Abstract
Genistein is an isoflavonic phyto-oestrogen contained in soya beans. It is thought to display anti-cancer effects. This study was designed to investigate its effect on human intestinal colon cancer Caco-2 cells. MTT assay, flow cytometric analysis and western blotting were used to investigate the effect of genistein on cell proliferation, cell cycle progression and protein alterations of selected cell cycle-related proteins in Caco-2 cells. Our results showed that genistein and daidzein significantly suppressed cell proliferation. Genistein treatment was demonstrated to modulate cell cycle distribution through accumulation of cells at G2/M phase, with a significant decreasing effect of Cyclin B1 and Serine/threonine-protein kinase 2 (Chk2) proteins expression. However, daidzein did not alter the cell cycle progression in Caco-2 cells. All these observation strongly indicate that genistein has anti-proliferative effect in human intestinal colon cancer Caco-2 cells through the down-regulation of cell cycle check point proteins, Cyclin B1 and Chk2.
Keywords: Genistein, Caco-2, Cyclin B1, Chk2
Introduction
Colon carcinoma is one of the most prevalent malignancies affecting millions of people yearly (Parker et al. 1997; Landis et al. 1999). Both genetic and environmental factors play a critical role in the pathogenesis of colon carcinoma, and diet seems to be a major player involved in its etiology. In this respect, a large number of studies suggest a relationship between the intake of high amount of fat and the incidence of colon carcinoma (Devesa et al. 1987). The potential of dietary constituents for preventing colon carcinoma is an important research field: high-fat diet generally represents a risk factor, whereas fiber- and vitamin-rich diets are considered to be protective factors (Mutanen et al. 2000; Kallay et al. 2002; Bocca et al. 2007). Treatments for recurrent and metastatic diseases remain a center of clinical attention. In this regard, recent researches demonstrated that molecular target therapy approach could be very promising (Surh 2003; Hurwitz and Kabbinavar 2005). While continuing efforts for discovering new molecular target-based molecules, there is an emerging interest in chemotherapeutic application of natural substances, such as tea polyphenols and resveratrol, for cancer treatment (Wolter et al. 2003).
Flavonoids display a wide range of biological activities, including anti-inflammatory and cytoprotective activities (Ross and Kasum 2002). Certain flavonoids are known to act as anti-cancer reagents. Especially, isoflavonoids are among the most promising potential anti-carcinogenic constituents, produced by pea family plants. Epidemiological studies indicate that high consumption of soybean is associated with a lower incidence of breast and prostate cancer in Asian people (Wang et al. 2002). Genistein and daidzein are the two major isoflavonoids in soybean, and were thought to play important roles in anti-cancer effects (Messina et al. 1994; Chiechi 1999). The main outcomes of genistein treatment are suppression of cell proliferation, induction of apoptosis and G2/M cell-cycle arrest (Shen et al. 2000; Chang et al. 2004; Mansour et al. 2004). The underlying molecular mechanism of action of genistein and daidzein were extensively investigated and are not fully understood (Ismail et al. 2006; Schmidt et al. 2005). The main upstream targets involved in cell cycle arrest; particularly increasing the cells at S phase after genistein treatment is not yet fully explained.
In the present study, we showed that genistein induces cell growth inhibition of human intestinal colon cancer Caco-2 cells and significantly inhibits the expression of Cyclin B1 and Chk2, cell cycle check point proteins for G2/M transition and for the cell to enter mitosis.
Materials and methods
Cell culture
Human intestinal colon cancer Caco-2 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10 % heat-inactivated fetal bovine serum (Sigma, St. Louis, MO, USA), 1 % non-essential amino acid (Cosmo Bio Co. Ltd., Tokyo, Japan) and 1 % penicillin (5,000 U/ml)-streptomycin (5,000 μg/ml) (Cosmo Bio Co. Ltd., Tokyo, Japan). Cells were incubated in an atmosphere of 5 % CO2 at 37 °C.
Cell proliferation assay
The effect of genistein and daidzein on the survival of Caco-2 cells was assessed using MTT proliferation assay and flow cytometry using the Guava ViaCount assay (Guava Technologies, Hayward, CA, USA). Caco-2 cells (2.0 × 104 cells/ml) were cultured in 96-well plates at 37 °C for 24 h with medium including genistein (Sigma, St. Louis, MO, USA) (0.1 and 0.2 μΜ) or daidzein (Sigma, St. Louis, MO, USA) (0.1 and 0.2 μΜ). The MTT assay was followed by the addition of 10 μl of MTT (3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H-tetrazolium bromide) (Dojin Laboratories, Kumamoto, Japan) at 5 mg/ml in PBS. After 6 h incubation, 10 % SDS (sodium dodecyl sulfate, Wako Pure Phamaceutical Co., Ltd., Japan) was added to dissolve the formazan and the plates were again incubated for 12 h. The amount of MTT formazan produced by the cells was determined at 570 nm wavelength. Cell viabilities are presented relative to those of the non-treated cells.
To evaluate the effect on cell count, Caco-2 cells (2.0 × 104 cells/ml) were exposed to genistein (0.1 and 0.2 μΜ) or daidzein (0.1 and 0.2 μΜ) treatments for 24 h and the treated cells were collected by trypsinisation. Cells were fixed with 70 % ethanol and stored at −20 °C. On the day of analysis, the cells were washed in 250 μl phosphate-buffered saline (PBS) (−). Following centrifugation the cells were resuspended in 500 μl ViaCount flex reagent and incubated in the dark at room temperature for 30 min. Cell count was determined by fluorescence-activated cell sorting analysis of propidium iodide-stained ethanol-fixed cells using a Guava EasyCyte (Guava Technologies).
Cell cycle analysis
The DNA content and cell-cycle distribution after genistein treatment were estimated by flow cytometry. Cell seeding, drug treatment and ethanol fixation were similar to cell proliferation assay. After washing with PBS, genistein and daidzein-treated and -fixed Caco-2 cells were suspended in 250 μl of PBS, then 1.0 ml phosphate-citrate buffer (0.05 M, pH 4.0) was added and the suspension was incubated at room temperature for 5 min to facilitate the extraction of low molecular weight DNA. Following centrifugation the cells were resuspended in 500 μl DNA staining solution (20 μg/ml propidium iodide, 200 μg/ml DNase (RNase-free), and 0.1 % Triton X-100) and incubated in the dark at room temperature for 30 min. Cell cycle distribution was determined by fluorescence-activated cell sorting analysis of propidium iodide-stained ethanol-fixed cells using a Guava EasyCyte (Guava Technologies).
Western blot analysis
Changes in proteins expression level after genistein treatment were evaluated by western blot. After treatment with genistein (0.1 μΜ) as indicated in figure legends, cells were washed twice with cold PBS (−) and total protein was extracted using RIPA buffer (20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1 % NP-40, 1 % sodium deoxycholate, 2.5 mM sodium pyrophosphate, 1 mM β-glycerophosphate, 1 mM Na3VO4, 1 μg/ml leupeptin). 15 μg of extracted protein sample was resolved by 12 % sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS–PAGE) and blotted using Invitrogen IBlot system. The blots were probed with anti-Cyclin B1 monoclonal antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) (1:3,500 dilution) and anti-Chk2 monoclonal antibody (Santa Cruz Biotechnology) (1:200 dilution) using Snap Id system (Millipore). The signal was visualized using Western blotting Chemiluminescent HRP Substrate (GE Healthcare, Piscataway, NJ, USA) Amersham ECL system.
Statistical analysis
All results were expressed as the mean ± standard error (SE). Statistical significance was analysed using the Student’s t test. P values less than 0.05 were considered statistically significant.
Results
Genistein and daidzein suppresses cell proliferation of Caco-2 cells
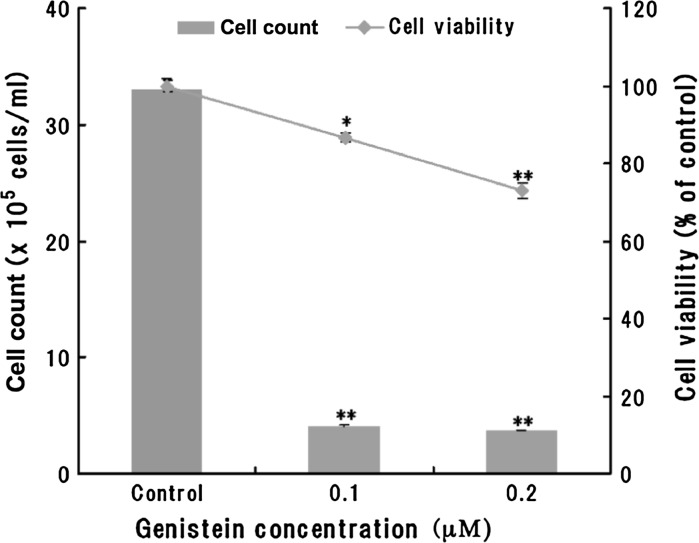
The effect of genistein and daidzein on the overall cell proliferation was assessed using the MTT assay and cell counting assay. Genistein at 0.1 and 0.2 μΜ significantly inhibited cell viability and the cell count of Caco-2 cells (Fig. 1). However, Daidzein at 0.1 and 0.2 μΜ significantly inhibited only the cell count (Fig. 2). A decrease of cell viability in dose-dependent manner was observed in genistein-treated Caco-2 cells, whereas only a decrease was observed at 0.1 μΜ daidzein-treated Caco-2 cells. Furthermore, genistein has an higher inhibition effect than daidzein in the cell count of Caco-2 cells. Phase-contrast microscope observation showed that genistein and daidzein irreversibly induced a morphological change after 3 days of treatment (data not shown).
Fig. 1.
Effect of genistein on cell viability and cell count of Caco-2 cells. Genistein (0.1 and 0.2 μΜ) suppressed cell viability and cell count of Caco-2 cells. Results represent the average of 5 independent wells ± S.D.; similar results were obtained from several independent trials. Stastistical significance was determined using the Student’s t-test, (*P < 0.05, **P < 0.01)
Fig. 2.
Effects of daidzein on the cell viability and cell count of Caco-2 cells. Daidzein suppressed the cell count of of Caco-2 cells. However, a significant decrease in the cell viability was not observed in 0.2 μΜ daidzein-treated Caco-2 cells. Results represent the average of 5 independent wells ± S.D.; similar results were obtained from several independent trials. Statistical significance was determined using the Student’s t-test, (*P < 0.05, **P < 0.01)
Genistein modulates cell cycle progression
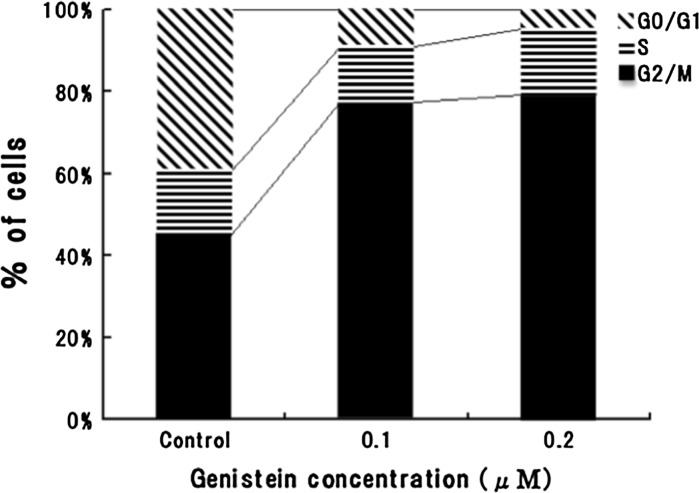
Flow cytometric analysis showed that genistein modulated the cell cycle progression by inducing cells to accumulate in G2/M phases with concurrent decrease of cells in G0/G1 phase (Fig. 3). The percentage of cells in G2/M phase significantly increased from 44 % in control cells to 76 and 77 % in 0.1 and 0.2 μΜ genistein-treated cells, respectively. In contrast, daidzein showed no significant effect in the distribution of cells in the cell cycle (Fig. 4).
Fig. 3.
Cell cycle distribution of Caco-2 cells treated with genistein (0.1 and 0.2 μΜ) for 24 h. The percentage of G2/M phase cells was increased by genistein treatment
Fig. 4.
Cell cycle distribution of Caco-2 cells treated with daidzein (0.1 and 0.2 μΜ) for 24 h. The percentage of cells at different cell cycle phase was not changed by daidzein treatment
Genistein down-regulates Cyclin B1 and Chk2
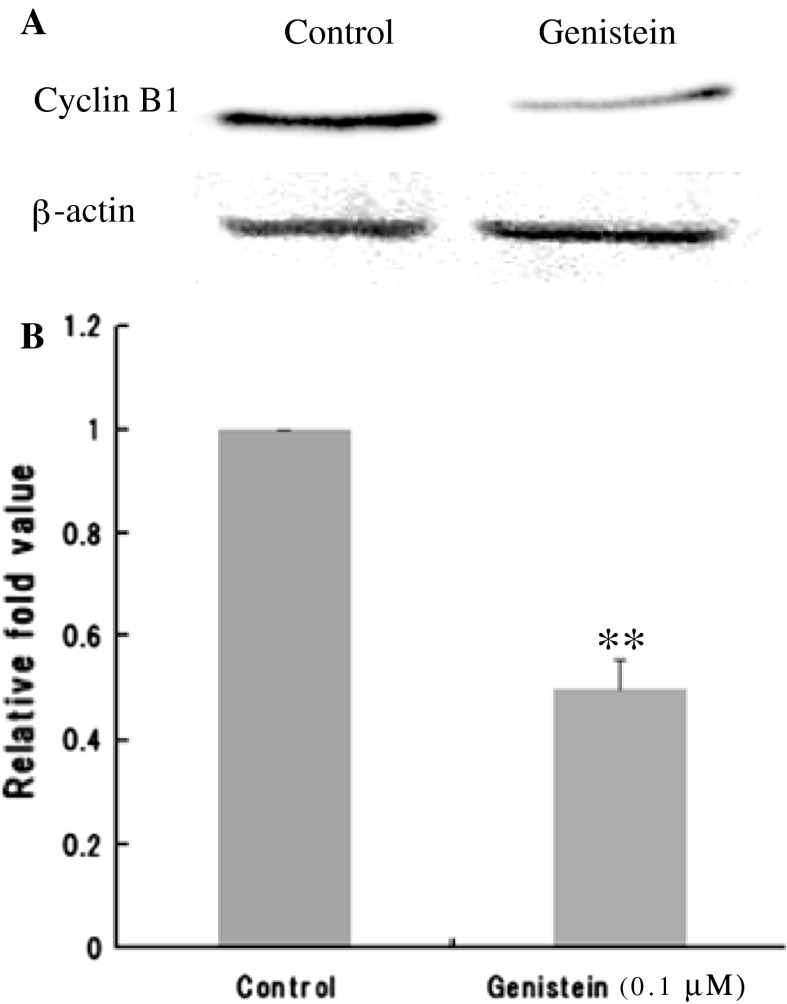
In order to understand the differential effect of genistein compared to daidzein on cell cycle progression, the expression of cell cycle check point proteins, Cyclin B1 and Chk2, were investigated. Genistein showed a significant down-regulation of Cyclin B1 protein expression (Fig. 5a, b). Treatment with 0.1 μΜ genistein induced a 49 % decrease (compared to control) in Cyclin B1 expression. In similar manner as the Cyclin B1 result, 0.1 μΜ genistein treatment showed a significant down-regulation of Chk2 protein expression to 29 % (Fig. 6a, b).
Fig. 5.
Expression of Cyclin B1 protein in 0.1 μΜ genistein-treated Caco-2 cells. The intensities of the protein expression were normalized to the β-actin expression of each treatment. Genistein induced the down-regulation of Cyclin B1 expression. The intensity values for the protein bands of interest were expressed as fold increase. All figures showing quantitative analysis include data from at least three independent trials. Values are means ± S. D. **P < 0.01
Fig. 6.
Expression of Chk2 protein in 0.1 μM genistein-treated Caco-2 cells. The intensities of the protein expression were normalized to the β-actin expression of each treatment. Genistein induced the down-regulation of Chk2 expression. The intensity values of the protein bands of interest were expressed as fold increase. All figures showing quantitative analysis include data from at least three independent trials. Values are means ± S. D. **P < 0.01
Discussion
The data presented in this paper demonstrate that genistein and daidzein are potently anti-proliferative toward human intestinal colon cancer Caco-2 cells. We have demonstrated that genistein induced cell cycle arrest predominantly at G2/M phase, but daidzein showed no effect on the cell cycle distribution of Caco-2 cells. In addition, genistein induced the down-regulation of Cyclin B1 and Chk2 expression, cell cycle check point proteins for G2/M transition and for the cell to enter mitosis.
Cyclin B1 was virtually undetectable in cells from G0/G1 phase to mid S phase, but became visible in the cytoplasm in late S phase (Kakino et al. 1996). As cells proceed to the G2 phase, the level of Cyclin B1 rapidly increased in the mitotic phase. The decision of cells to either remain in the G2/M phase or go through G2 phase into mitosis requires the activation of Cdc2 protein (Lindqvist et al. 2009) whose activity is regulated by synthesis of Cyclin B1 and subsequent complex formation. This complex formation is triggered by a 10-fold increase in Cyclin B1 expression in the G2 phase. Cyclin B1 expression changes through the cell cycle and cells with a prolonged G2 phase have reduced Cyclin B1 expression when compared to the ordinary expression (Gavet and Pines 2010). While the expression of Cyclin B1 is required for entry into mitosis, its destruction is required to exit from mitosis.
Chk protein is also implicated in cell cycle checkpoint control, DNA repair and DNA damage-induced apoptosis via phosphorylation of several downstream targets. Especially, Chk2 is required for preservation, but not start, of G2 phase arrest induced by DNA damage. Chk2 was shown to synergize with other genes or factors that perpetuate DNA damage repair during G2/M phase rather than inducing G2/M phase arrest (Hirao et al. 2000). Active Chk2 is known to phosphorylate ser-216 (negative regulatory site) of Cdc25C protein phosphatase, leading in turn to the inhibition of Cdc2 kinase and consequently G2/M blockade (Kastan and Bartek 2004; Niida and Nakanishi 2005). These findings are consistent with our results that only genistein has an effect on cell cycle through the down-regulation of Cyclin B1 and Chk2 in intestinal Caco-2 cells.
In this study, we found that genistein and daidzein, well known isoflavonoids from pea family plants, significantly inhibited the Caco-2 cell count. A dramatic decrease in cell viability was observed only in the genistein-treated cells. Furthermore, the percentages of S-phase cells and G0/G1 phase were increased by 1.7-fold and 4-fold, respectively, in 0.1 μΜ genistein treatment. However, daidzein did not cause an arrest in the cell cycle of Caco-2 cells. In agreement with our result, genistein has been reported to induce a G2/M cell cycle arrest on human neuroblastoma SK-N-MC cells through the down-regulation of cell cycle check point proteins, Cyclin B1, Cdc2, Cdc25 and Chk2 (Ismail et al. 2007). However, genistein concentrations-inducing G2/M cell cycle arrest on SK-N-MC cells, were 50 and 100 μΜ. These concentrations were 500 times higher than those concentrations we used to treat Caco-2 cells.
It was reported that genistein has an inhibitory effect on Epidermal Growth Factor Receptor (EGFR) and induced EGFR dimerization and down-regulation from the cell surface, and consequently inhibit EGFR signaling, leading to cell cycle arrest and/or cell death (Baselga 2001; Jiang et al. 2010). Furthermore, genistein was ineffective in extracellular-signal regulated kinase (ERK) activation in EGF-stimulated cell (Croisy-Delcey et al. 1997). Taken together, our results suggest that genistein treatment inhibits cell cycle progression and arrests EGFR-expressing cells at the G2 phase of the cell cycle.
In summary, genistein and daidzein suppressed Caco-2 cell proliferation. Furthermore, genistein treatment modulated the cell cycle distribution through the accumulation of cells at G2/M-phase. However, daidzein did not alter the cell cycle progression in Caco-2 cells. Western blotting results showed that genistein decreased the expression of Cyclin B1 and Chk2 proteins. To the best of our knowledge, our research suggests for the first time, the involvement of the downregulation of Cyclin B1 and Chk2 proteins expression in the anti-proliferative effect of geistein on Caco-2 cells, as well as its effect on cell cycle pathway.
References
- Baselga J. Targeting the epidermal growth factor receptor: a clinical reality. J Clin Oncol. 2001;19:41–44. [PubMed] [Google Scholar]
- Bocca C, Bozzo F, Gabriel L, Miglietta A. Conjugated linoleic acid inhibits Caco-2 cell growth via ERK-MAPK signaling pathway. J Nutri Biochem. 2007;18:332–340. doi: 10.1016/j.jnutbio.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Chang KL, Kung ML, Chow NH, Su SJ. Genistein arrests hepatoma cells at G2/M phase: involvement of ATM activation and upregulation of p21waf1/cip1 and wee1. Biochem Pharmacol. 2004;67:717–726. doi: 10.1016/j.bcp.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Chiechi LM. Dietary phytoestrogens in the prevention of long-term postmenopausal diseases. Intl J Gynaecol Obstet. 1999;67:39–40. doi: 10.1016/S0020-7292(99)00080-6. [DOI] [PubMed] [Google Scholar]
- Croisy-Delcey M, Croisy A, Mousset S, Letourneur M, Bisagni E, Jacquemin-Sablon A, Pierre J. Genistein analogues: effects on epidermal growth factor receptor tyrosine kinase and on stress-activated pathways. Biomed Pharmacother. 1997;51:286–294. doi: 10.1016/S0753-3322(97)83545-7. [DOI] [PubMed] [Google Scholar]
- Devesa LL, Silverman DT, Yough JL. Cancer incidence and mortality trends among whites in the United States, 1947–1984. J Natl Cancer Inst. 1987;79:701–708. [PubMed] [Google Scholar]
- Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell. 2010;18:533–543. doi: 10.1016/j.devcel.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao A, Kong YY, Matsuoka S, Wakeham A, Ruland J, Yoshida H, Liu D, Elledge SJ, Mak TW. DNA damage-induced activation of p53 by the checkpoint kinase Chk2. Science. 2000;287:1824–1827. doi: 10.1126/science.287.5459.1824. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Kabbinavar F. Bevacizumab combined with standard fluoropyrimidine-based chemotherapy regimens to treat colorectal cancer. Oncology. 2005;69:17–24. doi: 10.1159/000088480. [DOI] [PubMed] [Google Scholar]
- Ismail IA, Kang KS, Kim JW, Sohn YK. Genistein induces G2/M cell cycle arrest and apoptosis in rat neuroblastoma B35 cells; involvement of p21waf1/cip1, Bax and Bcl-2. Korean J Pathol. 2006;40:339–347. [Google Scholar]
- Ismail IA, Kang KS, Lee HA, Kim JW, Sohn YK. Genistein-induced neuronal apoptosis and G2/M cell cycle arrest is associated with MDC1 up-regulation and PLK1 down-regulation. Eur J Pharmacol. 2007;575:12–20. doi: 10.1016/j.ejphar.2007.07.039. [DOI] [PubMed] [Google Scholar]
- Jiang L, Jiang LS, Yan LN, Li FY, Wang W, Cheng NS, Wen TF. Effects of epidermal growth factor receptor inhibitor genistein on proliferative cholangitis in rats. J Surg Res. 2010;162:59–67. doi: 10.1016/j.jss.2009.04.048. [DOI] [PubMed] [Google Scholar]
- Kakino S, Sasaki K, Kurose A, Ito H. Intracellular localization of cyclin B1 during the cell cycle in glioma cells. Cytometry. 1996;24:49–54. doi: 10.1002/(SICI)1097-0320(19960501)24:1<49::AID-CYTO6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Kallay E, Bareis P, Bajna E, Kriwanek S, Bonner E, Toyokuni S, Cross HS. Vitamin D receptor activity and prevention of colonic hyperproliferation and oxidative stress. Food Chem Toxicol. 2002;40:1191–1196. doi: 10.1016/S0278-6915(02)00030-3. [DOI] [PubMed] [Google Scholar]
- Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- Landis SH, Murray T, Bolden S, Wingo PA. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- Lindqvist A, Rodriguez-Bravo V, Medema RH. The decision to enter mitosis: feedback and redundancy in the mitotic entry network. J Cell Biol. 2009;185:193–202. doi: 10.1083/jcb.200812045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansour A, McCarthy B, Schwander SK, Chang V, Kotenko S, Donepudi S, Lee J, Raveche E. Genistein induces G2 arrest in malignant B cells by decreasing IL-10 secretion. Cell Cycle. 2004;3:1597–1605. doi: 10.4161/cc.3.12.1293. [DOI] [PubMed] [Google Scholar]
- Messina MJ, Persky V, Kenneth KDR, Setchell DR, Bames S (1994) Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer 21:1l3–l31 [DOI] [PubMed]
- Mutanen M, Pajari AM, Oikarinen SI. Beef induces and rye bran prevents the formation of intestinal polyps in Apc (Min) mice: relation to β-catenin and PKC isozymes. Carcinogenesis. 2000;21:1167–1173. doi: 10.1093/carcin/21.6.1167. [DOI] [PubMed] [Google Scholar]
- Niida H, Nakanishi M. DNA damage checkpoints in mammals. Mutagenesis. 2005;21:3–9. doi: 10.1093/mutage/gei063. [DOI] [PubMed] [Google Scholar]
- Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin. 1997;47:5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- Ross JA, Kasum CM. Dietary flavonoids: bioavailability, metabolic effects, and safety. Ann Rev Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- Schmidt S, Michna H, Diel P. Combinatory effects of phytoestrogens and 17β-estradiol on proliferation and apoptosis in MCF-7 breast cancer cells. J Steroid Biochem Mol Biol. 2005;94:445–449. doi: 10.1016/j.jsbmb.2004.12.042. [DOI] [PubMed] [Google Scholar]
- Shen JC, Klein RD, Wei Q, Guan Y, Contois JH, Wang TT, Chang S, Hursting SD. Low-dose genistein induces cyclin-dependent kinase inhibitors and G (1) cell-cycle arrest in human prostate cancer cells. Mol Carcinog. 2000;29:92–102. doi: 10.1002/1098-2744(200010)29:2<92::AID-MC6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;10:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- Wang HZ, Zhang Y, Xie LP, Yu XY, Zhang RO. Effects of genistein and daidzein on the cell growth, cell cycle, and differentiation of human and murine melanoma cells. J Nutri Biochem. 2002;13:421–426. doi: 10.1016/S0955-2863(02)00184-5. [DOI] [PubMed] [Google Scholar]
- Wolter F, Turchanowa L, Stein J. Resveratrol-induced modification of polyamine metabolism is accompanied by induction of c-Fos. Carcinogenesis. 2003;24:469–474. doi: 10.1093/carcin/24.3.469. [DOI] [PubMed] [Google Scholar]