Abstract
Introduction:
Vertigo, dizziness, and nausea encompass a spectrum of balance-related symptoms caused by a variety of etiologies. Balance is affected by many systems: Proprioceptive pathways and visual, cerebellar, vestibulocochlear, and vascular / vasovagal systems. Vertigo is a subtype of dizziness, in which a subject, as a result to a dysfunction of the vestibular system, improperly experiments the perception of motion. The most useful clinical subdivision is to categorize vertigo into true vertigo and pseudovertigo, whereas from a pathophysiological point of view, vertigo can be classified into central, peripheral, and psychogenic. It is not easy to identify the cause of vertigo since the patients often are not able to precisely describe their symptoms. An impressive list of drugs may cause vertigo or dizziness.
Materials and Methods:
The aim of the present study was to analyze the data extracted from the reporting cards of the ADRs (adverse drug reactions), received at our Pharmacovigilance Regional Center (Calabria, Italy) in 2012. In particular, the data concerning the occurrence of vertigo and dizziness, after taking certain classes of drugs, have been considered.
Results:
Our results show that, among the side-effects of different classes of drugs such as anti-convulsants, anti-hypertensives, antibiotics, anti-depressants, anti-psychotics, and anti-inflammatory, also vertigo or dizziness are included.
Conclusions:
Spontaneous reports of vertigo or dizziness, as side-effect of certain drugs, received at our Pharmacovigilance Center, represented the 5% of all reports in 2012. Considering the high incidence of such an ADR for several drugs’ classes, it can be speculated that under-reporting also affect vertigo and dizziness. Despite the fact that these ADRs might not represent a direct threaten for life, indirectly they can cause secondary damage to patients such as falls, fractures etc. Balance should be accurately monitored during drug use and particularly in fragile patients.
Keywords: ADRs, drugs, dizziness, pharmacovigilance, reporting cards
INTRODUCTION
Dizziness and vertigo are among the most common disorders in medicine, affecting approximately 20-30% of people in the general population.[1,2,3] Dizziness is a general term used to express subjective complaints of the patients related to changes in sensation, movement, perception, or consciousness.[3,4] Vertigo is a subtype of dizziness, defined as an illusion of movement caused by asymmetric involvement of the vestibular system.[1,2,4,5,6] The incidence of vertigo increases with age and is about two to three times higher in women than in men.[2,6] Dizziness or vertigo originates from a mismatch between three sensory systems: The vestibular, the visual, and the somatosensory systems. Therefore, vertigo/dizziness is a multisensory syndrome and not a single disease entity.[3,4,5,6,7]
Different classifications of vertigo exist [Table 1] First of all, vertigo can be classified into true vertigo and pseudovertigo.[8,9,10] True vertigo denotes a sense of rotation in one direction, which may be “subjective,” when the subject feels that he is moving while the surrounding environment remains static or “objective” when he feels the surrounding objects are moving around him. The objective vertigo can itself be divided into rotary vertigo, in which the patient perceives that the space moves around him more or less vortically, into wavelike vertigo, in which the patient perceives that the space moves in a direction of oscillation on the transversal or longitudinal axis, and sussultatory vertigo, in which the patient perceives a feeling of dragging upward or downward (as in the elevator, or as floor which sinks). Pseudovertigo, more common than true vertigo, includes all sensations of imbalance, different from true vertigo. While true vertigo occurs as a result of primary neurological causes, non-neurological causes can often induce pseudovertigo. Furthermore, while true vertigo occurs as a single episode or as recurrent stereotyped attacks of defined durations, pseudovertigo, instead, occurs either as a very prolonged sensation lasting for hours or even days, or as momentary attacks.[11] Furthermore, vertigo may occur as a normal response to certain stimuli; this form of vertigo is called physiological vertigo. Healthy people may experience vertigo when they are travelling by car, boat, or spaceship (motion sickness) or on looking down from a mountain or from a tall building (height vertigo).[8,9,10,11] Vertigo may also be caused by diseases affecting the labyrinth (peripheral vestibular vertigo), the central vestibular system (central vestibular vertigo) or other functional systems (non-vestibular vertigo); this form of vertigo is generally called “pathological vertigo.”[8,9,10,11]
Table 1.
Characteristics of peripheral and central vertigo
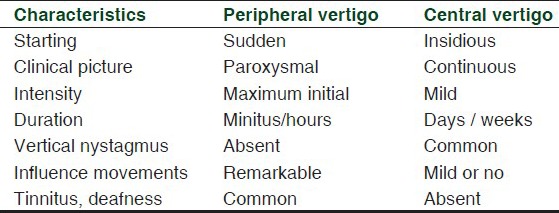
From a pathophysiological point of view, the vertigo can be classified into central, peripheral, and psychogenic; central vertigo is frequently accompanied by neurologic signs and symptoms, which are the result of a disease originating from the central nervous system (CNS).[10,11,12] The most important causes of central vertigo are cerebellar hemorrhage, brainstem ischemia, and vertebrobasilar insufficiency; other causes include infections, trauma, CNS tumors, and multiple sclerosis.[1,12,13,14,15,16,17] The most common form of vertigo in patients is the peripheral vertigo, often accompanied by nausea or vomiting, with no other neurologic signs.[12] The most common cause of peripheral vertigo is Benign Paroxysmal Positional vertigo (BPPV),[18] a clinical syndrome with unknown exact etiology, characterized by recurrent, brief episodes of severe vertigo and rotary nystagmus, precipitated by specific positions of the head relative to gravity. The physical symptoms of BPPV may interfere with normal daily functional activities, but it can be easily and effectively treated if identified.[18] It is known that BPPV incidence increases with age,[19,20,21] whereas the prevalence of BPPV in the young adult population is very low;[22] in older people, BPPV is often associated with depression, reduced activities of daily living, and falls.[23] Other causes of peripheral vertigo are acute labyrinthitis,[12] vestibular neuronitis,[12,24,25] Meniere's disease,[12,24,26] labyrinthic concussion,[12,24] cholesteatoma,[12,24] labyrinthine infarction /ischemia,[12,24,27] labyrinthine fistula,[12,24] malignant otitis externa,[12,24] cerumen impaction,[12,24] and tympanic membrane rupture.[12,24] The most important characteristics of peripheral and central vertigo are reported in Table 1.
Psychogenic vertigo is a form of vertigo, which may occur concurrently with hyperventilation, anxiety, panic attacks, claustrophobia, agoraphobia, and other psychiatric disturbances including schizophrenia.[28]
Several drugs and environmental chemicals, such as lead and mercury, can cause ototoxicity. The latter often causes damage to the inner ear or auditory nerve and leads to the occurrence of vertigo. The damage can be temporary or permanent.[29,30]
Since patients with vertigo often have difficulty to describe their symptoms, determination of causes can be very difficult. The diagnosis of vertigo is most often based upon history and clinical presentation of the patient. Very interesting clues for the diagnosis of vertigo, indeed, come from the patient's medical history, including drugs, trauma, or exposure to toxic substances. Age is associated with some underlying conditions that can cause vertigo. As an example, elderly patients, especially those with diabetes or hypertension, are at higher risk of cerebrovascular causes of vertigo. For the diagnosis of vertigo, the physician must first ask a series of questions regarding family history, including hereditary conditions such as migraine and risk factors for cerebrovascular diseases. After confirming that the patient has vertigo, the physician must determine whether the subject presents a peripheral or central origin.[5,15,17,31] Associated symptoms such as pain, nausea, vomiting, hearing loss, or neurologic symptoms can help differentiate the cause of vertigo: Indeed, in severe episodes of Ménière's disease, in acute vestibular neuronitis and in BPPV, vertigo often is associated with nausea or vomiting, whereas if the cause of vertigo is central, nausea and vomiting tend to be less severe.[17,23,24,25,31] Furthermore, most causes of vertigo with hearing loss are peripheral, whereas neurologic symptoms such as ataxia, weakness, changes in vision or hearing, paraesthesia, dysarthria, altered level of consciousness, or other changes in sensory and motor function favor the presence of a central cause of vertigo. In order to establish the cause of vertigo, it is also useful to know the severity of vertigo over time; indeed in Ménière's disease, vertigo attacks initially increase in severity, whereas their severity decreases later on; in acute vestibular neuronitis, instead, initial symptoms typically are severe but decrease during the subsequent days.[17,31]
Physicians should also pay particular attention to physical findings of the neurologic, head and neck, and cardiovascular systems. Laboratory tests such as electrolytes, glucose, blood counts, and thyroid function tests allow identifying the etiology of vertigo in less than 1 percent of patients. These tests result are appropriate when patients with vertigo exhibit signs or symptoms that suggest the presence of other causes.[31]
Finally, physicians should consider neuroimaging studies in patients with vertigo, which present neurologic signs and symptoms, progressive unilateral hearing loss, or risk factors for cerebrovascular disease. Magnetic resonance of the posterior fossa vasculature may be useful in diagnosing vascular causes of vertigo such as vertebrobasilar insufficiency, thrombosis of the labyrinthine artery, anterior or posterior inferior cerebellar artery insufficiency, and subclavian steal syndrome. Magnetic resonance can be used to exclude large bacterial infections, neoplasms, or developmental abnormalities, if other symptoms suggest one of these diagnoses. However, neuroimaging studies are not indicated in patients with BPPV and usually are not needed to diagnose acute vestibular neuronitis or Meniere's disease.[17,18,24,25,26,31]
The aim of this study was to analyze the data, received in our Regional Center of Documentation and Information on the Drug (Mater Domini Hospital, Catanzaro) in 2012, concerning the occurrence of vertigo and dizziness, as adverse effect of certain classes of drugs and report a brief review of the drugs that may induce such ADRs.
MATERIALS AND METHODS
We have analyzed 22 reports, received in our Regional Center of Documentation and Information on the Drug in 2012, concerning the occurrence of vertigo and dizziness, as ADRs of certain classes of drugs. The reporting cards of ADRs have been compiled by hospital doctors, specialists, general practitioners, and pharmacists who work in “Mater Domini” Hospital of Catanzaro (Calabria, Italy), in “Pugliese-Ciaccio” Hospital of Catanzaro (Calabria, Italy), in Hospital of Cosenza (Calabria, Italy), in Hospital of Reggio Calabria (Calabria, Italy), and in the LSC (Local Sanitation Company) of Catanzaro (Calabria, Italy), Crotone (Calabria, Italy), Vibo Valentia (Calabria, Italy), Reggio Calabria (Calabria, Italy), Paola (Calabria, Italy), Lamezia Terme (Calabria, Italy), Cosenza (Calabria, Italy), and Locri (Calabria, Italy).
RESULTS
Analyzing the data concerning the reports of vertigo and dizziness as ADRs in 2012, it was found that, half of these reports comes from the use of anti-epileptic drugs such as lamotrigine,[32] oxcarbazepine[32] and carbamazepine[32] (blockers of the voltage-gated sodium channels), lacosamide[33] (drug that, in vitro, selectively enhances slow inactivation of voltage-gated sodium channels, resulting in stabilization of hyperexcitable neuronal membranes), and clonazepam[34] (benzodiazepine with strong anticonvulsant properties) used in partial and generalized seizures, including tonic-clonic seizures, in focal seizures and mixed forms, alone or in combination with other drugs. Furthermore, from our analysis, it was found that drugs belonging to the class of antihypertensives may also cause vertigo or dizziness as adverse effect, more precisely the calcium antagonist amlodipine[35] (a long-acting calcium channel blocker, belonging to the class of dihydropyridines), and the association between irbesartan[36] (angiotensin-II receptor blockers or ARB) and hydrochlorothiazide[37] (thiazide diuretic). Our results have also shown that anti-depressant drugs, such as mirtazapine[38] (presynaptic α2-antagonist active at central level, which induces an increase in central noradrenergic and serotonergic neurotransmission and antagonist of H1-histaminergic receptors), paroxetine[39] (potent and selective serotonin reuptake inhibitor), and sertraline[39] (selective serotonin reuptake inhibitor) often are able to induce vertigo/dizziness accompanied by asthenia. Furthermore, from the analysis of our data, it was observed that antibiotics such as ciprofloxacin[40] (a fluoroquinolone antibacterial agent) and the association between amoxicillin and clavulanic acid[41] (β-lactam antibiotic) may induce adverse reactions characterized by dyspnea, nausea, and dizziness. Finally, after analyzing our data, it was noted that other drugs can possibly induce vertigo or dizziness, such as silodosin[42] (drug useful in the treatment of the signs and symptoms of benign prostatic hyperplasia, highly selective for α1A-adrenoreceptors), pantoprazole[43] (proton pump inhibitor), lornoxicam[44] (non-steroidal anti-inflammatory drug, belonging to the class of oxicams, with analgesic properties), aripiprazole[45] (effective antipsychotic in schizophrenia and in bipolar disorder type I), and atazanavir[46] (azapeptide HIV-1 protease inhibitor).
A list of drugs that may cause, among the side-effects, vertigo or dizziness is reported in Table 2.
Table 2.
Classification of drugs that may cause vertigo or dizziness as an ADR

DISCUSSION
The list of drugs that may cause vertigo or dizziness is impressive. It includes anti-convulsants, anesthetics, anti-depressants, analgesics, anti-diabetics, contraceptives, anti-inflammatory drugs, cardiovascular drugs, sedatives, tranquillizers, cytotoxic agents, and anti-hypertensive agents.[29,30] The main purpose of this study was that to examine the data, received in our Regional Center of Documentation and Information on the Drug (Mater Domini Hospital, Catanzaro, Calabria, Italy) in 2012, related to the occurrence of vertigo and dizziness, as adverse effect of certain drugs.
From the assessment of the reporting cards, it was found that drugs with anti-convulsant properties, such as oxcarbazepine, carbamazepine, lamotrigine, lacosamide may cause vertigo or dizziness. As already reported, oxcarbazepine, useful in the symptomatic treatment of pain associated with neuropathies, is well tolerated with the most common adverse events consisting of vertigo, tremor, somnolence, hypotension, and nausea.[47,48] Most of the side-effects associated with carbamazepine therapy are mild, transient, and reversible with an adjustment in dosage or rate of dosage increase. In a study of the incidence of side-effects in 220 children below the age of 16 years who were receiving carbamazepine, drowsiness, loss of coordination, and vertigo were the most commonly observed side-effects.[49] Under continuous, long-term use of valproate-lamotrigine, as found in a study, four patients presented heterogeneous disturbances, including vertigo.[50] Lacosamide, efficacy for treatment of patients with partial seizures, is well tolerated, and the most common adverse events are unspecific central nervous system and gastrointestinal effects such as dizziness, vertigo, nausea, and headache.[51,52]
From our analysis, it was also possible to verify that vertigo or dizziness can also be caused by anti-hypertensive drugs, such as amlodipine and the irbesartan-hydrochlorothiazide association. In a previous study, it was found that the overall incidence of adverse reactions due to the use of the telmisartan-amlodipine combination in the treatment of hypertension was 7.69% with headache (1.92%) and vertigo (1.44%) as the commonest side-effects.[53]
Furthermore, as found in reporting cards, among the side-effects of several anti-depressant (mirtazapine and SSRI) and antibiotics drugs (ciprofloxacin or amoxicillin-clavulanic acid association), even vertigo or dizziness were included. It is known that the abrupt discontinuation from the selective serotonin reuptake inhibitor (SSRI) category of anti-depressants commonly causes dizziness or vertigo.[54] The efficacy and toxicity of ciprofloxacin, an orally administered fluoroquinolone, were evaluated in 24 infections in 23 patients with osteomyelitis caused by aerobic gram-negative bacilli. Eosinophilia, nausea, mild elevation in transaminase levels, pruritus, diarrhea, rash, and mild leucopenia were minimal side-effects in most patients, whereas vertigo and acute renal failure were severe reactions.[55]
Finally, from our study, we found that drugs such as silodosin, pantoprazole, lornoxicam, aripiprazole, and atazanavir also induce adverse reactions characterized by vertigo or dizziness. Safety studies of silodosin, a new α1A-adrenoceptor selective antagonist, in healthy Chinese male subjects showed that mild adverse events, including dizziness, postural hypotension, and headache, were observed.[56] Pantoprazole (40 mg once daily) appears to be well tolerated during short term oral administration, with diarrhea (1.5%), headache (1.3%), dizziness (0.7%), pruritus (0.5%), and skin rash (0.4%) representing the most frequent adverse events.[57] Acute aripiprazole poisonings most commonly result in vertigo or dizziness, sedation, sinus tachycardia, nausea / vomiting, or dystonic reactions.[58]
Pharmacovigilance is the science, which allows physicians to have sufficient information related to drugs also increasing knowledge about drug safety. It is based on the spontaneous reporting of events that are supposed to be adverse effects of drugs.[59,60] According to the World Health Organization (WHO), an adverse drug reaction is “a response to a drug that is noxious and unintended and occurs at doses normally used in man for the prophylaxis, diagnosis or therapy of disease, or for modification of physiological function.”[61] Pharmacovigilance through, for example, spontaneous ADR reporting or large-scale databases, is used to generate hypotheses and signals about potential hazards of marketed drugs that require further investigation. Spontaneous reporting of suspected ADRs is particularly useful in identifying rare or delayed reactions; as such a system enables medicines to be monitored throughout their lifetime.[60]
In our study, the analysis of the spontaneous reports of vertigo or dizziness as side-effect of certain drugs, received at our Pharmacovigilance Center, located in Mater Domini Hospital (Catanzaro, Calabria, Italy) has been a precious instrument that has allowed to increase the knowledge concerning the safety and toxicity profile of these drugs; however, for some of them, their ability to induce vertigo or dizziness was already reported in literature. The fact that this group of ADRs only represents 5% of all reported in 2012 and that instead such ADRs are so common for several drug groups might indicate that they are under-reported. This is probably linked to the already known appearance of vertigo/dizziness for several drugs and that in most cases it is no serious or might disappear during treatment. Nevertheless, all ADRs represent a reduction in quality of life and more specifically, vertigo and dizziness might represent an indirect cause of serious adverse events due to falls and subsequent fractures or traumas.
In conclusion, all ADRs should not be under-estimated; safety improvement and patients’ quality of life remain one of the major objectives during pharmacological treatment.
ACKNOWLEDGMENT
The Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA) is kindly acknowledged for its financial and technical support.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Karatas M. Central vertigo and dizziness: Epidemiology, differential diagnosis and common causes. Neurologist. 2008;14:355–64. doi: 10.1097/NRL.0b013e31817533a3. [DOI] [PubMed] [Google Scholar]
- 2.Von Brevern M, Neuhauser H. Epidemiological evidence for a link between vertigo and migraine. J Vestib Res. 2011;21:299–304. doi: 10.3233/VES-2011-0423. [DOI] [PubMed] [Google Scholar]
- 3.Dieterich M. Dizziness. Neurologist. 2004;10:154–16. doi: 10.1097/01.nrl.0000126586.29463.c8. [DOI] [PubMed] [Google Scholar]
- 4.Saccomano SJ. Dizziness, vertigo and presyncope: What's the difference? Nurse Pract. 2012;37:46–52. doi: 10.1097/01.NPR.0000422206.92550.5b. [DOI] [PubMed] [Google Scholar]
- 5.Chawla N, Olshaker JS. Diagnosis and management of dizziness and vertigo. Med Clin North Am. 2006;90:291–304. doi: 10.1016/j.mcna.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Neuhauser HK, Lempert T. Vertigo: Epidemiologic aspects. Semin Neurol. 2009;29:473–81. doi: 10.1055/s-0029-1241043. [DOI] [PubMed] [Google Scholar]
- 7.Brandt T. 2nd ed. London: Springer-Verlag; 2003. Vertigo: Its multisensory Syndromes. [Google Scholar]
- 8.Cherian N. Vertigo as a migraine phenomenon. Curr Neurol Neurosci Rep. 2013;13:343. doi: 10.1007/s11910-013-0343-6. [DOI] [PubMed] [Google Scholar]
- 9.Singh Sura D, Newell S. Vertigo-diagnosis and management in the primary care. BJMP. 2010;3:a351. [Google Scholar]
- 10.Brandt T, Dieterich M, Strupp M. 5th ed. London: Springer-Verlag; 2009. Vertigo and dizziness: Common complaints. [Google Scholar]
- 11.Krishna Das KV. 4th ed. New Delhi, India: Jaypee; 2013. Clinical Medicine: A textbook of Clinical Mehods and Laboratory Investigations. [Google Scholar]
- 12.Thompson TL, Amedee R. Vertigo: A review of common peripheral and central vestibular disorders. Ochsner J. 2009;9:20–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Solomon D. Distinguishing and treating causes of central vertigo. Otolaryngol Clin North Am. 2000;33:579–601. doi: 10.1016/s0030-6665(05)70228-0. [DOI] [PubMed] [Google Scholar]
- 14.Choi KD, Lee H, Kim JS. Vertigo in brainstem and cerebellar strokes. Curr Opin Neurol. 2013;26:90–5. doi: 10.1097/WCO.0b013e32835c5edd. [DOI] [PubMed] [Google Scholar]
- 15.Smouha E. Inner ear disorders. NeuroRehabilitation. 2013;32:455–62. doi: 10.3233/NRE-130868. [DOI] [PubMed] [Google Scholar]
- 16.Schneider JI, Olshaker JS. Vertigo, vertebrobasilar disease, and posterior circulation ischemic stroke. Emerg Med Clin North Am. 2012;30:681–93. doi: 10.1016/j.emc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Lee AT. Diagnosing the cause of vertigo: A practical approach. Hong Kong Med J. 2012;18:327–32. [PubMed] [Google Scholar]
- 18.Parnes LS, Agrawal SK, Atlas J. Diagnosis and management of benign paroxysmal positional vertigo (BPPV) CMAJ. 2003;169:681–93. [PMC free article] [PubMed] [Google Scholar]
- 19.Neuhauser HK, von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, et al. Epidemiology of vestibular vertigo: A neurotologic survey of the general population. Neurology. 2005;65:898–904. doi: 10.1212/01.wnl.0000175987.59991.3d. [DOI] [PubMed] [Google Scholar]
- 20.Batuecas-Caletrio A, Trinidad-Ruiz G, Schaezk C, Del Pozo de Dios JC, De Toro Gil L, Martin-Sanchez V, et al. Benign paroxysmal positional vertigo in the enderly. Gerontology. 2013:408–12. doi: 10.1159/000351204. [DOI] [PubMed] [Google Scholar]
- 21.Von Brevern M, Radtke A, Lezius F, Feldmann M, Ziese T, Lempert T, et al. Epidemiology of benign paroxysmal positional vertigo: A population based study. J Neurol Neurosurg Psychiatry. 2007;78:710–5. doi: 10.1136/jnnp.2006.100420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerrigan MA, Costigan MF, Blatt KJ, Mathiason MA, Domroese ME. Prevalence of benign paroxysmal positional vertigo in the young adult population. PMR. 2013:ii. doi: 10.1016/j.pmrj.2013.05.010. S1934-148200283-9. [DOI] [PubMed] [Google Scholar]
- 23.Oghalai J, Manolidis S, Barth J, Stewart M, Jenkins H. Unrecognised benign paroxysmal positional vertigo in elderly patients. Otolaryngol Head Neck Surg. 2000;122:630–4. doi: 10.1016/S0194-5998(00)70187-2. [DOI] [PubMed] [Google Scholar]
- 24.Post RE, Dickerson LM. Dizziness: A diagnostic approach. Am Fam Psysician. 2010;82:361–9. [PubMed] [Google Scholar]
- 25.Kim YH, Kim KS, Kim KJ, Choi H, Choi JS, Hwang IK. Recurrence of vertigo in patients with vestibular neuritis. Acta Otolaryngol. 2011;131:1172–7. doi: 10.3109/00016489.2011.593551. [DOI] [PubMed] [Google Scholar]
- 26.Shin JE, Kim CH, Park HJ. Vestibular abnormality in patients with Meniere's disease and migrainous vertigo. Acta Otolaryngol. 2013;133:154–8. doi: 10.3109/00016489.2012.727469. [DOI] [PubMed] [Google Scholar]
- 27.Son EJ, Bang JH, Kang JG. Anterior inferior cerebellar artery infarction presenting with sudden hearing loss and vertigo. Laryngoscope. 2007;117:556–8. doi: 10.1097/MLG.0b013e3180303ed0. [DOI] [PubMed] [Google Scholar]
- 28.Shah H, Mukherjee S. Psychogenic Vertigo. Otorhinolaryngol Clin Int J. 2012;4:77–80. [Google Scholar]
- 29.Cianfrone G, Pentangelo D, Cianfrone E, Mazzei F, Turchetta R, Orlando MP, et al. Pharmacological drugs inducing ototoxicity, vestibular symptoms and tinnitus: A reasoned and updated guide. Eur Rev Med Pharmacol Sci. 2011;15:601–36. [PubMed] [Google Scholar]
- 30.Cianfrone G, Pace M, Turchetta R, Cianfrone F, Altissimi G. An update guide on drugs inducing ototoxicity, tinnitus and vertigo. Acta Otorhinolaryngol Ital. 2005;25(5 Suppl 81):3–31. [PubMed] [Google Scholar]
- 31.Labuguen RH. Initial evaluation of vertigo. Am Fam Physician. 2006;73:244–51. [PubMed] [Google Scholar]
- 32.Vohora D, Saraogi P, Yazdani MA, Bhowmik M, Khanam R, Pillai KK. Recent avances in adjunctive therapy for epilepsy: Focus on sodium canne blockers as third-generation antiepileptic drugs. Drug Today (Barc) 2010;46:265–77. doi: 10.1358/dot.2010.46.4.1445795. [DOI] [PubMed] [Google Scholar]
- 33.Beydoun A, D’Souza J, Hebert D, Doty P. Lacosamide: Pharmacology, mechanisms of action and pooled efficacy and safety data in partial-onset seizures. Expert Rev Neurother. 2009;9:33–42. doi: 10.1586/14737175.9.1.33. [DOI] [PubMed] [Google Scholar]
- 34.Jenner P, Pratt JA, Marsden CD. Mechanism of action of clonazepam in myoclonus in relation to effects on GABA and 5-HT. Adv Neurol. 1986;43:629–43. [PubMed] [Google Scholar]
- 35.Shields DL. Calcium channel blockers as initial therapeutic agents in hypertension: Relationship to incident heart failure. Biol Res Nurs. 2013 Jul 16; doi: 10.1177/1099800413494760. [DOI] [PubMed] [Google Scholar]
- 36.Forni V, Wuerzner G, Pruijm M, Burnier M. Long-term use and tolerability of irbesartan for control of hypertension. Integr Blood Press Control. 2011;4:17–26. doi: 10.2147/IBPC.S12211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Greathouse MK, Weir MR. The role of ARBs alone or with HCTZ in the treatment of hypertension and prevention of cardiovascular and renal complications. Postgrad Med. 2012;124:40–52. doi: 10.3810/pgm.2012.03.2535. [DOI] [PubMed] [Google Scholar]
- 38.Benjamin S, Doraiswamy PM. Review of the use of mirtazapine in the treatment of depression. Expert Opin Pharmacother. 2011;12:1623–32. doi: 10.1517/14656566.2011.585459. [DOI] [PubMed] [Google Scholar]
- 39.Fitzgerald KT, Bronstein AC. Selective serotonin reuptake inhibitor exposure. Top Companion Anim Med. 2013;28:13–7. doi: 10.1053/j.tcam.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Lode H, Borner K, Koeppe P. Pharmacodynamics of fluoroquinolones. Clin Infect Dis. 1998;27:33–9. doi: 10.1086/514623. [DOI] [PubMed] [Google Scholar]
- 41.Weber DJ, Tolkoff-Rubin NE, Rubin RH. Amoxicillin and potassium clavulanate: An antibiotic combination. Mechanism of action, pharmacokinetics, antimicrobial spectrum, clinical efficacy and adverse effects. Pharmacoterapy. 1984;4:122–36. doi: 10.1002/j.1875-9114.1984.tb03333.x. [DOI] [PubMed] [Google Scholar]
- 42.Osman NI, Chapple CR, Cruz F, Desgrandchamps F, Llorente C, Montorsi F. Silodosin: A new subtype selective alpha-1 antagonist for the treatment of lower urinary tract symptoms in patients with benign prostatic hyperplasia. Expert Opin Pharmacother. 2012;13:2085–96. doi: 10.1517/14656566.2012.714368. [DOI] [PubMed] [Google Scholar]
- 43.Horn J. The proton-pump inhibitors: Similarities and differences. Clin Ther. 2000;22:266–80. doi: 10.1016/S0149-2918(00)80032-6. [DOI] [PubMed] [Google Scholar]
- 44.Radhofer-Welte S, Rabasseda X. Lornoxicam, a new potent NSAID with an improved tolerability profile. Drugs Today (Barc) 2000;36:55–76. doi: 10.1358/dot.2000.36.1.566627. [DOI] [PubMed] [Google Scholar]
- 45.Hirose T, Uwahodo Y, Yamada S, Miwa T, Kikuchi T, Kitagawa H, et al. Mechanism of action of aripiprazole predicts clinical efficacy and a favourable side-effect profile. J Psychopharmacol. 2004;18:375–83. doi: 10.1177/026988110401800308. [DOI] [PubMed] [Google Scholar]
- 46.Bentuè-Ferre D, Arvieux C, Tribut O, Ruffault A, Bellissant E. Clinical pharmacology, efficacy and safety of atazanavir: A review. Expert Opin Drug Metab Toxicol. 2009;5:1455–68. doi: 10.1517/17425250903321514. [DOI] [PubMed] [Google Scholar]
- 47.McLean MJ, Schmutz M, Wamil AW, Olpe HR, Portet C, Feldmann KF. Oxcarbazepine: Mechanisms of action. Epilepsia. 1994;35(Suppl 3):S5–9. doi: 10.1111/j.1528-1157.1994.tb05949.x. [DOI] [PubMed] [Google Scholar]
- 48.Magenta P, Arghetti S, Di Palma F, Jann S, Sterlicchio M, Bianconi C, et al. Oxcarbazepine is effective and safe in the treatment of neuropathic pain: Pooled analysis of seven clinical studies. Neurol Sci. 2005;26:218–26. doi: 10.1007/s10072-005-0464-z. [DOI] [PubMed] [Google Scholar]
- 49.Pellock JM. Carbamazepine side effects in children and adults. Epilepsia. 1987;28(Suppl 3):S64–70. doi: 10.1111/j.1528-1157.1987.tb05780.x. [DOI] [PubMed] [Google Scholar]
- 50.Thome-Souza S, Moreira B, Valente KD. Late adverse effects of the coadministration of valproate and lamotrigine. Pediatr Neurol. 2012;47:47–50. doi: 10.1016/j.pediatrneurol.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 51.Kellinghaus C. Lacosamide as treatment for partial epilepsy: Mechanisms of action, pharmacology, effects, and safety. Ther Clin Risk Manag. 2009;5:757–66. doi: 10.2147/tcrm.s5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verrotti A, Loiacono G, Pizzolorusso A, Parisi P, Bruni O, Luchetti A, et al. Lacosamide in pediatric and adult patients: Comparison of efficacy and safety. Seizure. 2013;22:210–6. doi: 10.1016/j.seizure.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 53.Faruqui AA. Evaluation of safety and efficacy of telmisartan-amlodipine combination in treating hypertension. J Indian Med Assoc. 2008;106:612–4. [PubMed] [Google Scholar]
- 54.Smith PF, Darlington CL. A possible explanation for dizziness following SSRI discontinuation. Acta Otolaryngol. 2010;130:981–3. doi: 10.3109/00016481003602082. [DOI] [PubMed] [Google Scholar]
- 55.Hessen MT, Ingerman MJ, Kaufman DH, Weiner P, Santoro J, Korzeniowski OM, et al. Clinical efficacy of ciprofloxacin therapy for gram-negative bacillary osteomyelitis. Am J Med. 1987;82:262–5. [PubMed] [Google Scholar]
- 56.Zhou Y, Sun PH, Liu YW, Zhao X, Meng L, Cui YM. Safety and pharmacokinetic studies of silodosin, a new α1A-adrenoceptor selective antagonist, in healthy Chinese male subjects. Biol Pharm Bull. 2011;34:1240–5. doi: 10.1248/bpb.34.1240. [DOI] [PubMed] [Google Scholar]
- 57.Fitton A, Wiseman L. Pantoprazole. A review of its pharmacological properties and therapeutic use in acid-related disorders. Drugs. 1996;51:460–82. doi: 10.2165/00003495-199651030-00012. [DOI] [PubMed] [Google Scholar]
- 58.Young MC, Shah N, Cantrell FL, Clark RF. Risk assessment of isolated aripiprazole exposures and toxicities: A retrospective study. Clin Toxicol (Phila) 2009;47:580–3. doi: 10.1080/15563650902980027. [DOI] [PubMed] [Google Scholar]
- 59.Jeetu G, Anusha G. Pharmacovigilance: A worldwide master key for drug safety monitoring. J Young Pharm. 2010;2:315–20. doi: 10.4103/0975-1483.66802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scicchitano F, Giofrè C, Palleria C, Mazzitello C, Ciriaco M, Gallelli L, et al. Pharmacovigilance and drug safety 2011 in Calabria (Italy): Adverse events analysis. J Res Med Sci. 2012;17:872–5. [PMC free article] [PubMed] [Google Scholar]
- 61.Edwards IR, Aronson JK. Adverse drug reactions: Definitions, diagnosis, and management. Lancet. 2000;356:1255–9. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]


