Abstract
Primary neuroendocrine carcinoma (PNEC) of breast was an unknown pathologic entity till recently due its rare incidence and lack of definitive criteria for diagnosis. We present a case of PNEC of breast in a middle aged lady. A 34 years lady presented with a breast lump since 1 month, who underwent modified radical mastectomy with axillary clearance. Histopathological diagnoses were infiltrating ductal carcinoma-neuroendocrine (NE) type. Immunohistochemistry showed estrogen, progesterone positivity and NE markers positivity in more than 50% of tumor cells for chromogranin, synaptophysin, neuron specific enolase. On further investigation by whole body computed tomography and magnetic resonance imaging revealed no extra mammary primary tumor. Hence the diagnosis of PNEC of breast was confirmed. Patient received chemo and hormonal therapy and doing well after 6 months of follow up.
Keywords: Breast carcinoma, Immunohistochemistry, Neuroendocrine tumour
Introduction
Primary neuroendocrine carcinoma (PNEC) of breast were an unknown entity till recently because of its rare incidence accounting to only 2-5% of breast carcinoma and lack of definitive diagnostic criteria.[1,2] The World Health Organization (WHO) Classification of tumors in 2003 defined neuroendocrine carcinoma (NEC) of the breast as having more than 50% neoplastic tumor cells expressing neuroendocrine (NE) markers.[2] These tumors are usually seen in elderly women around sixth or seventh decade of life as reported in literatures.[1,3] Herein we report a case of primary NCE of breast in a middle aged lady.
Case Report
A 34 years lady, presented with history of breast lump since 1 month. On examination, a hard non tender lump of size 3 cm × 3 cm was noted below the nipple and areola. Clinically, the case was diagnosed as fibroadenoma. Fine needle aspiration cytology was done and reported as breast carcinoma. Mammography showed ill-defined irregular hypoechoic mass mentioning Breast Imaging-Reporting and Data System fifth category as malignant. Laboratory investigation like complete blood counts, liver and kidney function tests were within normal limits and serological tests for human immunodeficiency virus, Hepatitis B surface antigen, Venereal Disease Research Laboratory test was negative. Patient underwent modified radical mastectomy (MRM) with axillary clearance. Specimen of MRM with axillary pad of fat measured 16 cm × 9 cm × 3 cm. On dissection, there was a grey white irregular mass measuring 2.5 cm × 2 cm below the nipple and areola with areas of necrosis and cystic change. Adjacent breast tissue showed rubbery grey white fibrocystic areas. Thirteen lymph nodes were identified in axillary pad of fat. Histopathological examination of H and E sections showed tumor cells arranged in diffuse sheets, clusters and at places trabecular pattern separated by thin fibrous septae. Individual cells were uniform in shape and size with increased nuclear cytoplasmic ratio, hyperchromatic nucleus and scant cytoplasm. [Figure 1a and b] Areas of mucin and necrosis are seen. Adjacent breast tissue revealed in-situ component composed of similar cells as seen in main tumor. All thirteen lymph nodes showed sinus histiocytosis. By immunohistochemistry, tumor cells were positive for estrogen [Figure 2], progesterone receptors and cytokeratin (CK) 18. More than 50% of tumor cells were positive for NE markers like chromogranin A, synaptophysin and neuron specific enolase (NSE) [Figure 3]. Her 2/neu, S-100, Epithelial Membrane Antigen, CK 14, 7.5/6 were negative. Ki 67 proliferative index was less than 5%. So, the provisional diagnosis was infiltrating ductal carcinoma-NE type breast and its’ the molecular type was luminal A. Further investigations were done to rule out metastatic NEC. Hence computed tomography and magnetic resonance imaging head and neck, chest and abdomen were done and found no extra-mammary primary tumor. So, the final diagnosis of this case was Grade 1 solid primary NEC of breast with free all dissected lymph nodes-Stage 2A (pT2N0M0). The patient was given adjuvant chemotherapy-(cyclophosphamide, adriamycin and 5-fluorouracil) and hormonal therapy (Tamoxifen). During 6 months of follow-up, no local recurrence or metastasis was encountered.
Figure 1.
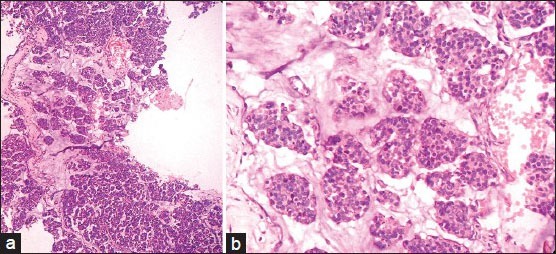
(a,b) Tumour cells arranged in diffuse sheets, clusters and at places trabecular pattern separated by thin fibrous septae (H and E, ×100 and ×400)
Figure 2.
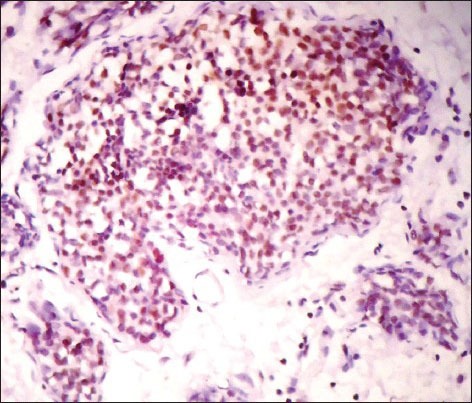
IHC stained section showing estrogen nuclear staining (×400)
Figure 3.
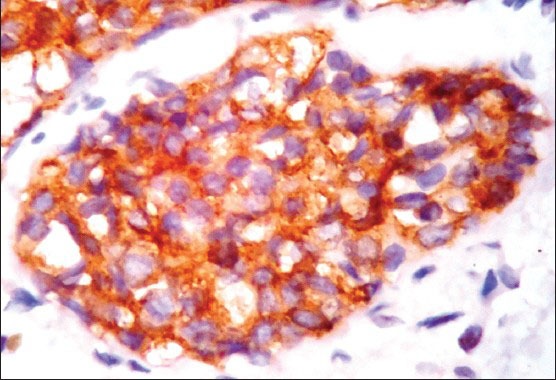
IHC stained section showing more than 50% of cells positive for synaptophysin cytoplasmic staining (×400)
Discussion
The existence of mammary tissue NE cells was shown by Volger in 1947,[4] later in 1977 Cubilla and Woodruff were the first to describe PNEC of breast.[5] They were initially named as argyrophilic breast carcinoma, breast carcinoid tumor or endocrine carcinoma, but after WHO laid the definitive criteria they labeled them as PNEC of breast.[2,4] The histogenesis of the tumor is still unclear; few postulated it to arise from endocrine differentiation of breast carcinoma rather than pre-existing endocrine cells in the breast. Others thought that they arise from multi potential stem cells which differentiate along NEC phenotype.[6,7]
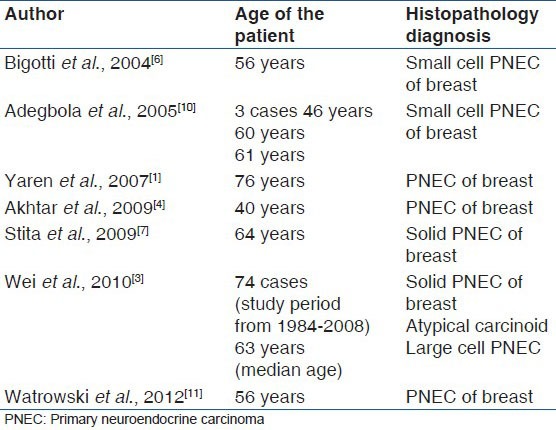
Small cell carcinomas are usually seen in lungs but also noticed in other extrapulmonary sites like stomach, small intestine, uterus, cervix, pancreas, larynx, trachea, prostate and breast.[1] The diagnosis of NEC primarily originating from breast can be confirmed by in-situ component and immunostaining for estrogen and progesterone receptor positivity as noted in our case, but many published cases lacked this finding.[1,6,7,8] Extensive literature search shows commonest age noted with NEC breast tumors were in sixth and seventh decade of life,[1,3,6,7,8,9] authors to the best of knowledge are first to report in the third decade [Table 1].
Table 1.
Reported cases of primary neuroendocrine carcinoma of breast
WHO classified NEC of breast as solid NEC, atypical carcinoid tumor, small cell/oat cell carcinoma and large cell NEC.[2] Histopathologically the tumor cells are arranged in sheets, ribbons and cords. Individual cells are uniform with round to ovoid nucleus, salt and pepper chromatin and scant cytoplasm.[4] Immunohistochemistry shows more than 50% tumor cells positive for NE markers like chromogranin, synaptophysin and NSE. Similar features were evident in our case. Akhtar et al., reported that grimelius staining is specific and shows argyrophilia in NEC, on electron microscopy electron dense granules are demonstrated. Metastatic NEC to breast have also been reported hence CT of thorax, head and neck and abdomen should be done to rule out primary in the lung or other sites,[1,2,3,4] which was carried out in our case and found no abnormalities. Hoang et al.,[12] studied the molecular features of two cases of primary small cell carcinoma which revealed identical molecular alterations at multiple chromosomal regions including Breast Cancer gene 1, BRCA-2, p53 and retinoblastoma gene loci. Weigelt et al. stated that out of six PNEC of breast, five NECs were of luminal A subtype, whereas one NE tumor was of luminal B phenotype.[13] Whereas Watrowski et al. studied a single case of PNEC of breast and classified as luminal B subtype also concluded that molecular classification helps in therapeutic interventions.[11]
The standard therapy for this case is controversial, and treatment is limited to surgery such as MRM with axillary clearance,[1,3] which was performed in our case. Since receptor positivity was determined adjuvant chemo and hormonal therapy was considered.
Previous reports of literature mention that NEC breast shows aggressive behavior compared to ductal carcinomas with a higher propensity for local and distant recurrence. But more recent data suggest this tumor has more favorable prognosis.[1,7] Higher grade, increased tumor size and regional lymph node metastasis are associated with poor prognosis and decreased disease free survival.[3] Mucinous differentiation and Estrogen/Progesterone receptors positivity are favorable prognostic factors.[7] Clinical outcomes reported in literature showed 15% of local recurrence by 5 years, with median recurrence free time of 177 months and 34% risk for distant recurrence within 5 years with median recurrence free time of 73 months among NEC patients. The common sites for distant metastasis are bone and liver, other sites include lungs, brain, pleura, mediastinal lymph nodes, adrenal glands, ovaries, fallopian tubes, colon, ileum and pancreas.[3]
Conclusion
This is the first reported case of primary NEC of breast in middle-aged lady in our institute and molecular genetic analysis should be done in large case numbers to know the early onset of this rare variant and also newer therapeutic interventions.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.Yaren A, Kelten C, Akbulut M, Teke Z, Duzcan E, Erdem E. Primary neuroendocrine carcinoma of the breast: A case report. Tumori. 2007;93:496–8. doi: 10.1177/030089160709300516. [DOI] [PubMed] [Google Scholar]
- 2.Tavassoli FA, Devilee P. Lyon: World Health Organization (WHO) Classification of Tumours; 2003. Pathology and genetics tumours of the breast and female genital organs; pp. 32–4. [Google Scholar]
- 3.Wei B, Ding T, Xing Y, Wei W, Tian Z, Tang F, et al. Invasive neuroendocrine carcinoma of the breast: A distinctive subtype of aggressive mammary carcinoma. Cancer. 2010;116:4463–73. doi: 10.1002/cncr.25352. [DOI] [PubMed] [Google Scholar]
- 4.Akhtar K, Zaheer S, Ahmad SS, Hassan MJ. Primary neuroendocrine carcinoma of the breast. Indian J Pathol Microbiol. 2009;52:71–3. doi: 10.4103/0377-4929.44970. [DOI] [PubMed] [Google Scholar]
- 5.Cubilla AL, Woodruff JM. Primary carcinoid tumor of the breast: A report of 8 patients. Am J Surg Pathol. 1977;1:283–92. [Google Scholar]
- 6.Bigotti G, Coli A, Butti A, del Vecchio M, Tartaglione R, Massi G. Primary small cell neuroendocrine carcinoma of the breast. J Exp Clin Cancer Res. 2004;23:691–6. [PubMed] [Google Scholar]
- 7.Stita W, Trabelsi A, Gharbi O, Mokni M, Korbi S. Primary solid neuroendocrine carcinoma of the breast. Can J Surg. 2009;52:E289–90. [PMC free article] [PubMed] [Google Scholar]
- 8.Papotti M, Gherardi G, Eusebi V, Pagani A, Bussolati G. Primary oat cell (neuroendocrine) carcinoma of the breast. Report of four cases. Virchows Arch A Pathol Anat Histopathol. 1992;420:103–8. doi: 10.1007/BF01605991. [DOI] [PubMed] [Google Scholar]
- 9.Shin SJ, DeLellis RA, Ying L, Rosen PP. Small cell carcinoma of the breast: A clinicopathologic and immunohistochemical study of nine patients. Am J Surg Pathol. 2000;24:1231–8. doi: 10.1097/00000478-200009000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Adegbola T, Connolly CE, Mortimer G. Small cell neuroendocrine carcinoma of the breast: A report of three cases and review of the literature. J Clin Pathol. 2005;58:775–8. doi: 10.1136/jcp.2004.020792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Watrowski R, Jäger C, Mattern D, Horst C. Neuroendocrine carcinoma of the breast: Diagnostic and clinical implications. Anticancer Res. 2012;32:5079–82. [PubMed] [Google Scholar]
- 12.Hoang MP, Maitra A, Gazdar AF, Albores-Saavedra J. Primary mammary small-cell carcinoma: A molecular analysis of 2 cases. Hum Pathol. 2001;32:753–7. doi: 10.1053/hupa.2001.25603. [DOI] [PubMed] [Google Scholar]
- 13.Weigelt B, Geyer FC, Horlings HM, Kreike B, Halfwerk H, Reis-Filho JS. Mucinous and neuroendocrine breast carcinomas are transcriptionally distinct from invasive ductal carcinomas of no special type. Mod Pathol. 2009;22:1401–14. doi: 10.1038/modpathol.2009.112. [DOI] [PubMed] [Google Scholar]


