Abstract
Diffuse intrinsic pontine glioma (DIPG) has a dismal prognosis with no chemotherapy regimen so far resulting in any significant improvement over standard radiotherapy. In this trial, a prolonged regimen (21/28 d) of temozolomide was studied with the aim of overcoming O6-methylguanine methyltransferase (MGMT) mediated resistance.
Forty-three patients with a defined clinico-radiological diagnosis of DIPG received radiotherapy and concomitant temozolomide (75 mg/m2) after which up to 12 courses of 21 d of adjuvant temozolomide (75–100 mg/m2) were given 4 weekly. The trial used a 2-stage design and passed interim analysis.
At diagnosis median age was 8 years (2–20 years), 81% had cranial nerve abnormalities, 76% ataxia and 57% long tract signs. Median Karnofsky/Lansky score was 80 (10–100). Patients received a median of three courses of adjuvant temozolomide, five received all 12 courses and seven did not start adjuvant treatment. Three patients were withdrawn from study treatment due to haematological toxicity and 10 had a dose reduction. No other significant toxicity related to temozolomide was noted. Overall survival (OS) (95% confidence interval (CI)) was 56% (40%, 69%) at 9 months, 35% (21%, 49%) at 1 year and 17% (7%, 30%) at 2 years. Median survival was 9.5 months (range 7.5–11.4 months). There were five 2-year survivors with a median age of 13.6 years at diagnosis.
This trial demonstrated no survival benefit of the addition of dose dense temozolomide, to standard radiotherapy in children with classical DIPG. However, a subgroup of adolescent DIPG patients did have a prolonged survival, which needs further exploration.
Keywords: Diffuse intrinsic pontine glioma (DIPG), Temozolomide
1. Introduction
Diffuse intrinsic pontine glioma (DIPG) is a devastating diagnosis for which there is no effective treatment strategy that results in cure. Nearly 90% of children are dead within 18 months of diagnosis and an average median survival of around 9 months is reported in a number of series [1–3].
Radiotherapy is the only treatment that has shown any degree of efficacy [4] but this usually merely delays the inevitable progression of the tumour. Hyperfractionation and increased doses of up to 78 Gy have had no additional effect [5–8]. A number of chemotherapeutic approaches have also been tried with limited or no effect. These have included radiosensitisers such as carboplatin [9–11], standard chemotherapy such as etoposide (oral and intravenous) [12,13], vincristine and high dose chemotherapy such as busulfan and thiotepa with stem cell support [14,15].
Temozolomide is an oral alkylating agent which crosses the blood brain barrier [16] and is used widely in the treatment of high grade gliomas, both in adults and children. It has been shown to be well tolerated and prolongs survival in glioblastoma [17]. Disappointingly, given in the standard way (alongside radiotherapy and subsequently for five out of every 28 d) this benefit has not been seen in DIPG in children [18–21] with median survival time of 11.7 and 9.6 months similar to historical controls treated with radiotherapy alone [1].
Temozolomide results in the depletion of O6-methylguanine methyltransferase (MGMT), a DNA repair protein. Administration with extended low dose schedules has been demonstrated in peripheral blood mononuclear cells to result in greater depletion of MGMT and thus a greater retention of O6-methylguanine in DNA that produces the predominant cytotoxic effect [22,23] as well as less time for protein replenishment to occur in between doses of temozolomide. This was extrapolated and an assumption made that the same effect may be seen in tumour cells. A prolonged dosing regime has been shown to be feasible and safe in a paediatric population [24].
This trial studied the effect of prolonged dose dense temozolomide both post radiotherapy (21/28 d) as well as concomitantly during radiotherapy (42 d) in children with newly diagnosed DIPG.
2. Patients and methods
2.1. Eligibility
Children and young people aged 2–21 years with a newly diagnosed diffuse intrinsic lesion centred in the pons and compatible with radiological criteria of DIPG on MRI imaging were eligible for the study. In addition, a clinical history of less than 6 months and the presence of at least one of the following (i) cranial nerve deficit, (ii) long tract signs or (iii) ataxia were required. The patients also were required to have a Karnofsky Performance Status or a Lansky play score of greater than or equal to 60 unless the reason for decrease in status was a direct result of neurological involvement of the brainstem glioma, a life expectancy of more than 12 weeks and adequate haematological, renal and hepatic function. Patients were not eligible for the study if they had a focal lesion of the brainstem, a predominantly exophytic tumour, had received previous chemotherapy or radiotherapy or had a condition which would have interfered with oral medication intake. Written informed parental consent (and assent for older children) was taken and the study was approved by a multicentre research ethics committee (Derby 1). Biopsy was not mandated for this study.
2.2. Treatment
This was a single arm open-label phase II study of concurrent and adjuvant single agent temozolomide alongside initial focal radiotherapy of 54 Gy given in 30 fractions over a 6 week period. An induction course of temozolomide was administered at an initial dose of 75 mg/m2 daily alongside radiotherapy (42 d). Following a minimum 4 week break after radiotherapy temozolomide was administered daily for 21 consecutive days out of every 28 at a starting dose of 75 mg/m2/d. After two cycles this dose was increased to 100 mg/m2/d for the remaining cycles up to a maximum of 12 months providing there was no clinical progression (Fig. 1).
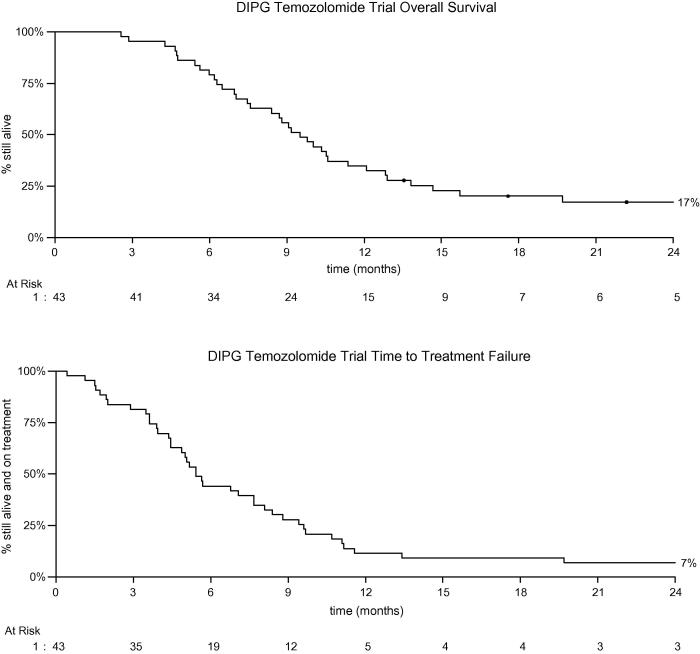
Fig. 1.
Overall and progression free survival of children treated on the diffuse intrinsic pontine glioma (DIPG) temozolomide trial.
2.3. Response evaluation
Response was evaluated (by the local team) clinically based on neurological examination (+2 = definitely better, +1 = possibly better, 0 = unchanged, −1 = possibly worse, −2 = definitely worse) and radiologically based on the RECIST criteria as outlined below: The RECIST criteria system was chosen as this was the most widely used system at the time of the trial.
-
•
CR (complete response) – disappearance of all tumour
-
•
PR (partial response) – at least a 30% decrease in the sum of the longest diameter (LD) of target lesions, taking as a reference the baseline sum LD and no new lesions.
-
•
SD (stable disease) – neither sufficient shrinkage to qualify for PR nor sufficient increase to qualify for progressive disease (PD), taking as reference the smallest sum LD since the treatment started
-
•
PD (progressive disease) – at least a 20% increase in the sum of the LD of target lesions, taking as reference the smallest sum LD recorded since the treatment started or the appearance of one or more new lesions.
No patient was withdrawn from the study purely on radiological grounds.
2.4. Quality of life
It was intended to assess quality of life and steroid administration as part of the study. The quality of life assessment (QoL) was planned to be performed prior to radiotherapy, at the beginning of adjuvant temozolomide and prior to each subsequent three cycles of temozolomide. The health utilities index (HUI) [25] and strengths and difficulties questionnaire (SDQ) [26] were used. Data collection from centres however was suboptimal for both QoL and steroid administration and inadequate for meaningful analysis and therefore cannot be reported reliably.
2.5. Statistical methods
This trial was originally designed as a Case-Morgan design [27]. This method was chosen to allow the trial to stop early for futility. It was a single arm study, testing a null hypothesis of 50% overall survival at 9 months against an alternate hypothesis of 70%. The level of significance for the study was set at a 1-tailed alpha of 0.05. There was one intermediate analysis for futility, which did not result in the trial stopping. The primary hypothesis test in the study was a 1-tailed test of the survival at 9 months. No other hypothesis testing was carried out – where appropriate, point estimates are presented with 2-tailed 95% confidence intervals.
Overall survival has been calculated as time from study entry to death from any cause, with patients censored at date last seen if lost to follow up. Time to treatment failure has been calculated as time from study entry to stopping temozolomide for any reason (except reaching the end of the maintenance course without progression) or death.
3. Results
3.1. Patient characteristics
Between February 2008 and July 2010, 43 patients from 16 centres with clinically and radiologically diagnosed DIPG were registered and treated according to the protocol described above (CCLG CNS 2007/4).
The median age at presentation was 8 years (2–20 years) and the male: female ratio was 24:19. Eighty-one percent of patients presented with cranial nerve abnormalities, 76% with ataxia and 57% with long tract signs. Median Karnofsky/Lansky score was 80 (mean 79; range 10–100). The median time from onset of symptoms to starting treatment was 32 d, range 9–194, (second highest 130).
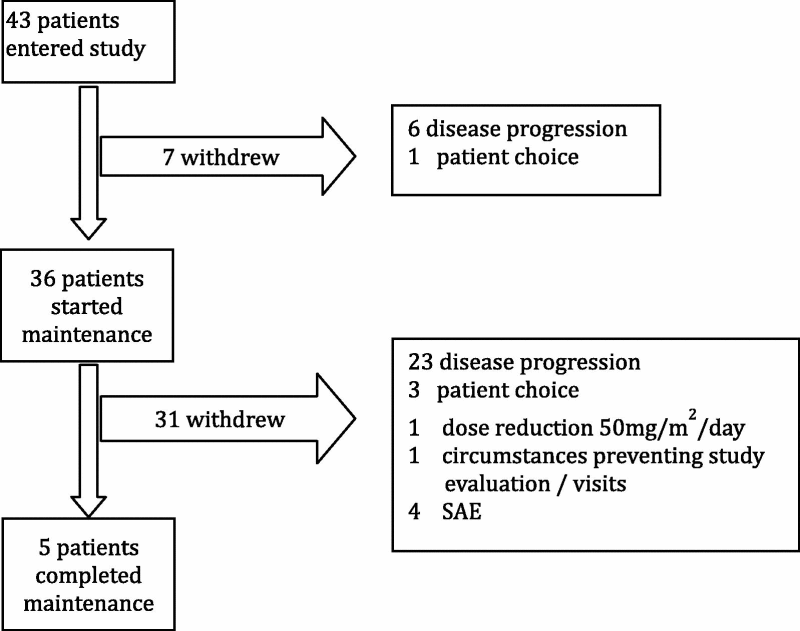
Thirty-eight patients received the full course of induction temozolomide, and a further three had dose reductions (two due to thrombocytopenia and one due to abdominal obstruction). In two cases it is unknown whether the full induction course was received. One child stopped radiotherapy after 18 Gy due to the need for surgical intervention (Ventriculoperitoneal shunt). Two others had short interruptions but received the total dose. Patients received a median of three courses of maintenance temozolomide, five received all 12 courses and seven did not start maintenance (six due to progression and one due to patient choice). Three patients were withdrawn from study treatment due to haematological toxicity and 10 had a dose reduction on at least one course. The most common grade 3/4 toxicities associated with induction temozolomide were: lymphopaenia (45% of patients with at least one episode), neutropaenia (12%), thrombocytopaenia (10%), infection (10%), leucopaenia (8%) and nausea (8%). Grade 3/4 toxicity associated with radiotherapy/temozolomide was very rare. One case each of ataxia, headaches/vomiting (both same patient) and elevated alanine aminotransferase (ALT). The most common grade 3/4 toxicities associated with maintenance temozolomide were: lymphopaenia (68% of patients with at least one episode), infection (24%), leukocytopaenia (21%) neutropaenia (15%), thrombocytopaenia (12%) and fatigue (12%). The reasons for withdrawal from treatment are detailed in Table 1.
Table 1.
Reasons for withdrawal of temozolomide.
 |
Based on the post radiotherapy assessment (RECIST), 11 children had partial response (PR), 26 stable disease (SD), four progressive disease (PD), and two not available. Seven children had an increased tumour size on their post radiotherapy scan (four PD and three SD on RECIST criteria). Of these, four had progressive disease by RECIST criteria and three stable disease. Of these seven children the survival time ranged from 87 to 420 days (median 212 d) that was not statistically significant from the cohort as a whole.
Overall survival (95% confidence interval (CI)) was 56% (40%, 69%) at 9 months. The comparison against the null hypothesis rate of 50% gave a 1-tailed p value of 0.23. A likelihood Bayesian approach suggested that there was a 77% chance that OS was >50% at 9 months. OS at 1 year was 0.35 (0.21, 0.49), and 0.17 (0.07, 0.30) at 2 years. Median survival was 9.5 months (7.5, 11.4) (Fig. 1). Median time to treatment failure (stopping temozolomide usually due to progression) was 168 d, with 88% of patients either dying or withdrawing from treatment by 1 year. Six patients had not died as at last follow up. These had been followed up for between 14 months and 3 years. All were last recorded as having had stable disease without additional treatment. There were five 2 year survivors with ages of 9, 12, 13, 16 and 18, the median age of 13.6 years being greater than the whole study median age of 8 years. One patient received two maintenance courses, one 6 courses and three all 12 courses. One had a partial response after radiotherapy, the other four had stable disease. There were no other unusual clinical or radiological features apart from age to predict that these children would be alive at 2 years.
4. Discussion
This study treating children and young people with DIPG with a prolonged dose dense temozolomide regimen in addition to focal radiotherapy showed no overall survival benefit over radiotherapy alone [4]. The median survival of 9.5 months is compatible with that reported in other trials in this disease [1,3,18–21]. This study also showed no improvement in 1 year survival, however the 2 year survival was 17% and better than the majority of reported series. This must be treated with caution as the numbers are small and may not be a true reflection of a definite survival advantage. Interestingly, the group of patients surviving for 2 years or longer has an older median age (13.6, range 9–18 years) than a typical DIPG patient. It is already known that children less than 3 years of age tend to have a better outlook [28], and it could be questioned as to whether there is another subgroup of older adolescent DIPG patients that may have a better outcome, possibly due to a difference in underlying biology. This raises important questions as to whether age stratification should be considered in DIPG trials to ensure an adequate sample size to identify possible age related prognostic factors.
The prolonged temozolomide regime was well tolerated with the main toxicity being haematological which resulted in three children withdrawing from treatment for this reason and 10 children having at least one dose reduction. There were no treatment related deaths and this regime did not have any greater toxicity than the 5/28 d regime [18–20]. Although, a prolonged administration schedule may make the drug more effective in maintaining depleted MGMT, our results did not support this treatment strategy. An on-going US trial with mandatory biopsy and biological analysis prior to treatment where temozolomide will be given in MGMT hypermethylated tumours, may help to answer this question [29,30].
A recently reported study in adults with recurrent high grade glioma, suggested a lower activity of this dose dense schedule compared to standard 5 days despite the strong theoretical rationale of using a prolonged dosing schedule [31]. It is possible although unlikely that high MGMT expression or other resistance mechanisms account for this lack of response but as biopsy was not mandated for this study this could not be tested. However in a study by Zarghooni [32] MGMT expression was not seen in DIPG patient material. Another explanation of lack of activity in DIPG may be poor drug delivery and it possible that temozolomide, although known to penetrate the central nervous system, fails to achieve sufficient tumour concentrations due to a greater integrity of the blood brain barrier in a typical DIPG. This is suggested by the lack of contrast enhancement in the majority of these tumours. A number of newer delivery strategies have been suggested such as convection-enhanced delivery of chemotherapeutic agents [33,34].
To date no novel therapeutic strategies have been shown to offer a survival benefit over and above standard radiotherapy (reviewed by Hargrave et al. [1] and Jansen et al. [3]). The reasons for this may include; lack of biological knowledge due to limited access to tumour samples, lack of DIPG preclinical models, tumour heterogeneity and poor drug delivery [35]. However, these issues are starting to be addressed and a better understanding of the molecular biology of DIPG is now emerging [32,36], with specific mutations in histone H3 characterising pontine glioma [37–39]. There is now a drive to develop appropriate cell culture and animal models to assist in developing DIPG specific targeted therapies.
The issue of biopsy of DIPG should now be reconsidered as part of future clinical trials as this would allow both prospective patient stratification/enrichment if a target exists and also retrospective analysis of patients based on biology to identify and explain subgroups of patients with longer survival that may benefit from a given therapy.
Unless further evidence becomes available, single agent temozolomide cannot be recommended for routine use in children with DIPG and its use in adolescents and in combination with other agents requires further evaluation. Future DIPG studies should consider possible emerging clinical and biological prognostic subgroups and be stratified and powered accordingly.
Conflict of interest statement
None declared.
Acknowledgements
We would like to thank the Brain Tumour Charity and CRUK for funding this trial. We would also like to thank Schering-Plough for providing the temozolomide. In addition we wish to thank the CCLG centres that recruited patients and most of all to the children and families who participated in this trial.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
S. Bailey, Email: simon.bailey@ncl.ac.uk.
A. Howman, Email: a.j.howman@bham.ac.uk.
K. Wheatley, Email: k.wheatley@bham.ac.uk.
D. Wherton, Email: d.wherton@bham.ac.uk.
N. Boota, Email: nazia.boota@gmail.com.
B. Pizer, Email: barry.pizer@alderhey.nhs.uk.
D. Fisher, Email: fishers@addenbrookes.nhs.uk.
P. Kearns, Email: p.r.kearns@bham.ac.uk.
S. Picton, Email: susan.picton@leedsth.nhs.uk.
F. Saran, Email: frank.saran@rmh.nhs.uk.
M. Gibson, Email: m.j.gibson@bham.ac.uk.
A. Glaser, Email: Adam.Glaser@leedsth.nhs.uk.
D.J.A. Connolly, Email: Daniel.Connolly@sth.nhs.uk.
D. Hargrave, Email: darren.hargrave@nhs.net.
References
- 1.Hargrave D., Bartels U., Bouffet E. Diffuse brainstem glioma in children: critical review of clinical trials. Lancet Oncol. 2006;7:241–248. doi: 10.1016/S1470-2045(06)70615-5. [DOI] [PubMed] [Google Scholar]
- 2.Qaddoumi I., Sultan I., Gajjar A. Outcome and prognostic features in pediatric gliomas: a review of 6212 cases from the surveillance, epidemiology, and end results database. Cancer. 2009;115:5761–5770. doi: 10.1002/cncr.24663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jansen M.H., van Vuurden D.G., Vandertop W.P., Kaspers G.J. Diffuse intrinsic pontine gliomas: a systematic update on clinical trials and biology. Cancer Treat Rev. 2012;38:27–35. doi: 10.1016/j.ctrv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Freeman C.R., Suissa S. Brain stem tumors in children: results of a survey of 62 patients treated with radiotherapy. Int J Radiat Oncol Biol Phys. 1986;12:1823–1828. doi: 10.1016/0360-3016(86)90325-1. [DOI] [PubMed] [Google Scholar]
- 5.Freeman C.R., Krischer J.P., Sanford R.A. Final results of a study of escalating doses of hyperfractionated radiotherapy in brain stem tumors in children: a Pediatric Oncology Group study. Int J Radiat Oncol Biol Phys. 1993;27:197–206. doi: 10.1016/0360-3016(93)90228-n. [DOI] [PubMed] [Google Scholar]
- 6.Kretschmar C.S., Tarbell N.J., Barnes P.D., Krischer J.P., Burger P.C., Kun L. Pre-irradiation chemotherapy and hyperfractionated radiation therapy 66 Gy for children with brain stem tumors. A phase II study of the Pediatric Oncology Group, Protocol 8833. Cancer. 1993;72:1404–1413. doi: 10.1002/1097-0142(19930815)72:4<1404::aid-cncr2820720441>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 7.Lewis J., Lucraft H., Gholkar A. UKCCSG study of accelerated radiotherapy for pediatric brain stem gliomas. United Kingdom Childhood Cancer Study Group. Int J Radiat Oncol Biol Phys. 1997;38:925–929. doi: 10.1016/s0360-3016(97)00134-x. [DOI] [PubMed] [Google Scholar]
- 8.Packer R.J., Boyett J.M., Zimmerman R.A. Hyperfractionated radiation therapy (72 Gy) for children with brain stem gliomas. A Childrens Cancer Group Phase I/II Trial. Cancer. 1993;72:1414–1421. doi: 10.1002/1097-0142(19930815)72:4<1414::aid-cncr2820720442>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Doz F., Neuenschwander S., Bouffet E. Carboplatin before and during radiation therapy for the treatment of malignant brain stem tumours: a study by the Societe Francaise d’Oncologie Pediatrique. Eur J Cancer. 2002;38:815–819. doi: 10.1016/s0959-8049(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 10.Jennings M.T., Sposto R., Boyett J.M. Preradiation chemotherapy in primary high-risk brainstem tumors: phase II study CCG-9941 of the Children’s Cancer Group. J Clin Oncol. 2002;20:3431–3437. doi: 10.1200/JCO.2002.04.109. [DOI] [PubMed] [Google Scholar]
- 11.Packer R.J., Krailo M., Mehta M. A phase I study of concurrent RMP-7 and carboplatin with radiation therapy for children with newly diagnosed brainstem gliomas. Cancer. 2005;104:1968–1974. doi: 10.1002/cncr.21403. [DOI] [PubMed] [Google Scholar]
- 12.Walter A.W., Gajjar A., Ochs J.S. Carboplatin and etoposide with hyperfractionated radiotherapy in children with newly diagnosed diffuse pontine gliomas: a phase I/II study. Med Pediatr Oncol. 1998;30:28–33. doi: 10.1002/(sici)1096-911x(199801)30:1<28::aid-mpo9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 13.Korones D.N., Fisher P.G., Kretschmar C. Treatment of children with diffuse intrinsic brain stem glioma with radiotherapy, vincristine and oral VP-16: a Children’s Oncology Group phase II study. Pediatr Blood Cancer. 2008;50:227–230. doi: 10.1002/pbc.21154. [DOI] [PubMed] [Google Scholar]
- 14.Bouffet E., Raquin M., Doz F. Radiotherapy followed by high dose busulfan and thiotepa: a prospective assessment of high dose chemotherapy in children with diffuse pontine gliomas. Cancer. 2000;88:685–692. doi: 10.1002/(sici)1097-0142(20000201)88:3<685::aid-cncr27>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 15.Broniscer A., Leite C.C., Lanchote V.L., Machado T.M., Cristofani L.M. Radiation therapy and high-dose tamoxifen in the treatment of patients with diffuse brainstem gliomas: results of a Brazilian cooperative study. Brainstem Glioma Cooperative Group. J Clin Oncol. 2000;18:1246–1253. doi: 10.1200/JCO.2000.18.6.1246. [DOI] [PubMed] [Google Scholar]
- 16.Sabharwal A., Waters R., Danson S. Predicting the myelotoxicity of chemotherapy: the use of pretreatment O6-methylguanine-DNA methyltransferase determination in peripheral blood mononuclear cells. Melanoma Res. 2011;20(6):502–508. doi: 10.1097/CMR.0b013e32832ccd58. [DOI] [PubMed] [Google Scholar]
- 17.Stupp R., Hegi M.E., Mason W.P. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 18.Chassot A., Canale S., Varlet P. Radiotherapy with concurrent and adjuvant temozolomide in children with newly diagnosed diffuse intrinsic pontine glioma. J Neurooncol. 2012;106:399–407. doi: 10.1007/s11060-011-0681-7. [DOI] [PubMed] [Google Scholar]
- 19.Cohen K.J., Heideman R.L., Zhou T. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: a report from the Children’s Oncology Group. Neuro Oncol. 2011;13:410–416. doi: 10.1093/neuonc/noq205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jalali R., Raut N., Arora B. Prospective Evaluation of Radiotherapy With Concurrent and Adjuvant Temozolomide in Children With Newly Diagnosed Diffuse Intrinsic Pontine Glioma. Int J Radiat Oncol Biol Phys. 2010;77:113–118. doi: 10.1016/j.ijrobp.2009.04.031. [DOI] [PubMed] [Google Scholar]
- 21.Sharp J.R., Bouffet E., Stempak D. A multi-centre Canadian pilot study of metronomic temozolomide combined with radiotherapy for newly diagnosed paediatric brainstem glioma. Eur J Cancer. 2010;46:3271–3279. doi: 10.1016/j.ejca.2010.06.115. [DOI] [PubMed] [Google Scholar]
- 22.Tolcher A.W., Gerson S.L., Denis L. Marked inactivation of O6-alkylguanine-DNA alkyltransferase activity with protracted temozolomide schedules. Br J Cancer. 2003;88:1004–1011. doi: 10.1038/sj.bjc.6600827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hegi M.E., Liu L., Herman J.G. Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol. 2008;26:4189–4199. doi: 10.1200/JCO.2007.11.5964. [DOI] [PubMed] [Google Scholar]
- 24.Baruchel S., Diezi M., Hargrave D. Safety and pharmacokinetics of temozolomide using a dose-escalation, metronomic schedule in recurrent paediatric brain tumours. Eur J Cancer. 2006;42:2335–2342. doi: 10.1016/j.ejca.2006.03.023. [DOI] [PubMed] [Google Scholar]
- 25.Furlong W.J., Feeny D.H., Torrance G.W., Barr R.D. The health utilities index (HUI) system for assessing health-related quality of life in clinical studies. Ann Med. 2001;33:375–384. doi: 10.3109/07853890109002092. [DOI] [PubMed] [Google Scholar]
- 26.Goodman R. The strengths and difficulties questionnaire: a research note. J Child Psychol Psychiatry. 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- 27.Case L.D., Morgan T.M. Design of phase II cancer trials evaluating survival probabilities. BMC Med Res Methodol. 2003;3:6. doi: 10.1186/1471-2288-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Broniscer A., Laningham F.H., Sanders R.P., Kun L.E., Ellison D.W., Gajjar A. Young age may predict a better outcome for children with diffuse pontine glioma. Cancer. 2008;113:566–572. doi: 10.1002/cncr.23584. [DOI] [PubMed] [Google Scholar]
- 29.Dunn J., Baborie A., Alam F. Extent of MGMT promoter methylation correlates with outcome in glioblastomas given temozolomide and radiotherapy. Br J Cancer. 2009;101:124–131. doi: 10.1038/sj.bjc.6605127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brandes A.A., Franceschi E., Tosoni A. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer. 2009;27(8):1275–1279. doi: 10.1002/cncr.24406. [DOI] [PubMed] [Google Scholar]
- 31.Brada M., Stenning S., Gabe R. Temozolomide versus procarbazine, lomustine, and vincristine in recurrent high-grade glioma. J Clin Oncol. 2010;28:4601–4608. doi: 10.1200/JCO.2009.27.1932. [DOI] [PubMed] [Google Scholar]
- 32.Zarghooni M., Bartels U., Lee E. Whole-genome profiling of pediatric diffuse intrinsic pontine gliomas highlights platelet-derived growth factor receptor alpha and poly (ADP-ribose) polymerase as potential therapeutic targets. J Clin Oncol. 2010;28:1337–1344. doi: 10.1200/JCO.2009.25.5463. [DOI] [PubMed] [Google Scholar]
- 33.Barua N.U., Lowis S.P., Woolley M., O’Sullivan S., Harrison R., Gill S.S. Acta Neurochir; Wien: 2013. Robot-guided convection-enhanced delivery of carboplatin for advanced brainstem glioma. [DOI] [PubMed] [Google Scholar]
- 34.Anderson R.C., Kennedy B., Yanes C.L. Convection-enhanced delivery of topotecan into diffuse intrinsic brainstem tumors in children. J Neurosurg Pediatr. 2013;11:289–295. doi: 10.3171/2012.10.PEDS12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hargrave D. Pediatric diffuse intrinsic pontine glioma: can optimism replace pessimism? CNS Oncol. 2012;1:137–1148. doi: 10.2217/cns.12.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paugh B.S., Broniscer A., Qu C. Genome-wide analyses identify recurrent amplifications of receptor tyrosine kinases and cell-cycle regulatory genes in diffuse intrinsic pontine glioma. J Clin Oncol. 2011;29:3999–4006. doi: 10.1200/JCO.2011.35.5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu G., Broniscer A., McEachron T.A. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat Genet. 2012;44:251–253. doi: 10.1038/ng.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khuong-Quang D.A., Buczkowicz P., Rakopoulos P. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 2012;124:439–447. doi: 10.1007/s00401-012-0998-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schwartzentruber J., Korshunov A., Liu X.Y. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature. 2012;482:226–231. doi: 10.1038/nature10833. [DOI] [PubMed] [Google Scholar]