Abstract
Introduction:
Spontaneous reporting of adverse drug reactions (ADRs) is the basis of pharmacovigilance. In fact, ADRs are associated with a high degree of morbidity and mortality. However, underreporting by all healthcare professionals remains the major problem in Italy and in the rest of the world. The dissemination of pharmacovigilance knowledge among Italian healthcare professionals, and the new pharmacovigilance regulations may promote the early detection and reporting of ADRs. This review examines the legislative framework concerning the pharmacovigilance in Italy.
Materials and Methods:
The information was collected from scientific articles and the websites of the Italian Ministry of Health and the Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA).
Results:
The pharmacovigilance system, both in Italy and Europe, has undergone profound changes. European legislation on pharmacovigilance has been changed in 2010 according to the EU Regulation 1235/2010 and Directive 2010/84/EU. Basically, the changes tend to increase the efficiency, speed and transparency of pharmacovigilance activities. The new Regulation (1235/2010) and the Directive (2010/84/EU) aim to strengthen the system of pharmacovigilance, establish more precisely who is obliged to do what, and allow faster and easier circulation and retrieval of information about ADRs.
Conclusion:
A greater knowledge on what is the Italian pharmacovigilance legislation will be useful to improve the status of ADRs reporting and spread the culture of spontaneous reporting.
Keywords: ADRs, AIFA, Italian, legislation, monitoring
INTRODUCTION
The information gathered during pre-marketing drug development is inevitably incomplete with regard to possible adverse reactions. This is mainly due to:[1]
Animal testing is insufficient to predict human safety;
Patients enrolled in clinical trials are selected and limited in number;
The conditions of use are different from those in clinical practice;
The period of testing is limited.
Clinical trials often lack important information about rare but serious adverse reactions, chronic toxicity, use in special groups (such as children, women, elderly or pregnant) or interactions with other drugs.[1] Therefore, post-marketing surveillance activities are important to allow the early detection of unexpected and/or serious adverse reactions.[1] ADRs occur frequently and lead to a significant number of deaths each year.[2]
It is estimated that only 6-10% of all ADRs are reported: This underestimation is a major problem.[3] Although the extent of under-reporting is widely variable depending on the estimates, it is certain that the number of reported ADRs represents only a small percentage of the total number of occurring ADRs.[4] ADRs have a great impact on public health and represent a significant economic burden on both healthcare systems and society.[2] In fact, it is estimated that in the United States, the total cost of ADRs may be comparable to that of diabetes, as well as it represents the fourth leading cause of death by disease.[5,6] In the European Union (EU), it is estimated that 5% of all hospital admissions are due to ADRs, which are the fifth leading cause of hospital death: Approximately 197,000 deaths per year in the EU are caused by ADRs, and the total cost to the society of ADRs in the EU is about € 79 billion.[7,8,9]
Based on these alarming data, on July 21th, 2012, a new EU pharmacovigilance legislation came into force in order to strengthen and rationalize the EU pharmacovigilance system, with the overall objectives of better protection of public health, ensuring proper functioning of the internal market and simplifying the existing procedures.[7]
History and definitions
Pharmacovigilance was officially born in December 1961 with the publication of a letter (case report) in the Lancet by W. McBride, the Australian doctor who first suspected a causal link between serious fetal deformities (phocomelia) and thalidomide drug used during pregnancy: Thalidomide was used as an antiemetic and sedative agent in pregnant women.[10]
In 1968, the World Health Organization (WHO) promoted the “Programme for International Drug Monitoring”, a pilot project aimed to the centralization of world data on ADRs. In particular, the main aim of the “WHO Programme” is to identify the earliest possible pharmacovigilance signals. Italy joined this international program in 1975.[11] As of April 2013, 112 countries have joined the WHO Programme for International Drug Monitoring, and in addition 32 ‘associate members’ are awaiting full membership [Table 1].[12]
Table 1.
Countries participating in the WHO Programme for International Drug Monitoring, with year of joining*[12]
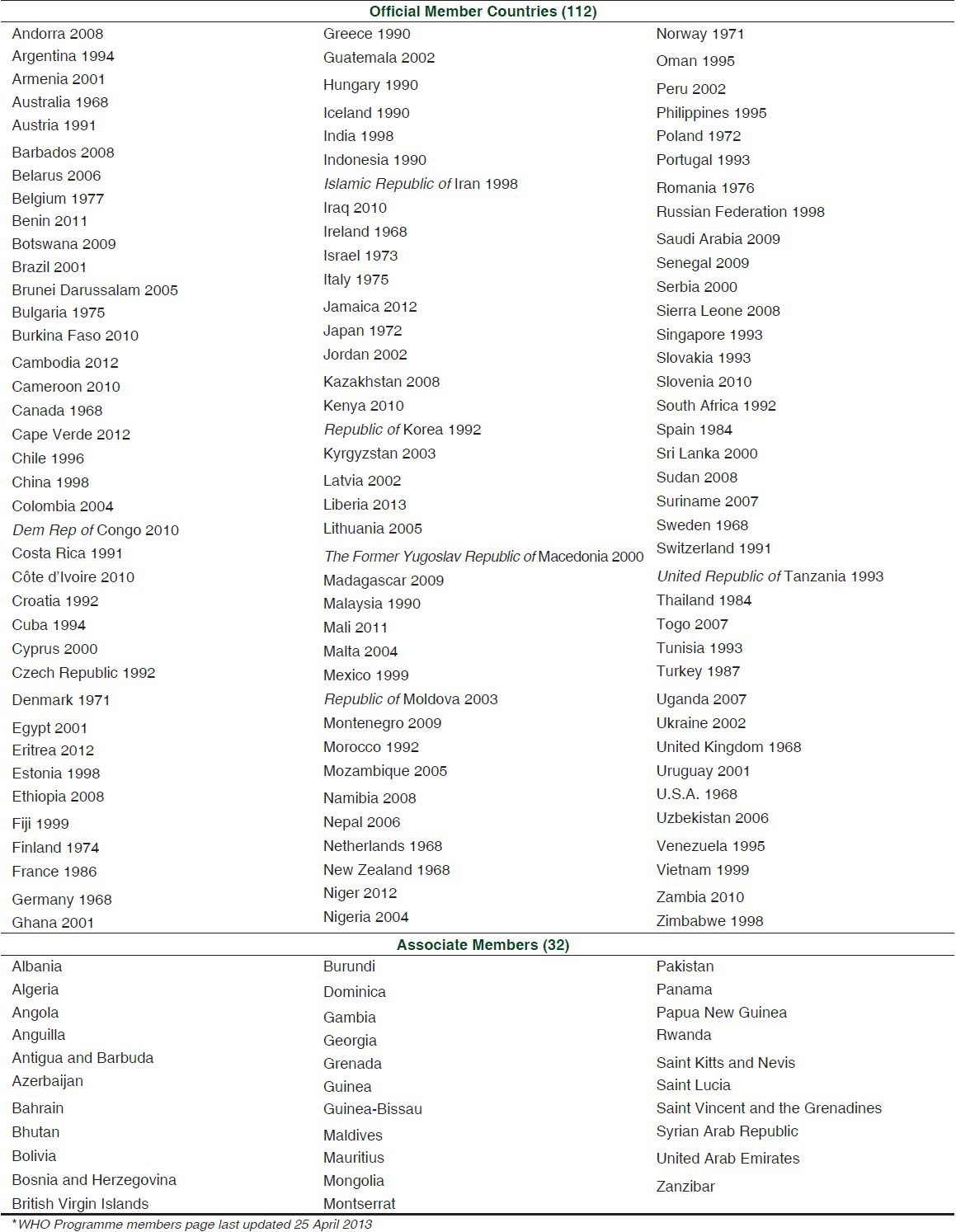
All member countries contribute sending at least quarterly individual case safety reports (ICSRs) to the WHO Global ICSR Database System, named VigiBase™. It is updated with incoming ICSRs on a continuous basis.
The WHO worldwide database of ADRs is located in Uppsala in Sweden, and it is developed and maintained by the Uppsala Monitoring Center for Pharmacovigilance (UMC) on behalf of the WHO.By April 2013 over 8 million reports were contained in the database.[12]
The term pharmacovigilance was proposed in the mid-70s by a French group of pharmacologists and toxicologists to define the activities promoting “The assessment of the risk of side effects potentially associated with drug treatment”.[13]
Pharmacovigilance is defined by the WHO as “the science and activities relating to the detection, assessment, understanding and prevention of adverse effects, or any other problem in the field of medicine”.[1] The monitoring of spontaneous suspected ADRs reports represents the key component of the integrated systems of pharmacovigilance.[14]
Pharmacovigilance has four main objectives:[15]
Recognize, as quickly as possible, new ADRs;
Improve and increase the information about already known or suspected ADRs;
Assess the benefits of a drug compared to other ones or other therapies;
Communicate the information in order to improve the therapeutic practice.
The main aim of pharmacovigilance is to provide an alarm signal through the early detection of new ADRs.[3] In pharmacovigilance a signal of suspected causality is defined as follows: “Information that arises from one or multiple sources, including observations and experiments, which suggests a new potentially causal association, or a new aspect of a known association, between an intervention and an event or set of related events, either adverse or beneficial, which would command regulatory, societal or clinical attention, and is judged to be of sufficient likelihood to justify verifiable and, when necessary, remedial actions”.[16,17]
Following signal, subsequent ‘ad hoc’ studies will be performed to test this hypothesis and possibly provide additional information, not achievable through spontaneous reporting systems, such as the incidence and relative risk for evaluated ADRs.[18,19,20] The purpose of ADRs monitoring is to ensure safe and effective products to the patients.[1]
The main limitations of spontaneous reporting are related to under-reporting, variable quality of the reported data and lack of information on drug exposure.[21]
Pharmacovigilance in the world
There are differences between countries (and also between regions within countries) in the occurrence of ADRs and other drug-related problems. This may be due to many factors such as:[22]
Disease and prescribing practices;
Genetics, diet, traditions of the communities;
Processes of drugs’ production influencing the quality of the pharmaceutical composition;
Drugs distribution and use including indications, doses and availability;[23]
The use of traditional medicines and supplements (for example, herbal remedies), which may constitute specific toxicological problems.[1,24] The data resulting from the whole country or single region may have greater relevance and educational value and may affect the national regulations.[23]
According to the circumstances, the information obtained in a country (for example, the country of origin of the drug) may not be relevant in other ones.[1]
Pharmacovigilance in Europe
At European level, government agencies responsible for pharmacovigilance in Member States are in contact with each other and with the European Medicines Agency (EMA).[16] EMA has established a web-based European network (EudraVigilance) for reporting andexchangingsuspected ADRs reports, during the development (pre-authorization phase) and following the marketing a-authorization (post-authorization phase) of medicinal products in the European Economic Area. EudraVigilance is an European database, which supports:[25,26]
The early detection of possible safety signals associated with medicinal products for human use.
The continual monitoring and evaluation of potential safety issues in relation to reported adverse reactions.
The decision making process, based on a broader knowledge of the adverse reaction profile of medicinal products.
Pharmacovigilance in Italy
In the early 80's, the Italian rules on the safety of marketed drugs identified the manufacturers as responsible for the communication to the Ministry of Health about possible drug-related adverse effects. In fact, in 1980, in Italy, the marketing-authorization holders were required to transmit to the Ministry of Health a periodical report in which they must specify the nature and frequency of any toxic effects and secondary consequences or correlated with drugs use.[27]
According to the Ministerial Decree (DM) of June 23th, 1981, (Article 8) and the DM of July 28th, 1984, the data collection forms on drugs use had to be filled in by physicians and collected by companies through sales representatives. Doctors could also decide to send the form directly to the Ministry of Health.[28,29] In 1987, two major changes occurred: The term Pharmacovigilance appeared and the local health units were involved.[30] Since then, the doctors were required to notify the local health units, which, in turn, communicated to the Ministry information about the most serious cases and deaths.[30] In 1997, the National System of Pharmacovigilance was established.[31]
By the Legislative Decree of April 8th, 2003, n. 95 was clarified that the detectors can be doctors and healthcare professionals, who may report suspected serious or unexpected adverse reactions to vaccines and drugs undergoing intensive monitoring.[32] In December 2003, a new pharmacovigilance form was approved and is still currently in use [Form 1].[33]
Form 1.

ADR Reporting Form
With the Legislative Decree no. 219 24/04/2006 - artt. 129-134 regions began to provide, within its competences, the dissemination of information to healthcare personnel and the training of operators in the field of pharmacovigilance. Regions can avail themselves of specific pharmacovigilance centers for their activities.[34]
In 2006, obligations on facilities and healthcare providers were defined. The healthcaresettings must nominate a qualified person responsible for pharmacovigilance (QPPV) for ADRs reports management.[35]
Physicians and other healthcare professionals are required to report all suspected serious and/or unexpected ADRs, which they learn as part of their activity.[35] They are in duty bound to report all suspected adverse reactions, serious, not serious, expected and unexpected from all vaccines and medicines under intensive monitoring.[35] All healthcare professionals are required to send reports of suspected adverse reactions by means of the appropriate form, promptly, to QPPV.[35] Upon verifying completeness and consistency of the reporting form data, the RAFV must store the report, no later than seven days from the date of receipt, in the database of the National Pharmacovigilance Network.[35]
The national pharmacovigilance system is headed by the Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA), acting in accordance with the rules laid down at EU level by the EMA. The AIFA is the national authority responsible for drugs regulation in Italy. It is a public body that operates autonomously, transparently and according to cost-effectiveness criteria, under the direction of the Ministry of Health and under the supervision of the Ministry of Health and the Ministry of Economy. AIFA collaborates with the regions, the National Institute of Health, Research Institutes, Patients’ Associations, Healthcare Professionals, Scientific Societies, the Pharmaceutical Industry and the Distributors.[16]
Specifically, the Agency:[16]
Guarantees access to the drugs and their safe and appropriate use in order to protect public health;
Provides an unified national pharmaceutical system in agreement with the Regions;
Provides pharmaceutical expenditure governance in the context of economic and financial compatibility and competitiveness of the pharmaceutical industry;
Ensures innovation, efficiency and simplification of the marketing authorization procedures, in order to allow rapid access to innovative drugs and drugs used for rare diseases;
Strengthens the relationships with the Agencies of other member countries, with the EMA and other international organizations;
Encourages and rewards investments in Research and Development (R and D) in Italy, promoting and rewarding innovation;
Interacts with the community of patients’ associations, the medical/scientific world, pharmaceutical companies and distributors;
Promotes pharmaceutical knowledge and culture.
The mission of the AIFA in pharmacovigilance is to ensure a positive risk/benefit ratio for all authorized drugs through the continuous monitoring of all safety information and ADRs. These data arise from different sources: Spontaneous reports of suspected ADRs, clinical trials, scientific literature, reports submitted by the Pharmaceutical Companies, and other sources.
In particular, all data of spontaneous ADRs reporting are managed and coordinated through the National Network of Pharmacovigilance (Rete Nazionale di Farmacovigilanza, RNF), a database that allows the collection, management and analysis of spontaneous reports of suspected ADRs. It is active since November 2001. The RNFisan AIFA-managed, extensive network throughout the national territory that includes the Regional Authorities and the Autonomous Provinces of Trento and Bolzano, the Regional Centers of Pharmacovigilance, 204 Local Health Authorities, 112 Hospitals, 43 Research Institutes, more than eight hundreds Pharmaceutical Companies and obviously AIFA.[36] Through this network, healthcare professionals reports unexpected ADRs observed in the Italian territory. Periodicallyt he RNF data are transmitted to Eudra Vigilance database (European database of suspected ADRs reports),[36] managed by the EMA. EudraVigilance collects all data provided at national level by the EU countries in a single database, which, in turn, communicates with Vigibase™,[37] the WHO global ICSR database.[38]
New pharmacovigilance legislation
In December 2010, European legislation on pharmacovigilance was changed with the adoption of the EU Regulation 1235/2010 and Directive 2010/84/EU amending, as regards pharmacovigilance, Regulation (EC) 726/2004 and Directive 2001/83/EC, respectively. This new legislation was the biggest change to the regulation of human medicines in the EU since 1995. EMA is responsible for implementing much of the new legislation, which has been effective since July 2012.[26]
In October 2012, the EU's new pharmacovigilance legislation was further amended by:[26]
Regulation (EU) No 1027/2012 (applies from 5 June 2013);
Directive 2012/26/EU (applies from 28 October 2013).
New legislation will greatly improve public-health protection and, ultimately, save lives by enhancing the overall efficiency and effectiveness of the pharmacovigilance system.[26]
Basically, changes tend to increase the efficiency, speed and transparency of pharmacovigilance tasks through rules that aim to:[16]
Strengthening pharmacovigilance systems (more clearly defined roles and responsibilities
of the many actors involved);
Streamline the activities among the Member States (distribution of tasks and work sharing, with less duplication of effort;
Increasing engagement of the patients and healthcare professionals;
Improving the communication systems of decision-making processes, giving proper justification to the public;
Increasing transparency.
First of all, the definition of “adverse reaction” has been changed and broadened to ensure that it now covers noxious and unintended effects resulting not only from the authorized use of a medicine at normal doses, but also from medication errors and uses outside the terms of the marketing authorization (off-label use), including overdose, misuse and abuse of the product, and from occupational exposure. As a consequence, new types of adverse reactions to be reported have been included. Therefore, there will be an increase of reports and resulting a greater monitoring activity.[16]
In all EU countries, the patients will be able to report directly suspected adverse reactions. In Italy, this possibility has already been considered since some years back by a paper form, however, from now on, also in accordance with the new directive, reports may also be sent by the web.[16,39] All reports of adverse reactions will merge into the European database EudraVigilance, but with different timing depending on the severity of the reaction (within 15 days for serious reports and within 90 days for non-serious ones). These reports will be accessible to the public.[16]
Reports from Pharmaceutical Companies will also be transmitted to the EudraVigilance database. Regarding some medicinal products authorized through the centralized procedure, it is already possible to consult the European database of adverse reactions at the following website http://www.adrreports.eu/.
In summary, Directive 2010/84/EU and Regulation (EU) 1235/2010 aim to maintain, reinforce and further develop the EudraVigilance database as the single — or at least the main- point of receipt, storage and exchange of information concerning the safety of medicinal products for human use authorized in the EU.[16]
The data collected in the EudraVigilance database will be monitored by the EMA in cooperation with the Member States, while monitoring of the data originated at the national level will be carried out by the Member State involved; these activities are aimed at the identification of changes in risk or new risks through the signals analysis. The methodology for the identification and management process of the signal were defined in the Implementing Regulation (EU) n. 520/2012 of 19 June 2012, concerning the performance of pharmacovigilance activities provided for in Regulation (EC) n.726/2004 of the European Parliament and of the Council and Directive 2001/83/EC of the European Parliament and of the Council.[16]
As afore mentioned, the new legislation aims to guarantee greater transparency and improve communication. In fact, on its web portal, AIFA makes available to the public at least the following information:[16]
Public assessment reports, together with a summary there of;
Summaries of product characteristics and package leaflets;
Summaries of risk management plans;
List of medicinal products that are subject to additional monitoring;
Information about the various procedures for the reporting of suspected ADRs to the competent authorities by the healthcare professionals and patients, including forms with web data entry mask.
Medicines subject to additional monitoring will be the products containing new active substances not included in the authorized medicines in the EU before the January 1st 2011; in particular, biologics and biosimilars,[40,41] but the list may also include products whose license is subject to particular conditions or products authorized in exceptional circumstances; e.g., products subject to safety studies after the granting of the marketing authorization (post-authorisation safety studies).[16]
These medicinal products subject to additional monitoring will be identifiable from the package leaflet that will bear the statement “This medicinal product is subject to additional monitoring”. This statement will be preceded by a black symbol and followed by an explanatory sentence explaining the concept of additional monitoring. The additional monitoring list will be regularly kept up to date by the EMA in collaboration with Member States.[16]
The new legislation has empowered the relevant authorities to impose on the marketing authorization holders the obligation to perform post-authorization safety and/or efficacy studies. These studies would be required at the time of the granting of the marketing authorization or later.[16,42]
According to another key point of the 2010 reform, each Member State and each marketing authorization holder is obliged to adopt a pharmacovigilance system with the aim of analyzing suspected ADRs and implementing provisions in order to prevent them.[16]
At last, the new EU pharmacovigilance legislation has established within the EMA a new scientific committee, “the Pharmacovigilance Risk Assessment Committee” (PRAC) in which all Member States are represented. The role of the PRAC in protecting public health requires close interaction with the Agency's Committee for Medicinal Products for Human Use (CHMP) and the Coordination Group for Mutual Recognition and Decentralized Procedures-Human (CMDh). The PRAC is responsible for assessing and monitoring the safety of human medicines. Its members include experts from regulatory authorities in EU Member States and independent scientific experts who provide additional expertise in particular scientific areas. Members from the patient organizations and healthcare professionals will be included in the near future further to appointment from the European Commission.
In its role in monitoring the safety of human medicines, the PRAC assesses all aspects of risk management, including the detection, assessment, minimization and communication of the risk of ADRs, giving due consideration to the benefits of the medicine. The PRAC is also involved in designing and evaluating post-authorization safety studies and in conducting pharmacovigilance audits.
When the PRAC has concluded an evaluation related to the safety of medicines, it prepares a recommendation that is sent either to the CHMP (if it concerns at least one centrally authorized medicine) or to the CMDh (if it only concerns medicines that are not centrally authorized in the EU).[16,43]
Pharmacovigilance regional centers
Recently, the Financial Law no. 296/2006 has provided to the AIFA an “ad hoc” fund to be allocated for activities related to pharmacovigilance. The Regional Authorities are responsible for the development and promotion of active pharmacovigilance programs, which agreed with AIFA and subsequently, allow the regions to access to funds.[16]
In this context, AIFA has promoted several projects and studies in Italy, with the aim of increasing knowledge on drugs and the “Culture of Pharmacovigilance”, through the establishment of Pharmacovigilance Regional Centers.[44] These centers have a key role in the most advanced national pharmacovigilance systems. The funding of pharmacovigilance projects by AIFA has contributed to the improvement of spontaneous reporting. In Italy, the situation of spontaneous ADRs reports in 2010 showed an increase of 39% in comparison to 2009. This result is in line with the average annual increase of 30% observed in the last five years,[6,45] and it also demonstrates the crucial role of active pharmacovigilance programs, funded by AIFA, in increasing knowledge on drugs, to better define their safety profile and to improve their safe use in clinical practice.
CONCLUSIONS
In conclusion, the Italian Pharmacovigilance System is based on local activities coordinated at National Level by AIFA under the control of the Ministry of Health. This national system represents a link among local experiences and its counterparts at the European level. Therefore, AIFA has the double opportunity to transfer and receive knowledge from other EU countries; furthermore, it is responsible in Italy of the entire campaign to improve pharmacovigilance knowledge and increase the number of ADRs reports up to the gold standard level established by the WHO. This system represents a good structure, however, several points need to be improved. This is particularly relevant for Italy, where too many differences exist between regional and local activities. The financial support received by AIFA and the future projects should help to achieve a more comprehensive pharmacovigilance activity.
ACKNOWLEDGMENTS
The Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA) is kindly acknowledged for its financial and technical support.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.A guide to detecting and reporting adverse drug reactions. Geneva, Switzerland: World Health Organization; 2002. World Health Organization. Safety of medicines. [Google Scholar]
- 2.White T, Arakelian A, Rho JP. Counting the cost of drug-related adverse events. Pharmacoeconomics. 1999;15:445–58. doi: 10.2165/00019053-199915050-00003. [DOI] [PubMed] [Google Scholar]
- 3.Vallano A, Cereza G, Pedròs C, Agustí A, Danés I, Aguilera C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol. 2005;60:653–8. doi: 10.1111/j.1365-2125.2005.02504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rawlins MD. Spontaneous reporting of adverse drug reactions. I: The date. Br J Clin Pharmacol. 1988;26:1–5. doi: 10.1111/j.1365-2125.1988.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morgan K, Morgan SN. 4th ed. Lawrence, Kansas: Morgan Quitno Press; 1996. Quito Health Care State Rankings 1996. [Google Scholar]
- 6.Giofrè C, Scicchitano F, Palleria C, Mazzitello C, Ciriaco M, Gallelli L, et al. Pharmacovigilance and drug safety 2012 in Calabria (Italy): Adverse events analysis. J Pharmacol Pharmacother. 2013 doi: 10.4103/0976-500X.120963. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baldinelli A, Ferrazin F, Pani L. Italian Medicines Agency (AIFA) Rome: Office of Pharmacovigilance; 2010-2011. Pharmacovigilance in the light of the new legislation: Implementation at national level. [Google Scholar]
- 8.Capuano A, Irpino A, Gallo M, Ferrante L, Illiano ML, Rinaldi B, et al. Regional surveillance of emergency-department visits for outpatient adverse drug events. Eur J Clin Pharmacol. 2009;65:721–8. doi: 10.1007/s00228-009-0641-8. [DOI] [PubMed] [Google Scholar]
- 9.Capuano A, Motola G, Russo F, Avolio A, Filippelli A, Rossi F, et al. Adverse drug events in two emergency departments in Naples, Italy: Anobservational study. Pharmacol Res. 2004;50:631–6. doi: 10.1016/j.phrs.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 10.McBride WG. Thalidomide and congenital abnormalities. Lancet. 1961;2:1358. [Google Scholar]
- 11.Olsson S. The role of the WHO program on international drug monitoring in coordinating worldwide drug safety efforts. Drug Saf. 1998;19:1–10. doi: 10.2165/00002018-199819010-00001. [DOI] [PubMed] [Google Scholar]
- 12. [Last accessed on 2013 Jun 2]. Available from: http://www.who-umc.org .
- 13.Bégaud B, Chaslerie A, Haramburu F. Organization and results of drug vigilance in France. Rev Epidemiol Sante Publique. 1994;42:416–23. [PubMed] [Google Scholar]
- 14.Oshikoya KA, Awobusuyi JO. Perceptions of doctors to adverse drug reaction reporting in a teaching hospital in Lagos, Nigeria. BMC Clin Pharmacol. 2009;9:14. doi: 10.1186/1472-6904-9-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards IR. Who cares about pharmacovigilance? Eur J Clin Pharmacol. 1997;53:83–8. doi: 10.1007/s002280050342. [DOI] [PubMed] [Google Scholar]
- 16. [Last accessed on 2013 Jun 2]. Available from: http://www.agenziafarmaco.gov.it .
- 17.Hauben M, Aronson JK. Defining ‘signal’ and its subtypes in pharmacovigilance based on a systematic review of previous definitions. Drug Saf. 2009;32:99–110. doi: 10.2165/00002018-200932020-00003. [DOI] [PubMed] [Google Scholar]
- 18.Carson JL, Strom BL, Maislin G. Screening for unknown effects of newly marketed drugs. In: Strom BL, editor. Pharmacoepidemiology. 2nd ed. Chichester West Sussex England: John Wiley and Sons Ltd; 1994. pp. 431–47. [Google Scholar]
- 19.Ferrajolo C, Verhamme KM, Trifirò G, ‘t Jong GW, Giaquinto C, Picelli G, et al. Idiopathic Acute Liver Injury in Paediatric Outpatients: Incidence and Signal Detection in Two European Countries. Drug Saf. 2013 doi: 10.1007/s40264-013-0045-7. In Press. [DOI] [PubMed] [Google Scholar]
- 20.Esposito K, Capuano A, Sportiello L, Giustina A, Giugliano D. Should we abandon statins in the prevention of bone fractures? Endocrine. 2013 doi: 10.1007/s12020-013-9924-z. In Press. [DOI] [PubMed] [Google Scholar]
- 21.Inman WH. Attitudes to adverse drug reaction reporting. Br J Clin Pharmacol. 1996;41:434–5. [PubMed] [Google Scholar]
- 22.Waller P. United States: A John Wiley & Sons, Ltd; 2010. An introduction to pharmacovigilance. [Google Scholar]
- 23.Gallelli L, Palleria C, De Vuono A, Mumoli L, Vasapollo P, Piro B, et al. Safety and efficacy of generic drugs respect to brand formulation. Brief review and case series presentation. J Pharmacol Pharmacother. 2013 doi: 10.4103/0976-500X.120972. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russo E, Scicchitano F, Whalley BJ, Mazzitello C, Ciriaco M, Esposito S, et al. Hypericum perforatum: Pharmacokinetic, mechanism of action, tolerability and clinical drug-drug interactions. Phytother Res. 2013 doi: 10.1002/ptr.5050. In Press. [DOI] [PubMed] [Google Scholar]
- 25. [Last accessed on 2013 Jun 2]. Available from: http://eudravigilance.ema.europa.eu/human/index.asp .
- 26. [Last accessed on 2013 Jun 2]. http://www.ema.europa.eu/ema/
- 27.Ministerial Decree. 1980 Mar 20; [Google Scholar]
- 28.Ministerial Decree. 1981 Jun 23; [Google Scholar]
- 29.Ministerial Decree. 1984 Jul 28; [Google Scholar]
- 30.Art.9 - DECREE 30 Oct 1987 (converted into Law 531/87) [Google Scholar]
- 31.D. Decree 18 Feb 1997, n. 44 (implementation of Dir 93/39/EEC) [Google Scholar]
- 32. D. Decree of 8 Apr 2003, n. 95. [Google Scholar]
- 33.2003 Dec 12; D. M. [Google Scholar]
- 34. Leg. 219 24/04/2006 - artt.129-134. [Google Scholar]
- 35. Legislative Decree no. 219 of 24/04/2006. [Google Scholar]
- 36. [Last accessed on 2013 Jun 2]. Available from: http://www.agenziafarmaco.gov.it/en/content/post-marketing-surveillance .
- 37.Ferrajolo C, Capuano A, Verhamme KM, Schuemie M, Rossi F, Stricker BH, et al. Drug-induced hepatic injury in children: A case/non-case study of suspected adverse drug reactions in VigiBase. Br J Clin Pharmacol. 2010;70:721–8. doi: 10.1111/j.1365-2125.2010.03754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. [Last accessed on 2013 Jun 2]. Available from: http://www.who-umc.org/graphics/24965.pdf .
- 39.Menniti M, Menniti A, Giofrè C, Russo E. Informaticsapplied to Pharmacovigilance. J Pharmacol Pharmacother. 2013 doi: 10.4103/0976-500X.120950. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bertuola F, Morando C, Menniti-Ippolito F, Da Cas R, Capuano A, Perilongo G, et al. Association between drug and vaccine use and acute immunethrombocytopenia in childhood: A case-control study in Italy. Drug Saf. 2010;33:65–72. doi: 10.2165/11530350-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Bianciotto M, Chiappini E, Raffaldi I, Gabiano C, Tovo PA, Sollai S, et al. Drug use and upper gastrointestinal complications in children: A case-control study. Arch Dis Child. 2013;98:218–21. doi: 10.1136/archdischild-2012-302100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruggiero S, Rafaniello C, Bravaccio C, Grimaldi G, Granato R, Pascotto A, et al. Safety of attention-deficit/hyper activity disorder medications in children: An intensive pharmacosurveillance monitoring study. J Child Adolesc Psychopharmacol. 2012;22:415–22. doi: 10.1089/cap.2012.0003. [DOI] [PubMed] [Google Scholar]
- 43. [Last accessed on 2013 Jun 2]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Other/2012/07/WC500129593.pdf .
- 44.Parretta E, Ianniello B, Ferrazin F, Rossi F, Capuano A. Italian post marketing surveillance for adverse event reports after MF59-adjuvanted H1N1 vaccination. Vaccine. 2011;29:3708–13. doi: 10.1016/j.vaccine.2011.02.097. [DOI] [PubMed] [Google Scholar]
- 45.Scicchitano F, Giofrè C, Palleria C, Mazzitello C, Ciriaco M, Gallelli L, et al. Pharmacovigilance and drug safety 2011 in Calabria (Italy): Adverse events analysis. J Res Med Sci. 2012;17:872–5. [PMC free article] [PubMed] [Google Scholar]


