Abstract
Taking into account that oxidative stress is among the factors causing cancer-related death; chemoprevention which consists in using antioxidant substances such as phenolics could prevent cancer formation and progression. In the present study, phenolic contents and antioxidant activities of methanolic extracts from the halophyte Tamarix gallica shoots were determined. Moreover, the anticancer effect of this species on human colon cancer cells and the likely underlying mechanisms were also investigated. Shoot extracts showed an appreciable total phenolic content (85 mg GAE/g DW) and a high antioxidant activity (IC50 = 3.3 μg/ml for DPPH test). At 50 and 100 μg/ml, shoot, leaf, and flower extracts significantly inhibited Caco-2 cell growth. For instance, almost all plant part extracts inhibited cell growth by 62 % at the concentration 100 μg/ml. DAPI staining results revealed that these extracts decrease DNA synthesis and confirm their effect on Caco-2 cells proliferation, principally at 100 μg/ml. More importantly, cell mitosis was arrested at G2/M phase. The changes in the cell-cycle-associated proteins (cyclin B1, p38, Erk1/2, Chk1, and Chk2) are correlated with the changes in cell cycle distribution. Taken together, our data suggest that T. gallica is a promising candidate species to be used as a source of anticancer biomolecules.
Keywords: Tamarix gallica, Phenolic contents, Antioxidant activities, Caco-2 cells, Anticancer effect, Cell cycle arrest
Introduction
Colorectal cancer is one of the major causes of cancer-related mortality in the populations of developed countries (Mao et al. 2011). Recently, research has been focusing on the cell-cycle arrest analysis as a major approach for the cancer eradication (Cárdenas et al. 2008; Liu et al. 2008). The cell-cycle progression is regulated by a number of Cdk/cyclin protein family which characterize the checkpoint controls of the cell division (Murray 2004; Wu et al. 2006). Chk1 is an essential kinase, regulated by Atr and required for the G2/M DNA damage checkpoint (Cho et al. 2005). It has been documented that Chk1 also plays a role in inducing the S-phase delay in response to DNA damage. In contrast, despite the marked increase in Chk2 activation, this kinase is not required for cell death activation (Wang et al. 2002). Cells recognize and respond to extracellular stimuli by engaging specific intracellular programs such as the signaling cascade that leads to activation of the mitogen-activated protein kinases (MAPKs). All eukaryotic cells possess multiple MAPK pathways which regulate diverse cellular activities from gene expression, mitosis, and metabolism to motility, survival, apoptosis and differentiation. The importance of MAPK pathways in cell proliferation and death is highlighted by the observation that regulation of these kinase cascades can result in cell transformation and cancer (Kennedy et al. 2007). Recently, three groups of MAPKs, which are responsible of the extracellular stimuli response cascade, have been characterized in cells as extracellular signal-regulated kinases (Erks1–5), c-Jun amino-terminal kinases (JNKs 1, 2, and 3) and p38 isoforms (Kennedy and Davis 2003). The extracellular signal-regulated kinase (Erk) pathway primarily directs a program of proliferation and survival, while the cJun NH2-terminal kinase (JNK) pathway can promote either proliferation or apoptosis (Kennedy and Davis 2003). The activation of Erk and JNK pathways can lead to the increase of proliferation and survival. Loss of JNK in some instances may also promote tumorigenesis (Kennedy and Davis 2003). In contrast, the p38 MAPK pathway is implicated in suppression of tumorigenesis since it can (1) inhibit cell growth by decreasing the expression of cyclin D (Lavoie et al. 1996), (2) inhibit the activity of Cdc25 phosphatases (Manke et al. 2005), and (3) engage the p16/Rb and p19ARF/p53 tumor suppressor pathways (Bulavin et al. 2002, 2004). Consequently, the p38 MAPK pathway is activated upon cellular stress and often engages pathways that can block proliferation (cell cycle arrest) or promote apoptosis (Bulavin and Fornace 2004).
Previous works reported that cell cycle progress is controlled by the balance between the reactive oxygen species (ROS) biosynthesis and the antioxidant system (Conour et al. 2004; Sarsour et al. 2009). The overproduction of ROS results in oxidative stress, a harmful process which can be an important mediator of cell structures damage and consequently initiation of various diseases such as cancer (Gülcin et al. 2004). Developing agents that can alter cell cycle events became an attractive alternative in cancer therapy and prevention (Malumbres and Barbacid 2007; Roberts and Der 2007). With this respect, studies show an increased global interest in traditional systems of medicine and herbal products over the past decades. The natural antioxidant substances are regarded as one of the most attractive alternatives to fight disease states issuing from oxidative stress (Pachón-Peña et al. 2009). The performance of halophytes growing in harsh climatic conditions (higher salinity and drought) is often ascribed to their acquired defense in relation with their contents in antioxidant substances. For instance, the powerful antioxidant activity of medicinal halophyte Tamarix gallica L. organs (leaves and flowers) which contain several phenolic compounds was mentioned (Ksouri et al. 2009). The inhibition of growth proliferation in cancer cells has been shown using several cancer cell lines with different phenolics by the inhibitory effect on cancer invasion and metastasis (Cai et al. 2009; Yang et al. 2009). Curcumin, resveratrol, and their related derivatives are the most studied compounds in this topic. Gallic acid, chlorogenic acid, caffeic acid, carnosol, capsaicin, 6-shogaol, 6-gingerol, and their corresponding derivatives are also suggested to be active members in the phenolic family on anti-invasion and anti-metastasis (Weng and Yen 2012). Besides, flavonoids show a remarkable spectrum of biochemical and pharmacological activities suggesting that they significantly affect basic cell functions such as growth, differentiation and/or programmed cell death (apoptosis). Although there is evidence that a high dietary intake of flavonoids could be associated with low cancer prevalence in humans, the cytotoxicity of some phytochemicals from the leaves of T. nilotica to human promyelocytic leukemia and human squamous carcinoma cell lines have been reported (Orabi et al. 2010). In addition, T. gallica extracts prevent the progression of liver cancer by restoring the level of antioxidant enzymes in rat liver (Sehrawat and Sultana 2006). Until now, there is no available information regarding the anticancer effects of T. gallica on human colon cancer.
Therefore, the present study focuses on the anticancer effect of the halophyte T. gallica extracts against Caco-2 cells in relation with phenolic contents and antioxidant capacities. Moreover, to better understand the effects of plant extracts on G2/M-phase delay and inhibition of cancer cell growth, the possible underlying mechanisms involving Erk1/2 and p38 MAPK on G2/M cell cycle arrest was investigated.
Materials and methods
Sampling and sample preparation
T. gallica (Tamaricaceae) shoots, leaves and flowers were harvested at full flowering stage from the sebkha of El Kelbia locality (20 km north-east Kairouan; superior semi-arid bioclimatic stage; mean annual rainfall: 400 mm) in May 2009. The harvested organs were rinsed with distilled water, left at room temperature for 7 days in the dark and grinded to fine powder. Extracts were obtained by soxhlet extraction of 20 g dry powder in 200 ml of 80 % methanol for 12 h. Extracts were kept for 24 h at 4 °C, filtered through a Whatman no. 4 filter paper, and evaporated under vacuum. Then, they were stored at 4 °C until analysis (Falleh et al. 2008). For the anticancer effect analysis, 10 g of powder were added to 100 ml 80 % methanol and allowed to stand for 1 week in obscurity at room temperature and then filtered through Millipore filter (0.2 μm). After drying, powder was dissolved in DMSO to get 5 % as stock concentration. Extracts were stored at −80 °C until analysis.
Cell maintenance
Caco-2 colon cancer cells isolated from a 72 year-old Caucasian male were grown in Dulbecco’s Modified Eagle Medium (Sigma, St. Louis, MO, USA) supplemented with 10 % heat-inactivated fetal bovine serum (FBS, Sigma), 1 % non essential amino acids (Cosmo Bio Co, LDT, Tokyo, Japan) and 1 % penicillin (5,000 IU/ml)-Streptomycin (5,000 μl/ml) solution (ICN Biomedicals, Irvine, CA, USA) at 37 °C in a 5 % CO2 atmosphere. The medium was replaced every 2 days after checking the cell growth under a microscope.
Quantification of phenolic fractions
The amount of total phenolics in methanolic extracts was determined with the Folin–Ciocalteu reagent (Dewanto et al. 2002). An aliquot of 125 μl of diluted extract was added to 500 μl of distilled water and 125 μl of the Folin–Ciocalteu reagent. The mixture was shaken, before adding 1,250 μl of Na2CO3 (7 %) and adjusting with distilled water to a final volume of 3 ml. After incubation for 90 min at 23 °C in the dark, the absorbance versus prepared blank was read at 760 nm. Total phenolic content was expressed as mg GAE (Gallic Acid Equivalent)/g DW (Dry Weight) using a calibration curve with gallic acid, ranged from 0 to 400 μg/ml. All samples were analysed in triplicate.
The measurement of flavonoid content in T. gallica shoots was based on the method of Dewanto et al. (2002). An aliquot of diluted sample or standard solution of (+)-catechin was added to 75 μl of 5 % NaNO2 solution, and mixed for 6 min. Then, 0.15 ml of 10 % AlCl3 solution was added. After 5 min, 0.5 ml of 1 N NaOH was added. The final volume was adjusted to 2.5 ml with distilled water and thoroughly mixed. Absorbance of the mixture was determined at 510 nm against the blank where the sample was omitted. Total flavonoid content was expressed as mg catechin per gram of DW (mg CE ((+)-catechin Equivalent)/g DW), through the calibration curve of (+)-catechin, ranging from 0 to 400 μg/ml. All samples were analysed in triplicate.
The content of condensed tannin was determined according to the method of Sun et al. (1998). To 50 μl of properly diluted sample, 3 ml of 4 % vanillin solution in methanol and 1.5 ml of concentrated hydrochloric acid were added. The mixture was allowed to stand for 15 min, and the absorption was measured at 500 nm against 80 % methanol as a blank. The amount of total condensed tannins is expressed as mg (+)-catechin/g DW. The calibration curve range of catechin was established between 0 and 400 μl g/ml. All samples were analysed in triplicate.
Antioxidant assays
Evaluation of total antioxidant capacity
An aliquot (0.1 ml) of sample was combined to 1 ml of reagent solution (0.6 M sulfuric acid, 28 mM sodium phosphate and 4 mM ammonium molybdate). The tubes were incubated in a thermal block at 95 °C for 90 min. Then, the mixture was cooled at room temperature and the absorbance of each solution was measured at 695 nm (Anthelie Advanced 2, SECOMAM, ALes, France) against a blank. The total antioxidant capacity was expressed as mg GAE/g DW. The calibration curve range was established between 0 and 500 μg/ml. All samples were analysed in triplicate.
DPPH (1,1-diphenyl-2-picrylhydrazyl) radical-scavenging activity
DPPH quenching ability of plant extracts was measured according to Hatano et al. (1988). One ml of the extract at different concentrations was added to 0.25 ml of a DPPH methanolic solution (2 mmol/l). The mixture was shaken vigorously and left standing at room temperature in the dark. The antiradical activity was determined by the decrease in absorbance at 517 nm against a blank of 80 % methanol. The DPPH radical-scavenging activity was reported after 30 min reaction time. The ability to scavenge the DPPH radical was calculated using the following equation:
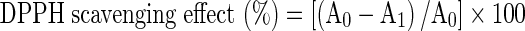 |
1 |
where A0 is the absorbance of the control at 30 min, and A1 is the absorbance of the sample at 30 min. All samples were analysed in three replications.
Iron reducing power
The iron(III) reductive capacity of the extract was assessed as described by Oyaizu (1986). Briefly, 1 ml of extract was mixed with 2.5 ml phosphate buffer (0.2 mol/l, pH 6.6) and 2.5 ml (1 g/100 ml) K3Fe (CN)6 solution. After 20 min at 50 °C, 2.5 ml (10 g/100 ml) trichloroacetic acid were added and the mixture was centrifuged for 10 min at 650×g. Finally, 2.5 ml aliquot were mixed with 2.5 ml ultra-pure water and 0.5 ml (0.1 g/100 ml) FeCl3 and the absorbance was recorded at 700 nm. A higher absorbance indicates a higher reducing power. EC50 value (mg/ml) is the effective concentration at which the absorbance was 0.5 for reducing power and was obtained from linear regression analysis.
Anti-proliferative effect by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay
The MTT assay (performed according to Mosmann 1983) was performed by seeding Caco-2 cells in a 96-well plate at the concentration of 2 × 104 cells/ml in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % fetal bovine serum. Cells were kept under 5 % CO2 at 37 °C. Caco-2 cells were treated with different concentrations of plant extracts ranging from 0.01 to 100 μg/ml. After 72 h incubation, 10 μl of MTT solution (5 mg/ml) were added and the cells were further incubated for 24 h. The MTT reduced to formazan was dissolved with 10 % SDS solution and then the cell viability was determined by measuring the absorbance at 570 nm by spectrophotometer (Dainnipon, Kyoto, Japan).
Cell cycle and cell viability analysis
Caco-2 cells (2 × 104 cells/ml) were exposed to 100 μg/ml of T. gallica extracts for 72 h and both suspended and attached cells were collected. The cells were washed with PBS, fixed with 70 % ice cold ethanol and stored at −20 °C until analysis. Before measurement, ethanol was removed; the cells were suspended in 500 μl cell cycle reagent (Guava technologies, Hayward, CA, USA) and incubated in the dark at room temperature for 30 min. Cell cycle distribution and cell viability analysis were determined by Guava flow cytometry (Guava Technologies).
DAPI staining
After 72 h treatment with extracts of T. gallica shoots, flowers and leaves (100 and 200 μg/ml), Caco-2 cells at the concentration of 2 × 104 cells/ml were washed two times with PBS. Then, the cells were fixed with 3.7 % formaldehyde in PBS for 10 min at room temperature. After washing two times with PBS and staining with DAPI (4′,6-diamidino-2-phenylindole) solution, cells were analysed using fluorescence microscopy.
Western blotting
Caco-2 cells (2 × 104 cells/ml) were incubated with 100 μg/ml shoot, flower and leaf extracts of T. gallica during 72 h. Then, cells were washed in PBS and lysed by RIPA buffer (Sigma Aldrich Co) with protease inhibitor cocktail (Sigma Aldrich Co). The mixture was centrifuged at 12,000 × g for 20 min at 4 °C. The protein-containing supernatant was kept and the quantification of the proteins was performed using Plus One 2D Quant kit (GE Healthcare, Piscataway, NJ, USA). Proteins (20 μg) were resolved on 12 % sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membrane using iBlot dry blotting system (Invitrogen/Life Technologies, Carlsbad, CA, USA). After blocking with 5 % non-dry fat milk, membrane was incubated at 4 °C overnight under shaking with primary antibodies anti-p38, β-actin (Sigma Aldrich Co), phospho-Erk1/2, Erk1/2, pp38, Cyclin B1, pChk1, Chk1, pChk2 and Chk2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA). The bands were detected by horseradish peroxidise-conjugated secondary antibodies using enhanced chemiluminescence system ECL (Amersham Biosciences/GE Healthcare).
Statistical analysis
For all plant parameters, all samples were analysed in three replications. Data are shown as mean ± SD. A one-way analysis of variance (ANOVA) using the post hoc analyse with Duncan’s test was carried out to test any significant differences at p < 0.05.
Results and discussion
Phenolic content and antioxidant activities of shoot extract
Based on the absorbance values, results of the colorimetric analysis of phenolic fractions are given in Table 1. The amount of total polyphenols was high in shoots (85 mg GAE/g DW). In addition, shoot methanolic extracts exhibited an important amount of flavonoids and condensed tannins, being about 10 and 7 % of total polyphenols, respectively. A previous study showed a very rich amount of phenolics in T. gallica leaf and flower extracts (Ksouri et al. 2009). Separately quantified, these organs displayed significant difference. The amount of total polyphenols was higher in flowers (135.3 mg GAE/g DW) than in leaves (34.4 mg GAE/g DW). The same tendency was observed for flavonoid content, being about three times more important in flowers than in leaves. Conversely, leaf extracts contained more condensed tannins than flower ones.
Table 1.
Phenolic contents and antioxidant activities of T. gallica shoot extracts related to the flowering period. The antioxidant activities of extracts were evaluated using total antioxidant activity, antiradical activity as well as the capacity of the extract to reduce the Fe3+
| T. gallica shoots | |
| Total phenolic content (mg GAE/g DW) | 85 |
| Flavonoid content (mg CE/g DW) | 8.12 |
| Tannin content (mg CE/g DW) | 12.14 |
| Total antioxidant activity (mg GAE/g DW) | 278.71 |
| Antiradical activity (IC50 values in μg/ml) | 3.3 |
| Reducing power (EC50 values in μg/ml) | 124 |
Concerning antioxidant activity, since there is no standardized method for the determination of antioxidant properties of certain foods and plants, the use of more than one method for evaluating antioxidant capacity is highly recommended. In this study, three different antioxidant assays (total antioxidant activity, DPPH, and Fe-reducing power tests) were applied for the evaluation of antioxidant capacity of T. gallica shoots (Table 1). Results showed that shoot extracts have strong total antioxidant activity (278 mg GAE/g DW) and antiradical ability to quench DPPH radical (IC50 = 3.3 μg/ml), together with an appreciable Fe-reducing power (EC50 = 124 μg/ml). This strong antioxidant activity of T. gallica might be attributed to the presence of phytochemicals such as phenolic compounds (Falleh et al. 2008). Recent studies showed that many flavonoids and related polyphenols contribute significantly to the total antioxidant activity of many fruits such as red grape (Negro et al. 2003), vegetables (Luo et al. 2002) and medicinal plants (Bourgou et al. 2008). Concerning leaf and flower organs, Ksouri et al. (2009) found better results notably for DPPH test and Fe-reducing power of flower extracts (IC50 and EC50 values equal to 2 and 45.2 μg/ml, respectively). This good antioxidant activity may be attributed to the specific presence of natural antioxidants such as phenolic compounds in T. gallica organs (Lee et al. 2008). Consequently, shoots, leaves and flowers were separately considered in the remaining investigation. In addition, the antioxidant activities against ABTS (2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid) or DPPH were correlated with the concentration, chemical structures, and polymerization degrees of organ antioxidants (Oszmianski et al. 2007).
Inhibitory effects of T. gallica on colon cancer cells
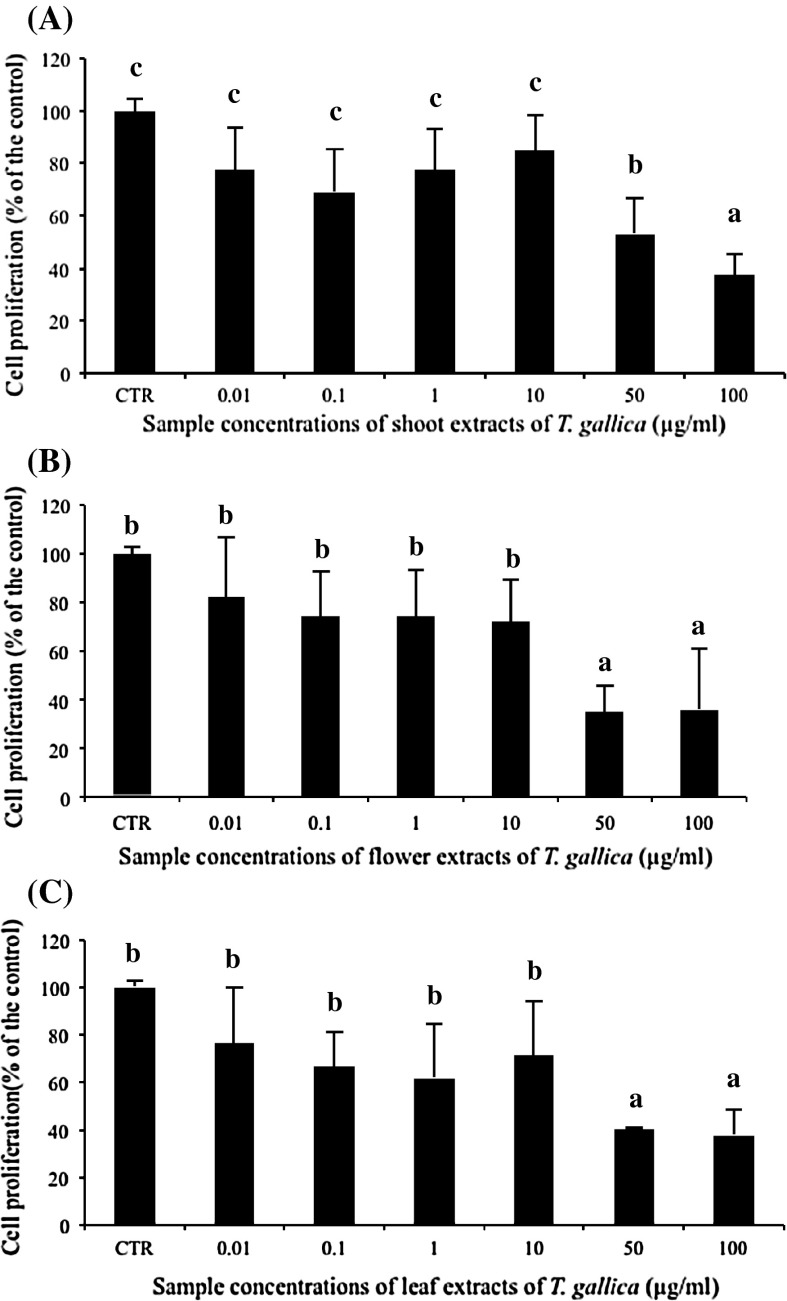
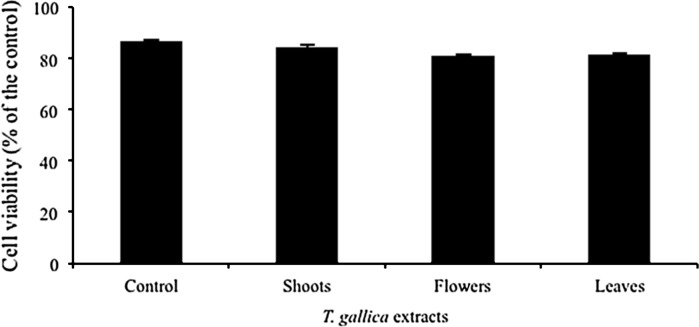
The anti-proliferative effect of T. gallica shoots, flowers and leaves were investigated against Caco-2 cells (Fig. 1). All methanolic extracts inhibited colon cancer cells in a dose-dependent manner. At the lower concentrations (0.01–10 μm/ml), statistically no significant decrease of cell growth was observed with these different extracts. However, at the concentrations of 50 and 100 μg/ml, all plant extracts significantly inhibited the growth of Caco-2 cells as compared to the control ones (Fig. 1). For instance, 50 μg/ml inhibited cell growth by 47, 64 and 59 %, respectively for shoot (Fig. 1a), flower (Fig. 1b) and leaf (Fig. 1c) extracts, whereas the application of extracts at 100 μg/ml inhibited the growth of Caco-2 cells at the same extent, independently from the plant organ (Fig. 1). This result incited us to look for another in vitro test to study the effect of extracts on Caco-2 cancer cell viability using the same Guava equipment. Therefore, all organ extracts were applied at the highest concentration (100 μg/ml) and results revealed that they have no significant effect on Caco-2 cancer cell (Fig. 2). This is in agreement with other studies on the interesting anticancer activity of Tamarix species (Sehrawat and Sultana 2006; Orabi et al. 2010). For instance, T. nilotica leaves showed anticancer capacity against human leukemia and squamous carcinoma cells. Besides, T. gallica shoots caused a marked inhibition of thioacetamide-induced hepatotoxicity, oxidative damage and early tumor promotion related events in male Wistar rat liver. Anticancer substances present in food phytochemicals are regarded as one of the most attractive strategies for cancer control, since certain natural compounds present in medicinal plants have efficiently inhibited tumor development in various human organs (Okita et al. 2002; Singh et al. 2004).
Fig. 1.
Measurements of cell proliferation using MTT assay in Caco-2 colon carcinoma cell line treated with different T. gallica plant parts. Cells at 2 × 104 cells/ml were cultured in the absence (control) or in the presence of 0.01, 0.1, 1, 10, 50 or 100 μg/ml of T. gallica for 72 h. The tested plant parts such as shoots (a), flowers (b) and leaves (c) were collected during the flowering stage. Values represent the means of three independent experiments ± standard deviation. The bars marked with different letters are significantly different at p < 0.05
Fig. 2.
Viability of Caco-2 cancer cells after treatment using 100 μg/ml of T. gallica extracts. Plant parts (shoots, flowers and leaves) were collected during the flowering stage. Guava equipment was employed during this study
Free radicals play a significant role in the biotransformation of chemical carcinogens and promotion stage of cancer. It implies that if any agent is potent enough to prevent the generation of these radicals, it will also be capable of delaying/preventing the occurrence of initiation, promotion and ultimately the development of cancer. For that, phenolic compounds as powerful antioxidant agents, are proven to prevent cancer disease. In this context, Jin et al. (1999) demonstrated that the two quercetin glycoside derivatives isolated from leaves of Ledum palustre were found to be cytotoxic against human mouth epidermal carcinoma. Moreover, Kuntz et al. (1999) have screened more than 30 flavonoids for their effects on cell proliferation and cytotoxic potential in the human colon cancer cell lines Caco-2, displaying features of small intestinal epithelial cells, and HT-29, resembling colonic crypt cells. All flavonoids showed antiproliferative activity in a dose-dependent manner. There was no obvious structure–activity relationship in the antiproliferative effects either on basis of the subclasses (i.e., isoflavones, flavones, flavonols, flavanones) or with respect to kind or position of substituent within a class. For example, the concentrations that caused 50 % inhibition of cell proliferation when compared to controls ranged between 40 and 200 μM. In Caco-2 cells, the flavones (baicalein, puerarin, kaempferide, tangeretin), the flavonol (fisetin), and flavanone were found to be the most potent compounds with EC50 values of approximately 60 μM. Whereas baicalein, tangeretin, fisetin, and flavanone showed strong growth inhibition of HT-29 cells, the flavones (puerarin and kaempferide) required concentrations was higher than 100 μM to promote 50 % growth inhibition in this cell line. Also for the flavone (diosmetin), EC50 values in HT-29 cells were twice as high as those obtained in Caco-2 cells (Kuntz et al. 1999).
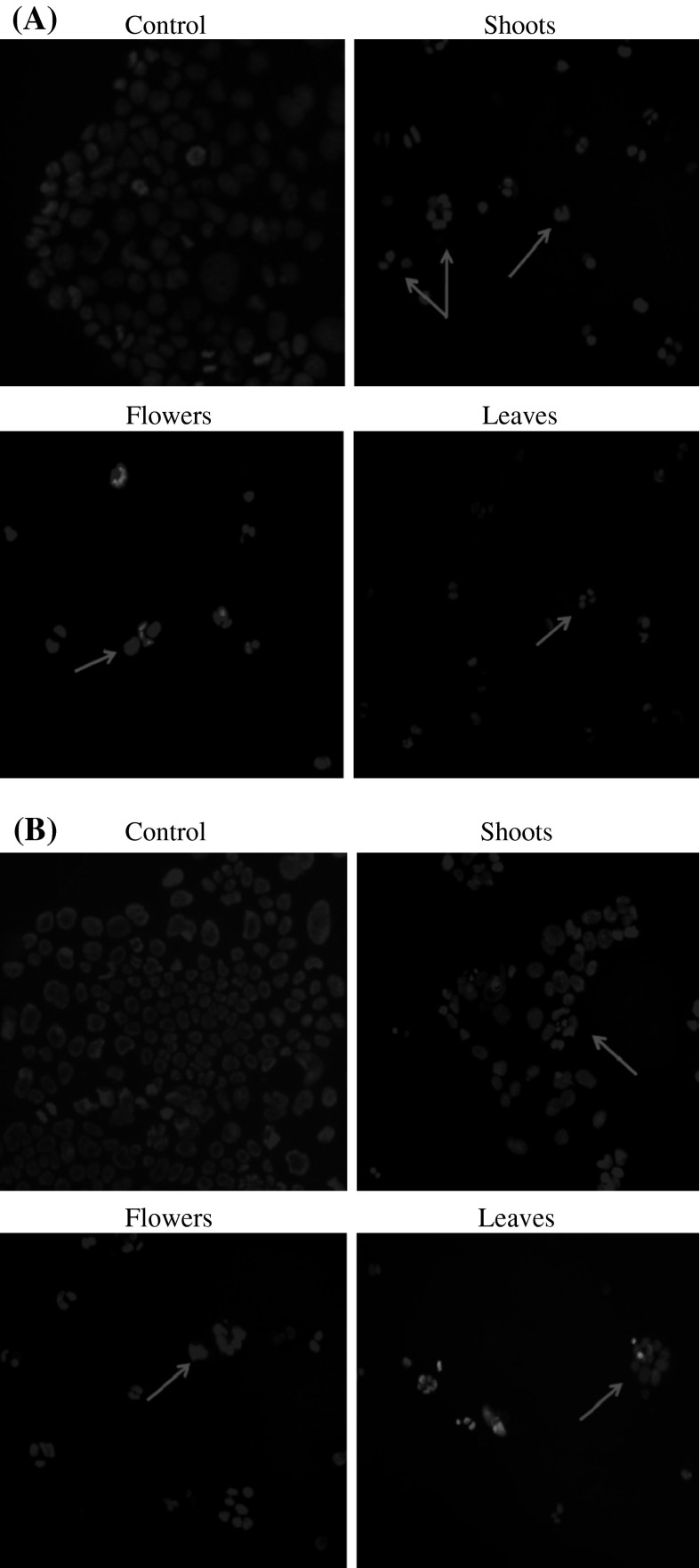
DAPI staining results, shown in the Fig. 3a, b, demonstrated that methanolic extracts at 100 and 200 μg/ml (Fig. 3a and b) exhibited significant effect on the DNA biosynthesis as compared to the control ones. The decease of DNA biosynthesis illustrates that the number of cells during treatment diminished and consequently proved the antiproliferative effect on Caco-2 cells. In this context, the study of Um et al. (2010) on Glehnia littoralis F. Schmidt ex Miquel (Apiaceae), a salt-tolerant plant, showed antiproliferative activity against HT-29 human colon cancer cells. They suggested that coumarines and polyacetylenes may be at least in part responsible for the antiproliferative activity displayed by crude extracts of the halophyte G. littoralis. To further characterize whether the antiproliferative activities of plant fractions on the Caco-2 cells were related to the induction of apoptosis or not, the presence of chromatin condensation was analyzed by fluorescent microscopy using the DNA-binding fluorescent dye DAPI. In our study, the fluorescence by DAPI staining did not show an apoptosis effect. Further experiments, like cell cycle distribution, are needed to clarify the mechanism of anticancer activities of those compounds.
Fig. 3.
DAPI staining of Caco-2 cancer cells treated with T. gallica. Cells were incubated for 72 h with T. gallica plant extracts (shoots, flowers and leaves) at a concentration of 100 (a) and 200 μg/ml (b). After incubation, the nuclear morphologies of the cells were examined using a fluorescent DNA-binding agent, DAPI. DNA was analysed using fluorescence microscopy. Arrows indicate mitotic cells with condensed chromosomes and no nuclear membrane. All photographs were taken at 200× magnification
Effect of T. gallia on cell cycle arrest using flow cytometry
The effect of shoot, flower and leaf extracts of T. gallica at the highest concentration (100 μg/ml) was investigated in order to demonstrate the suppressive mechanisms of these extracts on colon cancer cells (Table 2). Results showed that all sample extracts displayed the same distribution of the cell cycle. The percentage of cells in G0/G1 and S phases decreased under treatment. In contrast, DNA flow cytometric analysis indicated that treatment with T. gallica extracts induced G2/M arrest in colon cell lines. Shoot, flower and leaf extracts treatment (100 μg/ml) during 72 h produced maxima G2/M arrests of 47.3, 48.1 and 47.1 % in Caco-2 cells, respectively, in comparison with control cells (39 %). Sub-G0 was not detected after the treatment conditions. In addition, apoptosis was not found by flow cytometry in colon cell lines treated with any of the three extracts up to 72 h. As shown in a recent study (Wang et al. 2000), apigenin was a strong growth-inhibitory flavonoid in three different colon carcinoma cell lines (SW480, HT-29, and Caco-2) and selectively blocked cell-cycle progression at the G2/M phase. In this context, Ksouri et al. (2009) showed that flower and leaf extracts are rich in flavonoids as apigenin which may be responsible for the induction of G2/M arrest.
Table 2.
Effect of different organ extracts of T. gallica on the cell cycle arrest. Cells were treated with shoot, flower and leaf extracts of T. gallica at a concentration of 100 μg/ml in order to check the cell cycle distribution. Cells were incubated in the absence (control) or the presence of plant extracts during 72 h and then analysed by flow cytometry
| Control | Shoots | Flowers | Leaves | |
|---|---|---|---|---|
| G0/G1 | 42.4 ± 7.07 | 37.8 ± 2.12 | 36.6 ± 2.83 | 37.25 ± 6.86 |
| S | 17.65 ± 2.05 | 13.8 ± 2.4 | 14.45 ± 2.33 | 14.05 ± 2.9 |
| G2/M | 38.95 ± 5.02 | 47.3 ± 4.38* | 48.1 ± 4.95* | 47.1 ± 8.91* |
| Sub-G0 | 1.05 ± 0.07 | 1.1 ± 0.14 | 0.85 ± 0.21 | 1.55 ± 0.92 |
The treatment time was 72 h. Data of three independent experiments are presented as mean ± SD
* Statistical significance (p < 0.05) between treated and control cells
Changes in cell cycle-related proteins in colon cancer cells treated with T. gallica extracts
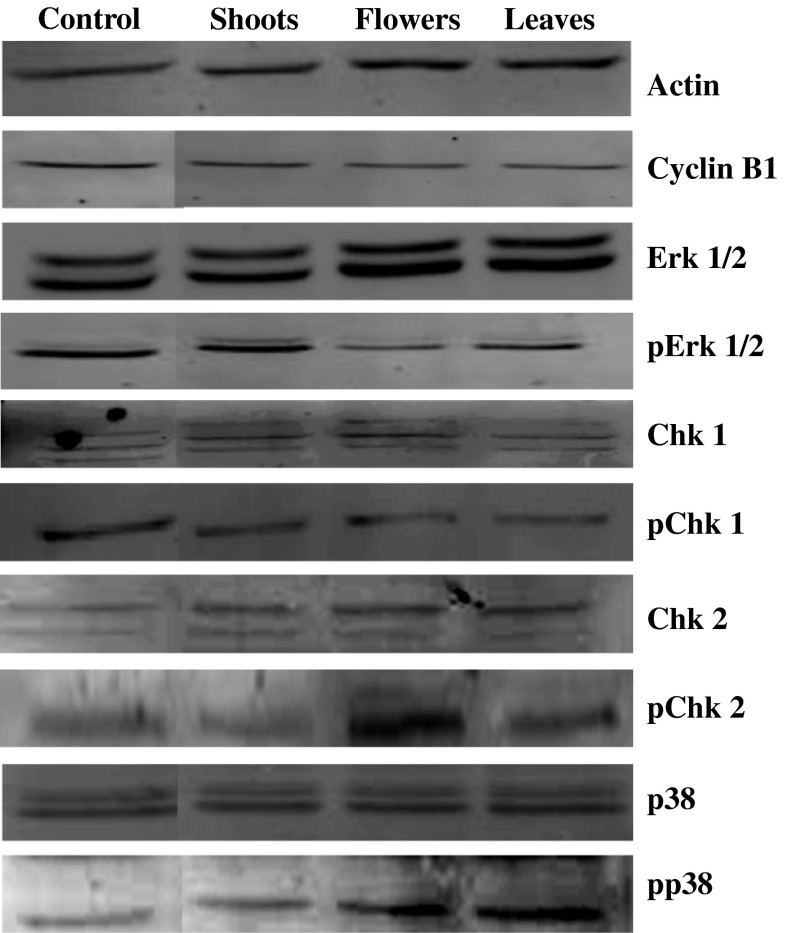
The present work showed a cell cycle arrest in the G2/M phase. In this context, and to better explain the mechanism of G2/M arrest, the expressions of regulatory proteins such as Erk1/2 and p38 MAP kinases, cyclin B1 and checkpoint kinases (Chk1 and Chk2) were analysed. Flower, leaf, and to a lesser extent shoot extracts influenced the levels of these regulatory proteins in different manners (Fig. 4). For instance, the expressions of pErk1/2 and pChk1 proteins were decreased while Erk1/2, Chk1, pChk2 and pp38 levels were enhanced in treated cells mainly by flower and leaf extracts. Moreover, a slight dwindle of cyclin B1 level was observed in Caco-2 cells. These data suppose that the complex of dephosphorylated CDC2 and cyclin B1, as a key factor in the G2/M arrest, was affected. Furthermore, data showed that the ratio pErk/total Erk in cells treated by flower and leaf extracts dropped off as a consequence of the inhibition of the phosphorylated Erk protein expression (activated form). This decrease has an effect on the antiproliferative mechanism process, especially on the inactivation of the Chk1 and cyclin B1 proteins leading to the G2/M cell arrest. In fact, the activity of MAP kinases is tightly controlled by a dual phosphorylation (Thr-183 and Tyr-185) which leads to over than 1,000-fold activation of Erk1 and Erk2 (Canagarajah et al. 1997). Several studies have shown that in many human cancers the activated Erk1/2 promotes the growth of tumor cells. In this context (Dumesic et al. 2000), showed that Erk1/2-depleted epidermal cells exhibit a G2/M cell cycle arrest with decreased expression of the transcription factor c-Fos and its target gene cyclin B1. Hence, the effect of T. gallica extracts on human colon cancer cells and the G2/M cell cycle arrest could be explained in this way. However, the high growth inhibition is associated with the increase of the pp38 (activated form) using flower and leaf extracts treated cells. According to these data, it has been shown that inhibition of the p38 kinase induces growth suppression of human ER-negative breast cancer cells (Chen et al. 2009). In the case of shoot extracts, the effect on some MAP kinases was not similar. In fact, the Erk1/2 activation was not suppressed and the activation of p38 was not observed. However, this extract also exhibited cell growth arrest and growth inhibition. The phosphorylation of Chk2 protein in cells treated with shoot extract is well correlated with observed G2/M arrest and growth inhibition of Caco-2 cells. Castedo et al. (2004) showed that Chk2 inhibition reduced the doxorubicin-induced G2 blocking and concomitantly increased the frequency of apoptotic cells in HCT116 colon cancer cells. The action of flower and leaf extracts was more significant as compared to shoots. This can be explained by the phenomenon of steric crowding of molecules in shoot parts. This observable fact was decreased using separated organs (flowers and leaves).
Fig. 4.
Western blotting showing changes of the levels of G2/M phase arrest associated proteins in Caco-2 cells after exposure to T. gallica shoots, flowers and leaves. Caco-2 cells (2 × 104 cells/ml) were incubated with 100 μg/ml of shoot, flower and leaf extracts of T. gallica during 72 h
In conclusion, our data revealed that all tested plant organ extracts significantly inhibited the growth of cancer cells, particularly flower and leaf extracts were the most active inhibitors of cell growth. Stopping cell growth was due to the cell cycle arrest at G2/M phase. The apoptosis was not clear after 72 h treatment by both morphological observation of DNA with DAPI staining and determination of sub-G0 phase by flow cytometry. We showed that T. gallica antioxidants induced a significant growth inhibition and selectively blocked cell-cycle progression at the G2/M phase, yet without causing apoptosis and necrosis. This fact may partially contribute to the well-known effect of cancer protection by plant antioxidants.
Acknowledgments
This study partially was supported by the JICA-JST Science and Technology Research Partnership for Sustainable Development (SATREPS) Project: “Valorization of Bio-resources in Semi-Arid and Arid Land for Regional Development”.
References
- Bourgou S, Ksouri R, Bellila A, Skandrani I, Falleh H, Marzouk B. Phenolic composition and biological activities of Tunisian Nigella sativa L. shoots and roots. C R Biol. 2008;331:48–55. doi: 10.1016/j.crvi.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Bulavin DV, Demidov ON, Saito S, Kauraniemi P, Phillips C, Amundson SA, Ambrosino C, Sauter G, Nebreda AR, Anderson CW, Kallioniemi A, Fornace AJ Jr, Appella E (2002) Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat Genet 31:210–215 [DOI] [PubMed]
- Bulavin DV, Fornace AJ., Jr p38 MAP Kinase’s emerging role as a tumor suppressor. Adv Cancer Res. 2004;92:95–118. doi: 10.1016/S0065-230X(04)92005-2. [DOI] [PubMed] [Google Scholar]
- Bulavin DV, Phillips C, Nannenga B, Timofeev O, Donehower LA, Anderson CW, Appella E, Fornace AJ Jr (2004) Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK-mediated activation of the p16(Ink4a)-p19(Arf) pathway. Nat Genet 36:343–350 [DOI] [PubMed]
- Cai XZ, Wang J, Li XD, Wang GL, Liu FN, Cheng MS, Li F (2009) Curcumin suppresses proliferation and invasion in human gastric cancer cells by downregulation of PAK1 activity and cyclin D1 expression. Cancer Biol Ther 8:1360–1368 [DOI] [PubMed]
- Canagarajah BJ, Khokhlatchev A, Cobb MH, Goldsmith EI. Activation mechanism of the MAP Kinase ERK2 by dual phosphorylation. Cell. 1997;90:859–869. doi: 10.1016/S0092-8674(00)80351-7. [DOI] [PubMed] [Google Scholar]
- Cárdenas MG, Blank VC, Marder M, Roguin LP. 2′-Nitroflavone induces cell cycle arrest and apoptosis in HeLa human cervical carcinoma cells. Cancer Lett. 2008;268:146–157. doi: 10.1016/j.canlet.2008.03.062. [DOI] [PubMed] [Google Scholar]
- Castedo M, Perfettini JL, Roumier T, Yakushijin K, Horne D, Medema R, Kroemer G. The cell cycle checkpoint kinase Chk2 is a negative regulator of mitotic catastrophe. Oncogene. 2004;23:4353–4361. doi: 10.1038/sj.onc.1207573. [DOI] [PubMed] [Google Scholar]
- Chen L, Mayer JA, Krisko TI, Speers CW, Wang T, Hilsenbeck SG, Brown PH (2009) Inhibition of the p38 kinase suppresses the proliferation of human ER-negative breast cancer cells. Cancer Res 69:8853–8861 [DOI] [PMC free article] [PubMed]
- Cho SH, Toouli CD, Fujii GH, Crain C, Parry D. Chk1 is essential for tumor cell viability following activation of the replication checkpoint. Cell Cycle. 2005;4:131–139. doi: 10.4161/cc.4.1.1299. [DOI] [PubMed] [Google Scholar]
- Conour JE, Graham WV, Gaskins HR. A combined in vitro = bioinformatic investigation of redox regulatory mechanisms governing cell cycle progression. Physiol Genomics. 2004;18:196–205. doi: 10.1152/physiolgenomics.00058.2004. [DOI] [PubMed] [Google Scholar]
- Dewanto V, Wu X, Adom KK, Liu RH. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014. doi: 10.1021/jf0115589. [DOI] [PubMed] [Google Scholar]
- Dumesic PA, Scholl FA, Barragan DI, Khavari PA. Erk1/2 MAP kinases are required for epidermal G2/M progression. J Cell Biol. 2000;185:409–422. doi: 10.1083/jcb.200804038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falleh H, Ksouri R, Chaieb K, Karray-Bouraoui N, Trabelsi N, Boulaaba M, Abdelly C. Phenolic composition of Cynara cardunculus L. organs, and their biological activities. C R Biol. 2008;331:372–379. doi: 10.1016/j.crvi.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Gülcin I, Uguz MT, Oktay M, Beydemir S, Küfrevioglu OI. Evaluation of the antioxidant and antimicrobial activities of Clary Sage (Salvia sclarea L.) Turk J Agric For. 2004;28:25–33. [Google Scholar]
- Hatano H, Kagawa T, Yasuhara J, Okuda T. Two new flavonoids and other constituents in licorice root: their relative astringency and radical scavenging effect. Chem Pharm Bull. 1988;36:1090–1097. doi: 10.1248/cpb.36.2090. [DOI] [PubMed] [Google Scholar]
- Jin C, Strembiski W, Kulchytska Y, Micetich RG, Daneshtalab M. Flavonoid glycosides from Ledum palustre subsp. decumbens. Daru. 1999;7:5–8. [Google Scholar]
- Kennedy NJ, Davis RJ. Role of JNK in tumor development. Cell Cycle. 2003;2:199–201. doi: 10.4161/cc.2.3.388. [DOI] [PubMed] [Google Scholar]
- Kennedy NJ, Cellurale C, Davis RJ. A radical role for p38 MAPK in tumor initiation. Cancer Cell. 2007;11:101–103. doi: 10.1016/j.ccr.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Ksouri R, Falleh H, Megdiche W, Trabelsi N, Mhamdi B, Chaieb K, Bakrouf A, Magné C, Abdelly C. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem Toxicol. 2009;47:2083–2091. doi: 10.1016/j.fct.2009.05.040. [DOI] [PubMed] [Google Scholar]
- Kuntz S, Wenzel U, Daniel H. Comparative analysis of the effects of flavonoids on proliferation, cytotoxicity, and apoptosis in human colon cancer cell lines. Eur J Nutr. 1999;38:133–142. doi: 10.1007/s003940050054. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, L’Allemain G, Brunet A, Muller R, Pouyssegur J. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J Biol Chem. 1996;271:20608–20616. doi: 10.1074/jbc.271.34.20608. [DOI] [PubMed] [Google Scholar]
- Lee YL, Yang JH, Mau JL. Antioxidant properties of water extracts from Monascus fermented soybeans. Food Chem. 2008;106:1128–1137. doi: 10.1016/j.foodchem.2007.07.047. [DOI] [Google Scholar]
- Liu WK, Cheung FWK, Liu BPL, Li C, Ye W, Che CT. Involvement of p21 and FasL in induction of cell cycle arrest and apoptosis by neochamaejasmin A in human prostate LNCaP cancer cells. J Nat Prod. 2008;71:842–846. doi: 10.1021/np8001223. [DOI] [PubMed] [Google Scholar]
- Luo XM, Basile MJ, Kennelly EJ. Polyphenolic antioxidants from the fruit of Chrysophyllum cainito L. (Star Apple) J Agric Food Chem. 2002;50:1379–1382. doi: 10.1021/jf011178n. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. Cell cycle kinases in cancer. Curr Opin Genet Dev. 2007;17:60–65. doi: 10.1016/j.gde.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Manke IA, Nguyen A, Lim D, Stewart MQ, Elia AE, Yaffe MB. MAPKAP kinase-2 is a cell cycle checkpoint kinase that regulates the G2/M transition and S phase progression in response to UV irradiation. Mol Cell. 2005;17:37–48. doi: 10.1016/j.molcel.2004.11.021. [DOI] [PubMed] [Google Scholar]
- Mao F, Xiao B, Jiang Z, Zhao J, Huang X, Guo J. Anticancer effect of Lycium barbarum polysaccharides on colon cancer cells involves G0/G1 phase arrest. Med Oncol. 2011;28:121–126. doi: 10.1007/s12032-009-9415-5. [DOI] [PubMed] [Google Scholar]
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63 [DOI] [PubMed]
- Murray AW. Recycling the cell cycle: cyclins revisited. Cell. 2004;116:221–234. doi: 10.1016/S0092-8674(03)01080-8. [DOI] [PubMed] [Google Scholar]
- Negro C, Tommasi L, Miceli A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresource Tech. 2003;87:41–44. doi: 10.1016/S0960-8524(02)00202-X. [DOI] [PubMed] [Google Scholar]
- Okita K, Sakaida I, Hino K. Current strategies for chemoprevention of hepatocellular carcinoma. Oncology. 2002;62:24–28. doi: 10.1159/000048272. [DOI] [PubMed] [Google Scholar]
- Orabi MAA, Taniguchi S, Yoshimura M, Yoshida T, Kishino K, Sakagami H, Hatano T. Hydrolyzable tannins of tamaricaceous plants. III. (1) Hellinoyl- and macrocyclic type ellagitannins from Tamarix nilotica. J Nat Prod. 2010;73:870–879. doi: 10.1021/np900829g. [DOI] [PubMed] [Google Scholar]
- Oszmianski J, Wojdylo A, Lamer-Zarawska E, Swiader K. Antioxidant tannins from Rosaceae plant roots. Food Chem. 2007;100:579–583. doi: 10.1016/j.foodchem.2005.09.086. [DOI] [Google Scholar]
- Oyaizu M. Studies on products of the browning reaction. Antioxidative activities of browning reaction products prepared from glucosamine. Jap J Nutr. 1986;44:307–315. doi: 10.5264/eiyogakuzashi.44.307. [DOI] [Google Scholar]
- Pachón-Peña G, Reyes-Zurita FJ, Deffieux G, Azqueta A, De Cerain AL, Centelles JJ, Creppy EE, Cascante M. Antiproliferative effect of flavomannin-6,6′-dimethylether from Tricholoma equestre on Caco-2 cells. Toxicol. 2009;264:192–197. doi: 10.1016/j.tox.2009.08.009. [DOI] [PubMed] [Google Scholar]
- Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer targeting the Raf-MEK-ERK MAPK cascade. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- Sarsour EH, Kumar MG, Chaudhuri L, Kalen AL, Goswami PC. Redox control of the cell cycle in health and disease. Antioxid Redox Signal. 2009;11:2985–3011. doi: 10.1089/ars.2009.2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehrawat A, Sultana S. Evaluation of possible mechanisms of protective role of Tamarix gallica against DEN initiated and 2-AAF promoted hepatocarcinogenesis in male Wistar rats. Life Sci. 2006;79:1456–1465. doi: 10.1016/j.lfs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Singh JP, Selvendiran K, Banu SM, Padmavathi R, Sakthisekaran D. Protective role of apigenin on the status of lipid peroxidation and antioxidant defense against hepatocarcinogenesis in Wistar albino rats. Phytomedicine. 2004;11:309–314. doi: 10.1078/0944711041495254. [DOI] [PubMed] [Google Scholar]
- Sun B, Richardo-da-Silvia JM, Spranger I. Critical factors of vanillin assay for catechins and proanthocyanidins. J Agric Food Chem. 1998;46:4267–4274. doi: 10.1021/jf980366j. [DOI] [Google Scholar]
- Um YR, Kong CS, Lee JI, Kim YA, Nam TJ, Seo Y. Evaluation of chemical constituents from Glehnia littoralis for antiproliferative activity against HT-29 human colon cancer cells. Process Biochem. 2010;45:114–119. doi: 10.1016/j.procbio.2009.08.016. [DOI] [Google Scholar]
- Wang W, Heideman L, Chung CS, Pelling JC, Koehler KJ, Birt DF. Cell-cycle arrest at G2/M and growth inhibition by apigenin in human colon carcinoma cell lines. Mol Carcinog. 2000;28:102–110. doi: 10.1002/1098-2744(200006)28:2<102::AID-MC6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Wang JL, Wang X, Wang H, Iliakis G, Wang Y. Chk1-regulated S-phase checkpoint response reduces camptothecin cytotoxicity. Cell Cycle. 2002;1:267–272. doi: 10.4161/cc.1.4.137. [DOI] [PubMed] [Google Scholar]
- Weng CJ, Yen GC. Chemopreventive effects of dietary phytochemicals against cancer invasion and metastasis: phenolic acids, monophenol, polyphenol, and their derivatives. Cancer Treat Rev. 2012;38:76–87. doi: 10.1016/j.ctrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Chien CM, Yang SH, Lin YH, Hu XW, Lu YJ, Wu MJ, Lin SR. Induction of G2/M phase arrest and apoptosis by a novel enediyne derivative, THDA, in chronic myeloid leukemia (K562) cells. Mol Cell Biochem. 2006;292:99–105. doi: 10.1007/s11010-006-9222-7. [DOI] [PubMed] [Google Scholar]
- Yang YT, Weng CJ, Ho CT, Yen GC. Resveratrol analog-3,5,4′-trimethoxy-trans-stilbene inhibits invasion of human lung adenocarcinoma cells by suppressing the MAPK pathway and decreasing matrix metalloproteinase-2 expression. Mol Nutr Food Res. 2009;53:407–416. doi: 10.1002/mnfr.200800123. [DOI] [PubMed] [Google Scholar]