the amount of Bd detected in samples were always lower in the 72-h grazing period compared with the 5-h grazing period (P < 0.02 for all comparisons, Fig. 3). Based on likelihood ratio tests (see Methods), we removed the grazing period x Daphnia density interaction term from both models during model selection. Figure S1 shows a comparison between the common treatments from experiments 1 and 2.
Figure 3.
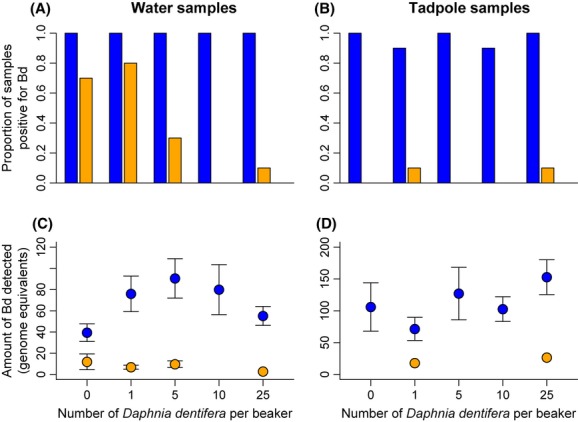
Results from experiment 2. Infection prevalence and amount of Batrachochytrium dendrobatidis (Bd) detected in samples from experiment 2 where we varied the number of Daphnia dentifera per beaker. Proportion of samples positive for Bd is shown for both (A) water and (B) tadpole samples (n = 10 for each bar). The average amount of Bd detected per treatment in Bd-positive samples for (C) water and (D) tadpoles is shown with error bars (±SE) for treatments with three or more positive samples. Dark (blue) bars and points represent the 5-h grazing period while light (orange) bars and points represent the 72-h grazing period. After the grazing period, we exposed all tadpoles to grazed water for 24 h.
Experiment 3
The proportion of water samples positive for Bd was not significantly affected by Daphnia or algae treatment (Fig. 4A). However, the amount of Bd detected in water samples was affected by the interaction between D. dentifera density and food density (X2 = 6.21, P = 0.01; Table 1, Fig. 4C). When no D. dentifera were present, there was less Bd in water when concentrations of algae were higher, but when D. dentifera were present there was less Bd detected in water when densities of algae were lower. The proportion of tadpole samples positive for Bd was lower when more D. dentifera were present (X2 = 7.70, P = 0.005), but was not affected by food density ( see Table 1, Fig. 4B). The amount of Bd detected in tadpole samples was affected by the interaction between D. dentifera and food density (X2 = 9.28, P = 0.002; Table 1, Fig. 4D). However, this significant interaction was driven by a single sample in the 25 Daphnia, low-food treatment. When we removed this point, neither Daphnia density nor the interaction was significant predictors of the amount of Bd detected in tadpoles.
Figure 4.
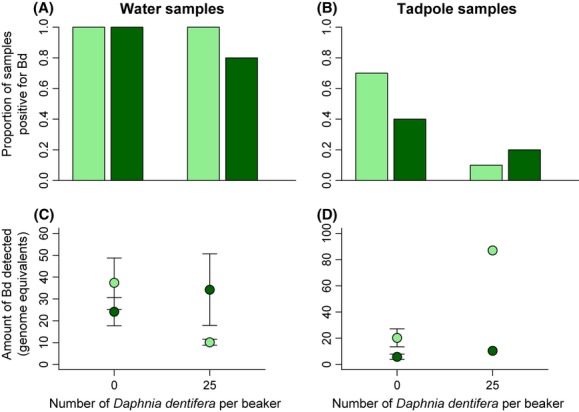
Results from experiment 3. Infection prevalence and amount of Batrachochytrium dendrobatidis (Bd) detected in samples from experiment 3. Dark bars and points represent the high-food treatments while light bars and points represent the low food treatments. Grazing period was 24 h for all treatments. Proportion of samples positive for Bd is shown for both (A) water and (B) tadpole samples (n = 10 for each bar). The average amount of Bd detected per treatment in Bd-positive samples for (C) water and (D) tadpoles is shown with error bars (±SE) for treatments with three or more positive samples.
Discussion
Our results demonstrate that direct predation upon parasites can reduce density of parasites in the environment and infection in hosts. Specifically, we found that Daphnia can reduce Bd levels in water and infection in tadpoles, but this effect was context-dependent. Daphnia abundance, Daphnia species identity, food concentration, and grazing period all affected the ability of Daphnia to reduce Bd in water and tadpole samples. Therefore, caution is warranted in assuming that Daphnia can successfully reduce infection in amphibians in natural systems.
In experiment 1, we found that high densities of both Daphnia species reduced Bd in water samples (see Fig. 2C, 3A), as previously demonstrated by Hamilton et al. (2012) and Woodhams et al. (2011). We also show that Daphnia can reduce tadpole infection (see Fig. 2B), but this effect only occurred for one of the two species we used in this study; direct comparison between the two Daphnia species (see Table 2) showed that only D. magna were able to reduce infection in tadpoles. As both species reduced the amount of Bd detected in water samples, this indicates that these species have similar rates of Bd consumption. However, zoospores may survive gut passage but be damaged and unable to infect tadpole hosts. Daphnia magna are larger than D. dentifera, and therefore are able to filter more water in a given time period (Burns 1969). Thus, zoospores are more likely to be consumed multiple times by D. magna than by D. dentifera. Therefore, D. magna may reduce infectiousness of zoospores at a greater rate than D. dentifera, even if the relative rates of Bd digestion are similar. Alternatively, Bd zoospores do not have thick cell walls or sheaths (Longcore et al. 1999), which suggests that they should not be particularly digestion resistant. Therefore, other mechanisms may drive the different effects of these two Daphnia species. Our results suggest that, when studying the effects of Daphnia on Bd in the field, it is important to consider the species identity and size of the Daphnia that are present.
High densities of Daphnia were able to reduce Bd in water and tadpole samples, but not in all circumstances. While the Daphnia densities we used in this experiment are within the range of densities found in natural systems, our highest densities (25 Daphnia per beaker; 100 Daphnia per L) and the highest densities in previous studies (1400 Daphnia per L; Hamilton et al. 2012; 1600 Daphnia per L; Woodhams et al. 2011) were likely above most natural densities. Field densities of Daphnia can occasionally reach over 100 individuals per L (e.g., ∼150: Luecke et al. 1990; up to 104: DeMott and Gulati 1999), but many field surveys have reported maximum densities below 50 individuals per L (Kwik and Carter 1975; DeMott 1983; Dawes et al. 1987). Thus, densities as high as those found in our 25 Daphnia treatments and as those used in previous studies are unlikely to be commonly found in nature. Limitations on Daphnia abundance from competition or predation may decrease the likelihood of Daphnia reducing Bd infection in natural systems.
In the absence of Daphnia, we found that the amount of Bd detected in water was lower when concentrations of algae were higher (see Fig. 4C). This pattern suggests a direct negative interaction between algae and Bd zoospores. This could occur if high concentrations of algae interfere with the ability of zoospores to swim through water through physical interference. Alternatively, some green algae exhibit allelopathy (Wolfe and Rice 1979), so A. falcatus may release chemicals that directly kill or impair Bd. Future studies are necessary to understand the direct impacts of algae on Bd zoospores. We also found that when Daphnia were present, the amount of Bd detected in water samples showed a different pattern; in this case, Bd was higher when densities of algae were higher (see Fig. 4C). It is possible that this pattern is driven by food saturation in the high-food treatments, where Daphnia were unable to consume all the algae and Bd in the water. However, individual D. dentifera under these conditions can filter over 10 mL water per day (Hall et al. 2010), so it is likely that the entire contents of our beakers would have been filtered at least once during the 24-h experiment. Gut passage time and food assimilation in Daphnia vary with food density; when food densities are low, gut passage time increases and assimilation efficiency of field-collected algae increases (DeMott et al. 2010). Therefore, even if Daphnia in the low-food treatments consumed the same number of zoospores as in the high-food treatments, a greater proportion of those zoospores may have been digested. This indicates that zoospores may be better able to survive passage through the gut of a Daphnia in high-food conditions. Alternatively, Daphnia can exhibit selective grazing (Burns 1968; Porter 1973; Haney 1987), so high densities of algae could have led to Daphnia consuming fewer Bd zoospores if A. falcatus is their preferred food. These results have implications for Bd disease risk in natural systems. In eutrophic lakes, for example, high densities of algae may have direct negative effects on Bd zoospores, reducing disease risk for amphibians. However, high densities of algae may reduce digestion of Bd zoospores, which would create an indirect positive effect of algae on Bd. It is unknown how these conflicting forces will affect Bd levels in eutrophic environments.
In both experiments 1 and 2, we found that treatments with longer grazing periods almost always reduced Bd in our samples. As we saw this pattern across all Daphnia densities (including treatments with no Daphnia), this is unlikely due to effects of Daphnia grazing. Bd zoospores were only added once at the beginning of the grazing period and have a limited lifespan in water (Piotrowski et al. 2004). Therefore, it is likely that we detected less Bd in water samples after 72 h due to increased time for zoospore mortality compared with the 5-h grazing periods. When zoospores die, their cells and DNA degrade, resulting in lower qPCR values. Additionally, when tadpoles were added after the beakers after 72 h of grazing, there were fewer surviving zoospores able to infect the tadpoles. It is possible that we would have found different results had we exposed tadpoles to Bd while simultaneously allowing Daphnia to graze. The ability of Daphnia to consume Bd in natural systems is likely affected by the length of time that zoospores remain in the water before finding hosts. Thus, if zoospores are able to find hosts quickly, then the effects of Daphnia grazing may be limited.
Another notable trend we observed is that patterns found in water samples were not necessarily similar to the observed patterns in tadpoles from the same experiment. For example, in both experiments 1 and 2, D. dentifera reduced Bd in water samples, but had no effect on tadpole samples. This indicates that infection in tadpoles is not necessarily dose-dependent. A previous experimental study demonstrated that only one of three amphibian species tested exhibited a dose-dependent response to Bd (Gervasi et al. 2013). Multiple factors may be involved in determining infection in tadpoles. For example, there is variation within amphibian species in anti-Bd microbial defenses (Harris et al. 2006; Lam et al. 2010), and different species exhibit behaviors that affect their chances of becoming infected (Rowley and Alford 2007). These factors may have large effects on Bd infection in tadpoles and sometimes outweigh the effects of zoospore densities. Therefore, it is essential to monitor Bd in both water bodies and amphibian hosts.
Our study demonstrated that direct predation on parasites can reduce infection of a deadly fungal parasite responsible for amphibian population declines and extirpations around the globe. However, this effect was context-dependent and varied with predator species, predator density and resource availability. Therefore, it cannot be assumed that predators will successfully act as biocontrol agents for infectious diseases, even if they have the ability to consume parasites. When attempting to understand the effects predation upon parasites, numerous biotic and abiotic conditions must be considered.
Acknowledgments
We would like to thank J.E. Longcore for providing Bd cultures and T.J. Little for providing the D. magna clone. We would also like to thank J. Housley Ochs, R.M. Penczykowski, S.K.J.R. Auld, D. Grippi, and S. Hernandez for assistance in performing the experiments, and R. Hill and J. Jensen for logistical advice. This project was funded by National Science Foundation–DEB 1305826 to MAD and the Georgia Institute of Technology.
Conflict of Interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Figure S1. Infection prevalence and amount of Bd detected in common treatments from experiments 1 and 2. Dark (blue) bars and points represent the 5 h grazing period and light (orange) bars and points represent the 72 h grazing period. Amount of Bd detected is shown as the average level for Bd-positive samples per treatment (±SE). Points without error bars were treatments with fewer than three positive samples.
Figure S2. Average whole-body wet mass of tadpoles used in each experiment (±SE). The number of tadpoles used in each experiment varied (experiment 1: n = 120, experiment 2: n = 100, experiment 3: n = 40).
Data S1. Methods and results.
References
- Bosch J, Martinez-Solano I, Garcia-Paris M. Evidence of a chytrid fungus infection involved in the decline of the common midwife toad (Alytes obstetricans) in protected areas of central Spain. Biol. Conserv. 2001;97:331–337. [Google Scholar]
- Bottrell HH, Duncan A, Gliwicz ZM, Grygierek E, Herzig A, Hillbright-Ilkowska A, et al. A review of some problems in zooplankton production studies. Norw. J. Zool. 1976;24:419–456. [Google Scholar]
- Boyle DG, Boyle DB, Olsen V, Morgan JAT, Hyatt AD. Rapid quantitative detection of chytridiomycosis (Batrachochytrium dendrobatidis) in amphibian samples using real-time Taqman PCR assay. Dis. Aquat. Org. 2004;60:141–148. doi: 10.3354/dao060141. [DOI] [PubMed] [Google Scholar]
- Buck JC, Truong L, Blaustein AR. Predation by zooplankton on Batrachochytrium dendrobatidis: biological control of the deadly amphibian chytrid fungus? Biodivers. Conserv. 2011;20:3549–3553. [Google Scholar]
- Burns CW. The relationship between body size of filter-feeding Cladocera and the maximum size of particle ingested. Limnol. Oceanogr. 1968;13:675–678. [Google Scholar]
- Burns CW. Relation between filtering rate, temperature, and body size in four species of Daphnia. Limnol. Oceanogr. 1969;14:693–700. [Google Scholar]
- Dawes CJ, Cowell BC, Gardiner WE, Scheda SM. Limnological characteristics of two eutrophic and four mesotrophic lakes in west-central Florida. Int. Rev. Hydrobiol. 1987;72:171–203. [Google Scholar]
- DeMott WR. Seasonal succession in a natural Daphnia assemblage. Ecol. Monogr. 1983;53:321–340. [Google Scholar]
- DeMott WR, Gulati RD. Phosphorus limitation in Daphnia: evidence from a long term study of three hypereutrophic Dutch lakes. Limnol. Oceanogr. 1999;6:1557–1564. [Google Scholar]
- DeMott WR, McKinney EN, Tessier AJ. Ontogeny of digestion in Daphnia: implications for the effectiveness of algal defenses. Ecology. 2010;91:540–548. doi: 10.1890/08-2103.1. [DOI] [PubMed] [Google Scholar]
- Duffy MA, Hall SR, Tessier AJ, Huebner M. Selective predators and their parasitized prey: are epidemics in zooplankton under top-down control? Limnol. Oceanogr. 2005;50:412–420. [Google Scholar]
- Duffy MA, Housley JM, Penczykowski RM, Caceres CE, Hall SR. Unhealthy herds: indirect effects of predators enhance two drivers of disease spread. Funct. Ecol. 2011;25:945–953. [Google Scholar]
- Geller W, Muller H. The filtration apparatus of Cladocera: filter mesh-sizes and their implications on food selectivity. Oecologia. 1981;49:316–321. doi: 10.1007/BF00347591. [DOI] [PubMed] [Google Scholar]
- Gervasi S, Gondhalekar C, Olson DH, Blasutein AR. Host identity matters in the amphibian-Batrachochytrium dendrobatidis system: fine-scale patterns of variation in responses to a multi-host pathogen. PLoS ONE. 2013;8:e54490. doi: 10.1371/journal.pone.0054490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grutter A. Parasite removal rates by the cleaner wrasse Labroides dimidiatus. Mar. Ecol. Prog. Ser. 1996;130:61–70. [Google Scholar]
- Hall SR, Sivars-Becker L, Becker C, Duffy MA, Tessier AJ, Caceres CE. Eating yourself sick: transmission of disease as a function of foraging ecology. Ecol. Lett. 2007;10:207–218. doi: 10.1111/j.1461-0248.2007.01011.x. [DOI] [PubMed] [Google Scholar]
- Hall SR, Becker CR, Duffy MA, Caceres CE. Variation in resource acquisition and use among host clones creates key epidemiological trade-offs. Am. Nat. 2010;176:557–565. doi: 10.1086/656523. [DOI] [PubMed] [Google Scholar]
- Hamilton PT, Richardson JML, Anholt BR. Daphnia in tadpole mesocosms: trophic links and interactions with Batrachochytrium dendrobatidis. Freshw. Biol. 2012;57:676–683. [Google Scholar]
- Haney JF. Field studies on zooplankton-cyanobacteria interactions. N. Z. J. Mar. Freshwater Res. 1987;21:467–475. [Google Scholar]
- Harris RN, James TY, Lauer A, Simon MA, Patel A. Amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. EcoHealth. 2006;3:53–56. [Google Scholar]
- Howard AFV, Zhou G, Omlin FX. Malaria mosquito control using edible fish in western Kenya: preliminary findings of a controlled study. BMC Public Health. 2007;7:199. doi: 10.1186/1471-2458-7-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Speare R. Survival of Batrachochytrium dendrobatidis in water: quarantine and disease control implications. Emerg. Infect. Dis. 2003;9:922–925. doi: 10.3201/eid0908.030145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagami M, Donk EV, Rijkeboer A, de Bruin M, Ibelings BW. Daphnia can protect diatoms from fungal parasitism. Limnol. Oceanogr. 2004;49:680–685. [Google Scholar]
- Keesing F, Holt RD, Ostfeld RS. Effects of species diversity on disease risk. Ecol. Lett. 2006;9:485–498. doi: 10.1111/j.1461-0248.2006.00885.x. [DOI] [PubMed] [Google Scholar]
- Kluttgen B, Dulmer U, Engels M, Ratte HT. ADaM, an artificial freshwater for the culture of zooplankton. Water Res. 1994;28:743–746. [Google Scholar]
- Kwik JK, Carter JCH. Population dynamics of limnetic Cladocera in a beaver pond. J. Fish. Res. Board Can. 1975;32:341–346. [Google Scholar]
- Lafferty KD. Fishing for lobsters indirectly increases epidemics in sea urchins. Ecol. Appl. 2004;14:1566–1573. [Google Scholar]
- Lafferty KD, Dobson AP, Kuris AM. Parasites dominate food web links. Proc. Natl Acad. Sci. USA. 2006;103:11211–11216. doi: 10.1073/pnas.0604755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam BA, Walke JB, Vredenburg VT, Harris RN. Proportion of individuals with anti-Batrachochytrium dendrobatidis skin bacteria is associated with population persistence in the frog Rana muscosa. Biol. Conserv. 2010;143:529–531. [Google Scholar]
- Lips KR, Brem F, Brenes R, Reeve JD, Alford RA, Voyles J, et al. Emerging infectious disease and the loss of biodiversity in a Neotropical amphibian community. Proc. Natl Acad. Sci. USA. 2006;103:3165–3170. doi: 10.1073/pnas.0506889103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longcore JE, Pessier AP, Nichols DK. Batrachochytrium dendrobatidis gen. et sp. nov., a chytrid pathogenic to amphibians. Mycologia. 1999;91:219–227. [Google Scholar]
- Luecke C, Vanni MJ, Magnuson JJ, Kitchell JF, Jacobson PT. Seasonal regulation of Daphnia populations by planktivorous fish: implications for the spring clear-water phase. Limnol. Oceanogr. 1990;8:1718–1733. [Google Scholar]
- Nelson XJ, Jackson RR. A predator from East Africa that chooses malaria vectors as preferred prey. PLoS ONE. 2006;1:e132. doi: 10.1371/journal.pone.0000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omacini M, Chaneton EJ, Ghersa CM, Muller CB. Symbiotic fungal endophytes control insect host-parasite interaction webs. Nature. 2001;409:78–81. doi: 10.1038/35051070. [DOI] [PubMed] [Google Scholar]
- Orlofske SA, Jadin RC, Preston DL, Johnson PTJ. Parasite transmission in complex communities: predators and alternative hosts alter pathogenic infections in amphibians. Ecology. 2012;93:1247–1253. doi: 10.1890/11-1901.1. [DOI] [PubMed] [Google Scholar]
- Packer C, Holt RD, Hudson PJ, Lafferty KD, Dobson AP. Keeping herds healthy and alert: implications of predator control for infectious disease. Ecol. Lett. 2003;6:797–802. [Google Scholar]
- Piotrowski JS, Annis SL, Longcore JE. Physiology of Batrachochytrium dendrobatidis, a chytrid pathogen of amphibians. Mycologia. 2004;96:9–15. [PubMed] [Google Scholar]
- Porter KG. Selective grazing and differential digestion of algae by zooplankton. Nature. 1973;244:179–180. [Google Scholar]
- Porter KG. Enhancement of algal growth and productivity by grazing zooplankton. Science. 1976;192:1332–1334. doi: 10.1126/science.192.4246.1332. [DOI] [PubMed] [Google Scholar]
- R Core Development Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2012. ISBN 3-900051-07-0, Available at http://www.R-project.org/ [Google Scholar]
- Ramirez RA, Snyder WE. Scared sick? Predator-pathogen facilitation enhances exploitation of a shared resource. Ecology. 2009;90:2832–2839. doi: 10.1890/08-1941.1. [DOI] [PubMed] [Google Scholar]
- Rowley JJL, Alford RA. Behaviour of Australian rainforest stream frogs may affect the transmission of chytridiomycosis. Dis. Aquat. Org. 2007;77:1–9. doi: 10.3354/dao01830. [DOI] [PubMed] [Google Scholar]
- Schloegel LM, Ferreira CM, James TY, Hipolito M, Longcore JE, Hyatt AD, et al. The North American bullfrog as a reservoir for the spread of Batrachochytrium dendrobatidis in Brazil. Anim. Conserv. 2009;13 s1:53–61. [Google Scholar]
- Searle CL, Gervasi SS, Jua J, Hammond JI, Relyea RA, Olson DH, et al. Differential host susceptibility to Batrachochytrium dendrobatidis, an emerging amphibian pathogen. Conserv. Biol. 2011;25:965–974. doi: 10.1111/j.1523-1739.2011.01708.x. [DOI] [PubMed] [Google Scholar]
- Skerratt LF, Berger L, Spear R, Cashins S, McDonald KR, Phillott AD, et al. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. EcoHealth. 2007;4:125–134. [Google Scholar]
- Wake DB, Vredenburg VT. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl Acad. Sci. USA. 2008;105:11466–11473. doi: 10.1073/pnas.0801921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe JM, Rice EL. Allelopathic interactions among algae. J. Chem. Ecol. 1979;5:533–542. [Google Scholar]
- Woodhams DC, Bosch J, Briggs CJ, Cashins S, Davis LR, Lauer A, et al. Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Front. Zool. 2011;8:8. doi: 10.1186/1742-9994-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R. 1st edn. New York, NY: Springer; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


