Abstract
To improve antibody production in Chinese hamster ovary (CHO) cells, the humanized antibody-producing CHO DP-12-SF cell line was transfected with the gene encoding activating transcription factor 4 (ATF4), a central factor in the unfolded protein response. Overexpression of ATF4 significantly enhanced the production of antibody in the CHO DP-12-SF cell line. The specific IgG production rate of in the ATF4-overexpressing CHO-ATF4-16 cells was approximately 2.4 times that of the parental host cell line. Clone CHO-ATF4-16 did not show any change in growth rate compared with the parental cells or mock-transfected CHO-DP12-SF cells. The expression levels of mRNAs encoding both the antibody heavy and light chains in the CHO-ATF4-16 clone were analyzed. This analysis showed that ATF4 overexpression improved the total production and specific production rate of antibody without affecting the mRNA transcription level. These results indicate that ATF4 overexpression is a promising method for improving recombinant IgG production in CHO cells.
Keywords: Activating transcription factor 4, Antibody production, Chinese hamster ovary cells, Specific production rate
Introduction
Since the 1990s, the production of antibodies and recombinant proteins has become much more efficient, and it is now possible to produce up to 10 g/l in some cases (Huang et al. 2010; Werner 2004; Wurm 2004). These higher yields are mainly attributed to increased viable cell concentrations (Wurm 2004), but remarkable progress in the improvement of specific production rates were recently reported. For instance, the specific production rate of recombinant cell lines reaches around 50 pg/cell/day (Butler and Meneses-Acosta 2012), or around 90 pg/cell/day (Kober et al. 2013). However, a plasma cell can secrete several thousands of immunoglobulin M molecules (Randall et al. 1992), indicating that a 2.5–12-fold increase in specific productivity is possible (Khan and Schroder 2008).
Three main engineering strategies have been used for improving biopharmaceutical production in Chinese hamster ovary (CHO) cell lines (Omasa et al. 2010). First, the conventional methods focused on process and reactor design and media formulation development (Wurm 2004) to increase viable cell concentration. Second, gene amplification methodologies, including the construction of gene-amplified cell lines, target integration into hot spots on CHO chromosomes, or the use of strong promoters and/or stable elements in vectors, can increase yield by increasing mRNA translation (Barnes and Dickson 2006; Omasa 2002; Omasa et al. 2010; Park et al. 2010). Third, protein folding, post-translational modification and secretion have been improved, which is of particular importance because the amount of secreted heterologous protein does not always increase proportionally with gene copy number (Parekh et al. 1995; Yoshikawa et al. 2000), mRNA (Barnes et al. 2004) or even the amount of intracellular heterologous protein (Schroder and Friedl 1997). Thus, it appears that the bottleneck for high protein production involves folding, posttranslational modification and protein secretion. These steps occur mainly in the endoplasmic reticulum (ER) and Golgi apparatus. In this study, we highlight the importance of the third engineering strategy for improving antibody production.
Immunoglobulin (Ig) is complicated in its structure and function compared with other recombinant proteins produced in CHO cells. IgG is a heterodimeric molecule composed of two heavy and two light chains. The light chains consist of about 214 amino acids in 2 domains, while the heavy chains consist of 445 amino acids in 4 domains. The heavy and light chains are connected by several inter- and intra-disulfide bonds. The assembly of heavy and light chains inside the ER is strictly controlled (O’Callaghan et al. 2010). The heavy chains cannot be secreted without being assembled with the light chains because the CH1 domain controls the assembly and secretion of IgG (Feige et al. 2009). After assembly, N-glycosylation takes place at the Asn residues of the Asn-X-Ser/Thr sequons in the Golgi apparatus (Zheng et al. 2011) (Fig. 1).
Fig. 1.
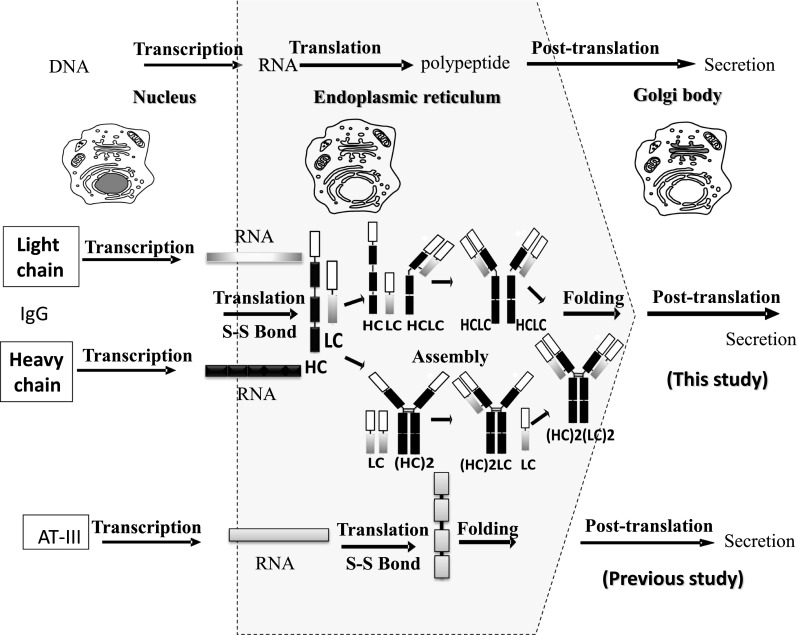
Schematic diagram for the intracellular production of both AT-III and IgG (modified from Omasa et al. 2010; O’Callaghan et al. 2010). Immunoglobulin G requires two mRNAs for the heavy (HC) and light (LC) chains. In the case of IgG, assembly in the ER is necessary after the translation of both mRNAs
The endoplasmic reticulum is the major organelle involved in the synthesis of proteins and it forms a membranous network throughout the cell. According to a previous report, about one-third of the total proteins produced are synthesized in the ER (Shimizu and Hendershot 2007). The ER lumen possesses a unique environment for high quality control of protein folding and assembly. It contains high concentrations of molecular chaperones, folding enzymes and ATP, which aid in the proper maturation of proteins (Gomez et al. 2008). However, if the amount of proteins to be folded exceeds the capacity of the folding machineries, unfolded proteins will accumulate in the ER and the unfolded protein response (UPR) is triggered. The UPR restores homeostasis inside the ER by attenuating protein translation, activating the folding machineries, degrading unfolded proteins, and finally inducing apoptosis (Brewer and Hendershot 2005).
Many UPR genes and chaperones provide an enhancing effect on recombinant protein production. PDI overexpression increased the secretion of heterologous protein (Mohan et al. 2007). BiP overexpression also increased heterologous protein secretion (Hsu and Betenbaugh 1997). In CHO-K1 cells, XBP-1s overexpression improved the secretion of several heterologous proteins (Ku et al. 2008; Tigges and Fussenegger 2006). Our previous study reported that the overexpression of ATF4 in antithrombin III-expressing CHO cells remarkably increased the specific production rate of antithrombin III (Ohya et al. 2008). However, activating transcription factor 4 (ATF4) technology has not been tested with complicated proteins such as IgG, which is strictly assembled in the ER. In this study, we investigated whether the ATF4 overexpression approach could improve IgG production in CHO cells to determine whether this approach is product-specific.
Materials and methods
Cell line and medium
The serum-free adapted CHO cell line, CHO-DP12-SF, producing human anti-IL-8 IgG was used in this study (Kim et al. 2010). The cell culture medium was Dulbecco’s modified Eagle’s medium DMEM (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 10 % dialyzed fetal bovine serum FBS (SAFC Biosciences, Lenexa, KS, USA) and 200 nM methotrexate (Sigma-Aldrich, St. Louis, MO, USA). Methotrexate at is added at this concentration during all the experiments.
Construction of the ATF4-overexpressing CHO cell line
The CHO ATF4 cDNA was cloned as described previously (Ohya et al. 2008). The cDNA was inserted into the KpnI/XbaI sites of pcDNA3.1/Hygro(+)® (Invitrogen, Carlsbad, CA, USA) to construct the pcDNA3.1/Hygro(+)-ATF4 vector. ATF4 was constitutively expressed under the control of the human cytomegalovirus (CMV) immediate early promoter. The pcDNA3.1/Hygro(+)-ATF4 vector was transfected into the CHO DP-12-SF cell line using TransIT-LT1 transfection reagent (Mirus Bio Madison, WI USA). Single cell clones were obtained using the limiting dilution method. The transfected cell lines were selected using 200 μg/ml hygromycin (Wako, Osaka, Japan). Chromosomal DNA was isolated from the obtained single cell clones after 72 h of cultivation using DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany). The primers 5′-TAATACGACTCACTATAGGG-3′ and 5′-TAGAAGGCACAGTCGAGG-3′ were employed for the amplification of the non-coding region of pcDNA3.1/Hygro(+)-ATF4 to detect the integration of the designated plasmid into the CHO chromosome. PCR was performed using Ex Taq polymerase (Takara Bio, Otsu, Shiga JAPAN) with 100 ng of genomic DNA. RNA was also isolated after 72 h of cultivation of the obtained clones using the RNeasy mini kit (Qiagen). cDNA equivalent to 1 μg of RNA was prepared using the Omniscript RT kit (Qiagen), and PCR was performed using the same primers used for chromosomal detection.
Determination of specific growth and IgG production rates
CHO cells were grown in 6-well plates containing DMEM supplemented with 10 % FBS, 200 nM methotrexate and 200 μg/ml of hygromycin using the replica culture method (Lee et al. 2013). Cell concentration was determined using a Coulter Vi-Cell automated cell viability analyzer (Beckman Coulter, Inc., Fullerton, CA, USA). IgG concentration was determined by sandwich enzyme linked immunosorbent assay (ELISA) according to a previous report (Kim et al. 2010). In brief, a 96-well plate (Corning, Corning, NY, USA) was coated with a goat anti-human IgG-Fc polyclonal antibody (100 μl/well, 10 μg/ml; Bethyl Laboratories, Montgomery, TX, USA) in 0.1 M sodium hydrogen carbonate buffer overnight at room temperature. The plate was washed three times with phosphate-buffered saline (PBS) containing 0.05 % (v/v) Tween 20 (PBS-T). The unbound active sites were blocked using 300 μl/well of 1 % (v/v) bovine serum albumin BSA (KPL, Gaithersburg, MD, USA) in PBS for 1 h at room temperature. The plate was then washed with PBS-T. Human serum reference (Athens Research & Technology, Athens, GA, USA) and samples were added to the plate and incubated for 2 h at room temperature. After incubation, the plate was washed three times with PBS-T and then incubated for 1 h at room temperature with horseradish peroxidase-conjugated goat anti-human IgG-Fc polyclonal antibody solution (100 μl/well, 1 μg/ml; Bethyl Laboratories) in 1 % (v/v) BSA/PBS. Finally, the plate was washed three times with PBS-T, and 100 μl/well of enhanced chemiluminescence solution (KPL, Geithersburg, MD, USA) was added. The reaction was stopped after 30 min at room temperature by adding 100 μl/well of peroxidase stop solution. The absorbance was measured at 405 nm using a microplate reader (Tecan, Männedorf, Switzerland). The specific growth and IgG production rates were calculated as described previously (Ohya et al. 2008). Statistical analysis was performed using the two-sided unpaired t test.
Quantification of mRNA
In order to determine mRNA levels, total RNA was isolated from CHO cells at 72 h for ATF4 and C/EBP-homologous protein (CHOP) mRNAs and at 24, 48, 72, 96, 120 and 144 h for heavy and light chain mRNAs using the RNeasy Mini Kit (Qiagen). mRNA quantification was performed using the SYBR® Green quantitative real-time polymerase chain reaction (PCR) and the Step One Plus Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). The efficiency of reverse transcription was verified by standardization to the house-keeping gene, ACTB (β-actin). The mRNA expression level is evaluated by delta delta Ct method according to the Step One Plus program as described previously (Lee et al. 2013). The primers used for real time PCR are shown in Table 1. Statistical analysis was performed using the two-sided unpaired t test.
Table 1.
Real time PCR primers
| Primer name | Sequence |
|---|---|
| T7-promoter | 5′-TAATACGACTCACTATAGGG-3′ |
| BGH | 5′-TAGAAGGCACAGTCGAGG-3′ |
| Heavy chain-F | 5′-ACGGTGTCGTGGAACTCAG-3′ |
| Heavy chain-B | 5′-ACGCTGCTGAGGGAGTAGAG-3′ |
| Light chain-F | 5′-ACCAAGGTGGAGATCAAACG-3′ |
| Light chain-B | 5′-ATTCAGCAGGCACACAACAG-3′ |
| β-actin-F | 5′-ACTCCTACGTGGGTGACGAG-3′ |
| β-actin-B | 5′-AGGTGTGGTGCCAGATCTTC-3′ |
| CHOP-F | 5′-GGGAGCTGGAAGCCTGGTAT-3′ |
| CHOP-B | 5′-GGGACCCCCATTTTCATCTG-3′ |
| ATF4-F | 5′-TTCTCCAGCGACAAGGCTAAG-3′ |
| ATF4-B | 5′-GCACTGACCAACCCATCCA-3′ |
Results and discussion
Evaluation of the effect of ATF4 overexpression on IgG production
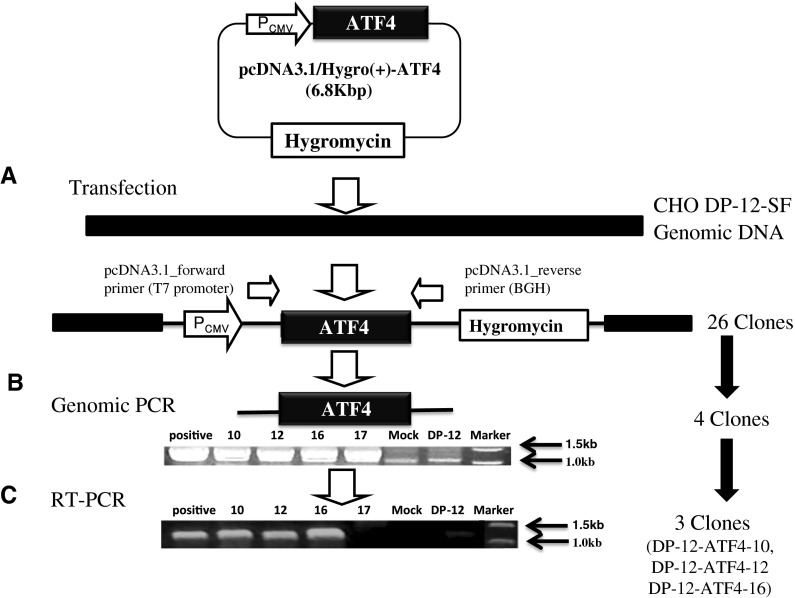
After introducing pcDNA3.1-ATF4/Hygro(+) into CHO DP-12-SF cells, 26 clones were selected in DMEM supplemented with 10 % FBS and 200 μg/ml hygromycin using the limiting dilution method. In order to confirm that the construct was integrated into the CHO genome, PCR analysis was performed. By using primers from the noncoding region of the vector (T7 promoter and BGH as shown in Fig. 2a and Table 1), the genome integration of ATF4 was confirmed in the obtained CHO single clones. Out of the 26 clones, only 4 clones were confirmed to possess a genomic insert of about 1.2 kb in the CHO DP-12-SF chromosome (Fig. 2b). Reverse transcriptase PCR analysis revealed that only three clones possessed the insert (Fig. 2c).
Fig. 2.
Summary of the construction of the ATF4-overexpressing CHO cell line. a Transfection of IgG producing CHO DP-12-SF cells with the ATF4 expression plasmid, pcDNA3.1/Hygro(+)-ATF4, and the position of forward and backward primers for genomic PCR. b Detection of the ATF4 transgene integrated into the CHO genome. Numbers 10, 12, 16 and 17 are clone numbers. Positive indicates the positive control, pcDNA3.1(+)-ATF4. DP-12-SF is the genomic content of the host cell before transfection. Mock is the genomic content of the host cell (CHO DP-12-SF) after transfection with empty vector pcDNA3.1(+)/Hygro. c Analysis of ATF4 expression after 72 h of cultivation. RNA was extracted, reverse transcribed into cDNA, and then amplified by PCR with the pcDNA3.1 Forward and Backward primers
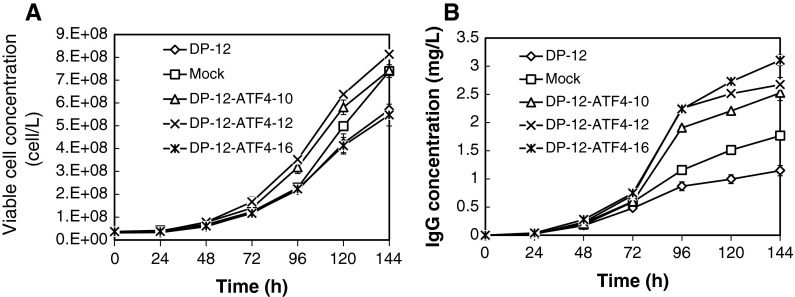
To evaluate cell growth and IgG production in the three clones, batch cultivations were performed using 6-well plates. The clones, DP-12-ATF4-12, -10, and the mock transfected cells showed similar high viable cell concentrations at 144 h of 8.1 × 105, 7.4 × 105, and 7.4 × 105 cells/ml, respectively. In addition, DP-12-SF and DP-12-ATF4-16 showed lower viable cell concentrations after 144 h of 5.7 × 105 and 5.5 × 105 cells/ml, respectively (Fig. 3a). A significant increase in IgG concentration was observed in clones DP-12-ATF4-10, -12, and -16, which showed positive expression of the pc-DNA3.1-ATF4/Hygro(+) insert (Fig. 3b).
Fig. 3.
Time course of viable cell and IgG concentrations in cultured CHO DP-12-SF cells, mock-transfected cells and ATF4-overexpressing clones 10, 12 and 16 (n = 3). a Viable cell concentration. b IgG concentration
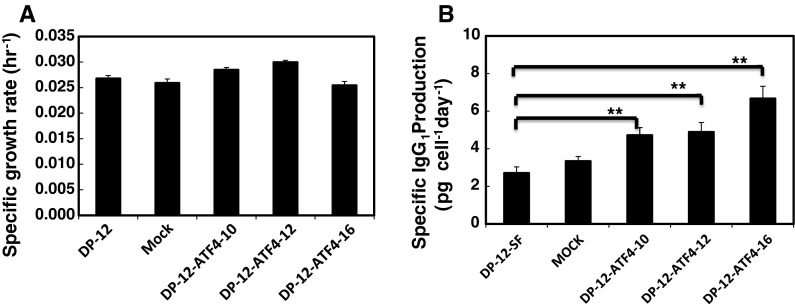
The specific production rate was evaluated from time courses of viable cell and IgG concentrations (Fig. 4). The results showed that clones DP-12-ATF4-10, -12, and -16 showed high specific production rates of 4.8, 4.9 and 7.2 pg/cell/day, respectively, compared with the mock transfected cells and the DP-12-SF clone, which produced 2.8 and 3.6 pg/cell/day, respectively. Therefore, the specific rates for clones 10, 12, 16 increased approximately 1.7, 1.7 and 2.5 times, respectively, compared with the original DP-12-SF cell line (Fig. 4b).
Fig. 4.
Specific IgG1 production rates and the specific growth rates of the confirmed clones, in addition to the DP-12-SF clone and the mock-transfected cells (n = 3). Comparison of the specific growth and IgG production rates between CHO DP-12-SF cells, mock-transfected cells and ATF4 overexpressing clones 10, 12 and 16. Error bars represent the standard error of the mean (n = 3; triple determination from each of three independent culture samples). a Specific growth rate. b Specific IgG production rate **P <0.001, two sided unpaired t test
Regarding the specific growth rates of the ATF4-expressing clones, clones DP-ATF4-10, -12 showed slightly higher specific growth rates of 0.029 and 0.030 h−1, respectively (Fig. 4a). Clone DP-ATF4-16 showed the same specific growth rate, 0.025 h−1, as the DP-12-SF clone and the mock transfected cell line (0.026 h−1 for both). Clone DP-ATF4-16 was selected for further study of ATF4 overexpression because of its high productivity and growth rate. The results of this experiment were in agreement with our previous results which showed that overexpression of ATF4 increased the total production of antithrombin III and the specific production rate of antithrombin III (Ohya et al. 2008).
Quantification of ATF4, CHOP and IgG mRNAs
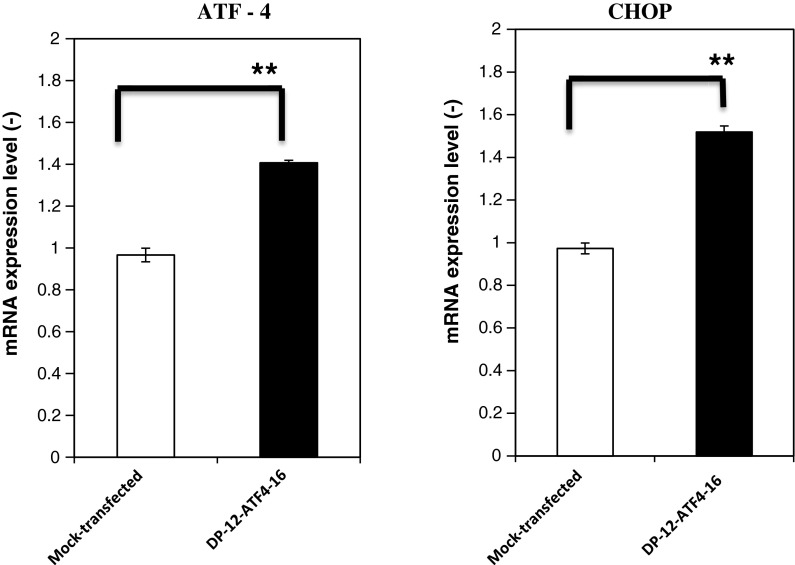
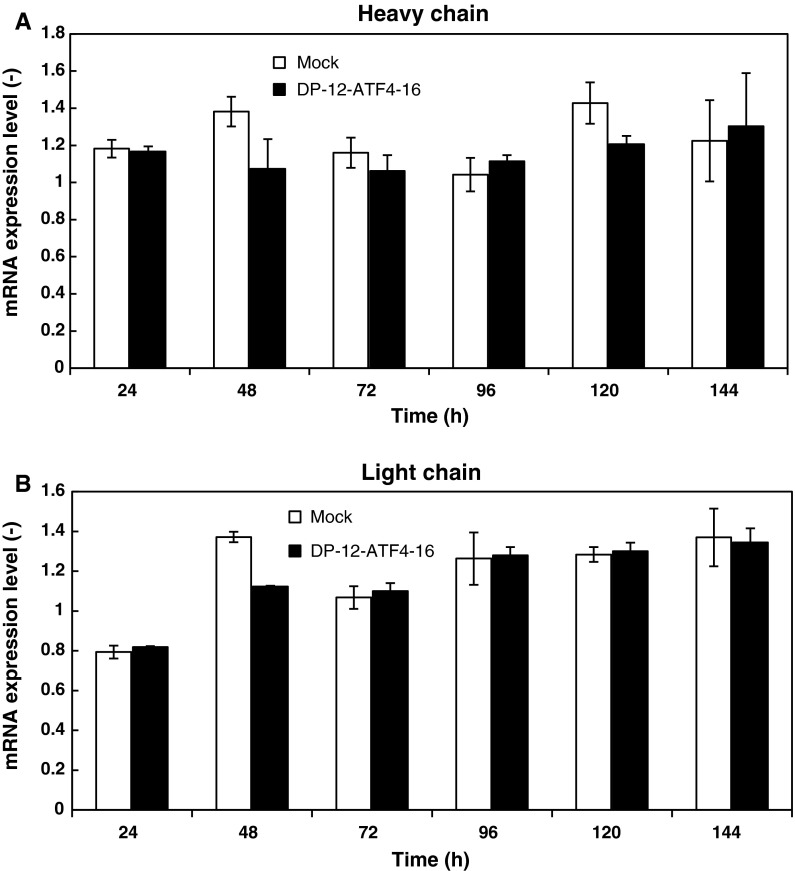
It has been reported that ATF4 expression is able to induce the expression of subsets of UPR target genes, including CHOP (Harding et al. 2002). In order to elucidate the reason for the enhancement of IgG production by ATF4 overexpression, the levels of ATF4 and CHOP mRNAs expressed by clone DP-12-ATF4-16 were measured. The results showed that ATF4 expression was slightly increased in the overexpressing cells by approximately 1.4 times compared with the mock-transfected cell line (Fig. 5). CHOP expression reached approximately 1.58 times compared with the mock-transfected cell line (Fig. 5). These results are in accordance with our previous results showing that ATF4 expression was slightly increased, while CHOP expression was increased further (Ohya et al. 2008). In order to confirm that the levels of heavy and light chain mRNAs were not affected by the overexpression of ATF4, quantitative mRNA measurement was performed during batch cultivation (Fig. 6). The relative expression levels of heavy and light chain mRNAs in DP-12-ATF4-16 did not change significantly through the time course of batch culture compared with the mock-transfected cell line.
Fig. 5.
Real-time RT-PCR analysis of ATF4 and CHOP mRNAs in the DP-12-ATF4-16 clone. Total RNA was isolated from CHO cells after 72 h of cultivation. The mRNA levels were standardized to β-actin in CHO cells and were then compared to the expression level in the original host cell line at 72 h. Error bars represent the standard error of the mean (n = 3; single determination from each of three independent culture samples). **P < 0.001, two sided unpaired t test
Fig. 6.
Time course of heavy chain and light chain relative mRNA expression levels in both mock transfected and DP-12-ATF4-16 cells. The mRNA levels were standardized to β-actin expression in CHO DP-12-SF cells and were then compared to the expression level in the original host cell line at the same time interval. Error bars represent the standard error of the mean (n = 3)
UPR is thought to be associated with the production of most recombinant proteins (Feige et al. 2010; Nishimiya et al. 2013). In order to obtain productive cells with higher specific production rates, it is important to engineer the secretory pathway (Hou et al. 2012; Le Fourn et al. 2013). ATF4 overexpression can enhance the dephosphorylation of phosphorylated eIF2α by activating the expression of GADD34 (Ohya et al. 2008; Omasa et al. 2008). In addition, the production of heterologous protein is always accompanied by overproduction of disulfide bonds inside the ER, which leads to the production of reactive oxygen species (ROS) (Shimizu and Hendershot 2009). Although ROS are formed in all cellular compartments, the ER appears to be a major place for their production. Oxidative protein folding in the ER is responsible for about one-fourth of the ROS produced in a professional secretory cell (Tu and Weissman 2004).
Studies on ATF4-null cells revealed that ATF4 regulates a number of genes important for protection against oxidative stress (Lange et al. 2008). These include genes involved in the import and metabolism of thiol-containing amino acids that serve as precursors to glutathione, and proteins involved in redox reactions such as heme oxygenase-1 (Harding et al. 2003), glutathione peroxidase, and peroxiredoxin-1 (Kitiphongspattana et al. 2007), which protect the cell from oxidative stress (Shimizu and Hendershot 2009). ATF4 acts to relieve this ROS stress inside the cell in different ways. Overexpression of ATF4 was shown to enhance CHOP expression in mouse embryonic stem cells (Harding et al. 2003) and in antithrombin III secreting CHO cells (Ohya et al. 2008).
In this study, all clones with confirmed expression of ATF4 showed a higher total antibody production. ATF4 and CHOP were significantly elevated in clone DP-12-ATF4-16, which revealed 2.5 times increased productivity with no change in growth rate. Recently, it was reported that CHOP might help to improve antibody secretion (Nishimiya et al. 2013). Although CHOP is considered to be a pre-apoptotic gene, it did not affect the growth of any of the confirmed clones in this study. We used CMV promoter for evaluation of ectopic CHO ATF4 expression. The suitable expression level for ATF4 for enhancing antibody secretion without inducing apoptosis is not clear. To elucidate this point, the effect of ATF4 expression should be investigated using controllable expression system, i.e., Tet-on/off system in future.
Regarding the quantitative measurement of the heavy and light chain mRNAs during cultivation, the results were in agreement with our previous data showing that antithrombin III mRNA did not change significantly but the specific production rate increased (Omasa et al. 2008). The increased specific production rate correlated with the release of translation attenuation by dephosphorylation of phosphorylated eIF2-α. This leads to the conclusion that the overexpression of ATF4 did not affect the transcription of the heavy and light chains. Overexpression of ATF4 might enhance its translation, leading to increased secretion of antibody as with AT-III production. These results suggest that the assembly process of heavy and lights chain is not the rate-limiting step in antibody production. Recently, new type of complicated artificial antibody with the Fc portion (i.e., single-chain diabody Fc) has been attractive molecules as new therapeutic reagents (Asano et al. 2008; Onitsuka et al. 2012). It is estimated that the assembly process is rate-limiting step for these complicated artificial antibody production in CHO cells. We are now trying to investigate the effect of the overexpression of ATF4 on the production of these artificial antibodies.
Finally, we can conclude that ATF4-mediated translational control is likely a promising strategy for improving the production of secreted protein pharmaceuticals in CHO cells. The action of ATF4 in increasing the specific productivity is not cell line specific. Not only did ATF4 enhance the specific productivity of antithrombin III by twofold compared with the parental 13D-35D cell line, but it also enhanced the specific productivity of IgG by 2.5-fold compared with the parental DP-12-SF cell line.
Acknowledgments
This work was partially supported by the Program for the Promotion of Fundamental Studies in Health Sciences of National Institute of Biomedical Innovation (NIBIO), and a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS).
References
- Asano R, Kawaguchi H, Watanabe Y, Nakanishi T, Umetsu M, Hayashi H, Katayose Y, Unno M, Kudo T, Kumagai I. Diabody-based recombinant formats of humanized IgG-like bispecific antibody with effective retargeting of lymphocytes to tumor cells. J Immunother. 2008;31:752–761. doi: 10.1097/CJI.0b013e3181849071. [DOI] [PubMed] [Google Scholar]
- Barnes LM, Dickson AJ. Mammalian cell factories for efficient and stable protein expression. Curr Opin Biotechnol. 2006;17:381–386. doi: 10.1016/j.copbio.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Barnes LM, Bentley CM, Dickson AJ. Molecular definition of predictive indicators of stable protein expression in recombinant NS0 myeloma cells. Biotechnol Bioeng. 2004;85:115–121. doi: 10.1002/bit.10893. [DOI] [PubMed] [Google Scholar]
- Brewer JW, Hendershot LM. Building an antibody factory: a job for the unfolded protein response. Nat Immunol. 2005;6:23–29. doi: 10.1038/ni1149. [DOI] [PubMed] [Google Scholar]
- Butler M, Meneses-Acosta A. Recent advances in technology supporting biopharmaceutical production from mammalian cells. Appl Microbiol Biotechnol. 2012;96:885–894. doi: 10.1007/s00253-012-4451-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige MJ, Groscurth S, Marcinowski M, Shimizu Y, Kessler H, Hendershot LM, Buchner J. An unfolded CH1 domain controls the assembly and secretion of IgG antibodies. Mol Cell. 2009;34:569–579. doi: 10.1016/j.molcel.2009.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feige MJ, Hendershot LM, Buchner J. How antibodies fold. Trends Biochem Sci. 2010;35:189–198. doi: 10.1016/j.tibs.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez E, Powell ML, Bevington A, Herbert TP. A decrease in cellular energy status stimulates PERK-dependent eIF2alpha phosphorylation and regulates protein synthesis in pancreatic beta-cells. Biochem J. 2008;410:485–493. doi: 10.1042/BJ20071367. [DOI] [PubMed] [Google Scholar]
- Harding HP, Calfon M, Urano F, Novoa I, Ron D. Transcriptional and translational control in the Mammalian unfolded protein response. Annu Rev Cell Dev Biol. 2002;18:575–599. doi: 10.1146/annurev.cellbio.18.011402.160624. [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, Stojdl DF, Bell JC, Hettmann T, Leiden JM, Ron D. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell. 2003;11:619–633. doi: 10.1016/S1097-2765(03)00105-9. [DOI] [PubMed] [Google Scholar]
- Hou J, Tyo KE, Liu Z, Petranovic D, Nielsen J. Metabolic engineering of recombinant protein secretion by Saccharomyces cerevisiae. FEMS Yeast Res. 2012;12:491–510. doi: 10.1111/j.1567-1364.2012.00810.x. [DOI] [PubMed] [Google Scholar]
- Hsu TA, Betenbaugh MJ. Coexpression of molecular chaperone BiP improves immunoglobulin solubility and IgG secretion from Trichoplusia ni insect cells. Biotechnol Prog. 1997;13:96–104. doi: 10.1021/bp960088d. [DOI] [PubMed] [Google Scholar]
- Huang YM, Hu W, Rustandi E, Chang K, Yusuf-Makagiansar H, Ryll T. Maximizing productivity of CHO cell-based fed-batch culture using chemically defined media conditions and typical manufacturing equipment. Biotechnol Prog. 2010;26:1400–1410. doi: 10.1002/btpr.436. [DOI] [PubMed] [Google Scholar]
- Khan SU, Schroder M. Engineering of chaperone systems and of the unfolded protein response. Cytotechnology. 2008;57:207–231. doi: 10.1007/s10616-008-9157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WD, Tokunaga M, Ozaki H, Ishibashi T, Honda K, Kajiura H, Fujiyama K, Asano R, Kumagai I, Omasa T, Ohtake H. Glycosylation pattern of humanized IgG-like bispecific antibody produced by recombinant CHO cells. Appl Microbiol Biotechnol. 2010;85:535–542. doi: 10.1007/s00253-009-2152-z. [DOI] [PubMed] [Google Scholar]
- Kitiphongspattana K, Khan TA, Ishii-Schrade K, Roe MW, Philipson LH, Gaskins HR. Protective role for nitric oxide during the endoplasmic reticulum stress response in pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2007;292:E1543–E1554. doi: 10.1152/ajpendo.00620.2006. [DOI] [PubMed] [Google Scholar]
- Kober L, Zehe C, Bode J. Optimized signal peptides for the development of high expressing CHO cell lines. Biotechnol Bioeng. 2013;110:1164–1173. doi: 10.1002/bit.24776. [DOI] [PubMed] [Google Scholar]
- Ku SC, Ng DT, Yap MG, Chao SH. Effects of overexpression of X-box binding protein 1 on recombinant protein production in Chinese hamster ovary and NS0 myeloma cells. Biotechnol Bioeng. 2008;99:155–164. doi: 10.1002/bit.21562. [DOI] [PubMed] [Google Scholar]
- Lange PS, Chavez JC, Pinto JT, Coppola G, Sun CW, Townes TM, Geschwind DH, Ratan RR. ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J Exp Med. 2008;205:1227–1242. doi: 10.1084/jem.20071460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Fourn V, Girod PA, Buceta M, Regamey A, Mermod N (2013) CHO cell engineering to prevent polypeptide aggregation and improve therapeutic protein secretion. Metab Eng. doi:10.1016/j.ymben.2012.12.003 [DOI] [PubMed]
- Lee KH, Onitsuka M, Honda K, Ohtake H, Omasa T (2013) Rapid construction of transgene-amplified CHO cell lines by cell cycle checkpoint engineering. Appl Microbiol Biotechnol 97:5731–5741. doi:10.1007/s00253-013-4923-9 [DOI] [PubMed]
- Mohan C, Park SH, Chung JY, Lee GM. Effect of doxycycline-regulated protein disulfide isomerase expression on the specific productivity of recombinant CHO cells: thrombopoietin and antibody. Biotechnol Bioeng. 2007;98:611–615. doi: 10.1002/bit.21453. [DOI] [PubMed] [Google Scholar]
- Nishimiya D, Mano T, Miyadai K, Yoshida H, Takahashi T. Overexpression of CHOP alone and in combination with chaperones is effective in improving antibody production in mammalian cells. Appl Microbiol Biotechnol. 2013;97:2531–2539. doi: 10.1007/s00253-012-4365-9. [DOI] [PubMed] [Google Scholar]
- O’Callaghan PM, McLeod J, Pybus LP, Lovelady CS, Wilkinson SJ, Racher AJ, Porter A, James DC. Cell line-specific control of recombinant monoclonal antibody production by CHO cells. Biotechnol Bioeng. 2010;106:938–951. doi: 10.1002/bit.22769. [DOI] [PubMed] [Google Scholar]
- Ohya T, Hayashi T, Kiyama E, Nishii H, Miki H, Kobayashi K, Honda K, Omasa T, Ohtake H. Improved production of recombinant human antithrombin III in Chinese hamster ovary cells by ATF4 overexpression. Biotechnol Bioeng. 2008;100:317–324. doi: 10.1002/bit.21758. [DOI] [PubMed] [Google Scholar]
- Omasa T. Gene amplification and its application in cell and tissue engineering. J Biosci Bioeng. 2002;94:600–605. doi: 10.1016/s1389-1723(02)80201-8. [DOI] [PubMed] [Google Scholar]
- Omasa T, Takami T, Ohya T, Kiyama E, Hayashi T, Nishii H, Miki H, Kobayashi K, Honda K, Ohtake H. Overexpression of GADD34 enhances production of recombinant human antithrombin III in Chinese hamster ovary cells. J Biosci Bioeng. 2008;106:568–573. doi: 10.1263/jbb.106.568. [DOI] [PubMed] [Google Scholar]
- Omasa T, Onitsuka M, Kim WD. Cell engineering and cultivation of Chinese hamster ovary (CHO) cells. Curr Pharm Biotechnol. 2010;11:233–240. doi: 10.2174/138920110791111960. [DOI] [PubMed] [Google Scholar]
- Onitsuka M, Kim WD, Ozaki H, Kawaguchi A, Honda K, Kajiura H, Fujiyama K, Asano R, Kumagai I, Ohtake H, Omasa T. Enhancement of sialylation on humanized IgG-like bispecific antibody by overexpression of alpha2, 6-sialyltransferase derived from Chinese hamster ovary cells. Appl Microbiol Biotechnol. 2012;94:69–80. doi: 10.1007/s00253-011-3814-1. [DOI] [PubMed] [Google Scholar]
- Parekh R, Forrester K, Wittrup D. Multicopy overexpression of bovine pancreatic trypsin inhibitor saturates the protein folding and secretory capacity of Saccharomyces cerevisiae. Protein Expr Purif. 1995;6:537–545. doi: 10.1006/prep.1995.1071. [DOI] [PubMed] [Google Scholar]
- Park JY, Takagi Y, Yamatani M, Honda K, Asakawa S, Shimizu N, Omasa T, Ohtake H. Identification and analysis of specific chromosomal region adjacent to exogenous Dhfr-amplified region in Chinese hamster ovary cell genome. J Biosci Bioeng. 2010;109:504–511. doi: 10.1016/j.jbiosc.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Randall TD, Parkhouse RM, Corley RB. J chain synthesis and secretion of hexameric IgM is differentially regulated by lipopolysaccharide and interleukin 5. Proc Natl Acad Sci USA. 1992;89:962–966. doi: 10.1073/pnas.89.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder M, Friedl P. Overexpression of recombinant human antithrombin III in Chinese hamster ovary cells results in malformation and decreased secretion of recombinant protein. Biotechnol Bioeng. 1997;53:547–559. doi: 10.1002/(SICI)1097-0290(19970320)53:6<547::AID-BIT2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Hendershot LM. Organization of the functions and components of the endoplasmic reticulum. Adv Exp Med Biol. 2007;594:37–46. doi: 10.1007/978-0-387-39975-1_4. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Hendershot LM. Oxidative folding: cellular strategies for dealing with the resultant equimolar production of reactive oxygen species. Antioxid Redox Signal. 2009;11:2317–2331. doi: 10.1089/ars.2009.2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tigges M, Fussenegger M. Xbp1-based engineering of secretory capacity enhances the productivity of Chinese hamster ovary cells. Metab Eng. 2006;8:264–272. doi: 10.1016/j.ymben.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Tu BP, Weissman JS. Oxidative protein folding in eukaryotes: mechanisms and consequences. J Cell Biol. 2004;164:341–346. doi: 10.1083/jcb.200311055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner RG. Economic aspects of commercial manufacture of biopharmaceuticals. J Biotechnol. 2004;113:171–182. doi: 10.1016/j.jbiotec.2004.04.036. [DOI] [PubMed] [Google Scholar]
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22:1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T, Nakanishi F, Ogura Y, Oi D, Omasa T, Katakura Y, Kishimoto M, Suga K. Amplified gene location in chromosomal DNA affected recombinant protein production and stability of amplified genes. Biotechnol Prog. 2000;16:710–715. doi: 10.1021/bp000114e. [DOI] [PubMed] [Google Scholar]
- Zheng K, Bantog C, Bayer R. The impact of glycosylation on monoclonal antibody conformation and stability. MAbs. 2011;3:568–576. doi: 10.4161/mabs.3.6.17922. [DOI] [PMC free article] [PubMed] [Google Scholar]