Abstract
Introduction:
The epidermal growth factor receptor inhibitors (EGFRIs), cetuximab and panitumumab, represent an effective treatment option for patients affected by metastatic colorectal cancer (mCRC); furthermore, they are relatively devoid of systemic toxicities, which are commonly observed with standard cytotoxic chemotherapy. However, the majority of patients treated with these monoclonal antibodies (mAbs), will experience dermatologic toxicities, most notably the papulopustular skin rash, which can impact quality-of-life and affect adherence to therapy. This paper reviews the most recent practices in the management of skin rash related to anti-epidermal growth factor receptor (EGFR) mAbs, cetuximab and panitumumab, in the treatment of mCRC.
Materials and Methods:
We reviewed relevant literature regarding dermatologic toxicities associated with anti-EGFR mAbs in order to give important indications about prevention and reactive treatment of skin rash.
Results:
Two case reports were presented to show how skin rash could hamper mAb EGFRIs use in clinical practice, underscoring the need of implementing a comprehensive management strategy of skin toxicity in order to promote patients’ compliance with anti-EGFR therapy and maintain quality-of-life. Based on randomized data, recent guidelines established by the Multinational Association for Supportive Care in Cancer Skin Toxicity Study Group suggest that prophylactic use of oral doxycycline or minocycline reduces the risk and severity of skin rash, improving clinical outcomes.
Conclusions:
At the start of treatment with cetuximab and panitumumab, the proper patient education about the skin rash associated with these mAbs and the implementation of a pre-emptive, comprehensive skin toxicity program significantly contribute to improve adherence to therapy, optimize anti-EGFR therapy and maintain quality-of-life.
Keywords: Cetuximab, dermatologic toxicity, panitumumab, rash, safety
INTRODUCTION
Epidermal growth factor receptor (EGFR) is a trans-membrane glycoprotein that is a member of a subfamily of type I receptor tyrosine kinases including EGFR or human epidermal growth factor receptor-1 (HER1), HER2, HER3, and HER4.[1]
EGFR-mediated signaling pathway is essential for physiological organ development, contributing to cell proliferation, differentiation, apoptosis inhibition and angiogenesis in normal epithelial tissues, including the epidermis and hair follicles.[2] In normal cells, pleiotropic EGFR signaling activates a strong proliferative cascade that is strictly controlled. However, in tumor cells, EGFR signaling is uncontrolled, stimulating events, which are responsible for tumor cell growth and progression, such as cell proliferation, angiogenesis, cell adhesion, invasion of surrounding normal tissues, metastasis and resistance to apoptosis.[2,3,4]
The discovery that EGFR is over-expressed in a variety of solid tumors, including colorectal cancer (CRC), and that its over-expression correlates with decreased survival, poor prognosis and resistance to cytotoxic agents, has resulted in the development and use of EGFR inhibitors (EGFRIs) in tumor therapy of patients refractory or intolerant to chemotherapy.[4,5,6]
This article focuses on the two monoclonal antibody (mAb) EGFRIs, cetuximab and panitumumab, which have been both approved by the European Medicines Agency and the U.S. Food and Drug Administration as monotherapy for the treatment of EGFR-expressing metastatic colorectal cancer (mCRC) with disease progression after conventional oxaliplatin and irinotecan-based chemotherapy regimens. They are also indicated for use in combination with chemotherapy. The approval of both antibodies is limited to patients with a wild-type copy of the Kirsten rat sarcoma-2 virus oncogene (KRAS).[1,2]
Although both antibodies share an anti-EGFR monoclonal antibodies (mAbs), mechanism, cetuximab is a recombinant, chimeric (mouse/human) antibody, whereas panitumumab, is a fully human antibody.[2]
Lack of many side effects commonly observed with cytotoxic chemotherapy has contributed to mAb EGFRIs integration into protocols.[6] However, these agents are associated with a set of unique and class-specific dermatologic toxicities in more than 90% of patients, most notably a papulopustular skin rash, xerosis, pruritus and paronychia, due to EGFR blockade on normal epithelial tissues.[2,7]
The papulopustular skin rash, in some cases, may be accompanied by few elements or in other cases may spread with many lesions in a large part of body surface. Therefore, according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (NCI-CTCAE v4.0),[8] it may be classified from Grade 1 to Grade 3 depending on the number of papules or pustules, the number of areas of erythema or edema <1 cm and the presence of symptoms of pain or pruritus. Grade 1 is when the number of papules or pustules is <5 or area of erythema or edema only 1; Grade 2 is when the number of papules or pustules is 5-20 or areas of erythema or edema 2-5; Grade 3 is when the number of papules or pustules is >20 or areas of erythema or edema >5. For every grade, the letter A or B is posed if symptoms such as pain or pruritus are absent or present.
This paper reviews the best practices in the management of skin rash related to the anti-EGFR monoclonal antibodies (mAbs), cetuximab and panitumumab, in the treatment of mCRC. Starting from the description of two cases of mAb EGFRIs-related skin rash,[9] we underline the importance of a correct assessment of grading of papulopustular skin eruption as well as a proactive and early management of skin toxicity, in order to maintain adherence to therapy and an acceptable quality-of-life.
MATERIALS AND METHODS
A computer-aided search of Medline, PubMed, Embase, Cochrane library databases, American Society of Clinical Oncology Meetings and European Society for Medical Oncology Congresses was performed to identify relevant literature regarding the signs and symptoms, assessment of severity and best strategies available to prevent and manage dermatologic toxicities associated with the anti-EGFR mAbs, cetuximab and panitumumab, especially in mCRC. The upper limit date for the search was January 31th, 2013, without lower limit.
Secondary search included articles cited in reference lists identified by the primary search. Records were first screened by title/abstract before full-text articles were retrieved for eligibility evaluation. Remaining articles were then subject to a citation search before a final hand-search of all reference lists. Papers were deemed eligible if they included any form of words: “cetuximab or Erbitux,” “panitumumab or Vectibix,” “EGFRIs or anti-EGFR agents,” “colorectal cancer or carcinoma or colon cancer or rectal cancer,” “cutaneous toxicity or skin rash or skin toxicity or dermatologic toxicity,” and “management or prevention or treatment.”
All citations were downloaded into Endnote® software version 14 (Thomson Reuters) and duplicates deleted. All articles were screened by title/abstract to determine their eligibility and then a random sample of 15% was reviewed in order to evaluate the reliability of the selection process. In order to avoid a bias of exclusion, the full-text articles were retrieved following first round exclusions and were also subject to two independent eligibility reviews, this time with perfect agreement. The studies evaluated as eligible were enclosed in the present review.
RESULTS
Case report 1
A 75-year-old man was switched to a new line tumor therapy with panitumumab (Vectibix® - Amgen Europe B.V.) (6 mg/kg of bodyweight given once every 2 weeks) for the treatment of EGFR-expressing mCRC (stage IV) with KRAS wild-type, after failure of the following chemotherapy regimens: capecitabine/oxaliplatin, capecitabine/irinotecan, capecitabine/oxaliplatin/bevacizumab, capecitabine/bevacizumab.
The patient had no history of severe or life-threatening hypersensitivity to panitumumab and its excipients.
On the morning of October 30th, 2012, he was admitted to the oncology outpatient service to receive his first infusion of panitumumab (520 mg given once every 2 weeks). His skin was healthy and the drug was well-tolerated.
Several hours after initial drug infusion, he developed a pruritic, erythematous eruption over anterior chest and face. Over the time, the skin reaction worsened; but the patient was not treated with any drug. Two weeks after starting panitumumab treatment and before the second infusion, the patient reported to his oncologist an intense, pruritic, skin eruption that was appeared on the face [Figure 1], scalp, neck and upper chest. Therefore, he was referred to a dermatologist for an in-depth clinical evaluation. Physical examination revealed wide erythematous areas almost entirely covered by crusted papulopustular and pustular necrotic lesions.
Figure 1.

Clinical image of pruritic papulopustular skin rash that patient developed 2 weeks after starting panitumumab treatment
Excluding any drug-drug interactions (DDIs), a diagnosis of panitumumab-induced Grade 3B papulopustular skin rash, according to NCI-CTCAE v4.0, was made. Hence, the oncologist delayed the subsequent panitumumab dose until the severity of rash was reduced to at least Grade 2 and recommended a treatment with oral levofloxacin (500 mg once daily for 5 days), topical hydrocortisone 0.1% (cream: twice daily application for 5 days) and methylprednisolone 0.1% (cream: once daily application for 5 days).
Two weeks later, the severity of skin symptoms reduced to Grade 2B and clinical conditions of patient allowed the administration of a subsequent drug dose. However, the second panitumumab infusion was not performed because the patient refused it, fearing a second occurrence of dermatologic toxicities. Cutaneous side-effects had a complete resolution over the next several weeks.
Case report 2
A 74-year-old man was undergoing treatment with fluoropyrimidine and irinotecan-based chemotherapy regimen (continuous infusion every 14 days) and cetuximab (Erbitux® - Merck KGaA) (once a week) for EGFR-expressing rectal carcinoma with KRAS wild-type. The patient had no history of severe hypersensitivity reactions to anti-EGFR agent.
On the morning of October 29th, 2012, he was admitted to the oncology outpatient service to receive his first infusion of cetuximab (720 mg, 400 mg/m2 body surface area). Prior to infusion, the patient was administered with premedication drugs: ondansetron (8 mg intravenously) and atropine sulfate (0.25 mg subcutaneously). Four days after beginning the therapy, he developed a moderately intense erythema with papules on the face. After clinical evaluation, the skin reaction was not attributed to DDIs. A diagnosis of a cetuximab-related Grade 2B papulopustular skin rash (NCI-CTCAE v4.0) was made while a decision to continue initial treatment protocol, without dose modifications, was taken, according to manufacturer's guidelines for management of drug-related skin toxicities. The patient was advised both systemic and topical treatment: oral levofloxacin (500 mg once daily for 5 days); moisturizing, non-perfumed and soothing cream (twice daily application for 1 week); fusidic acid and betamethasone cream – 2% + 0.1% (twice daily application for 1 week). He was also advised to take sun-protective measures and avoid activities and products that were likely to dry skin, in order to reduce the risk of rash exacerbation.
These pre-emptive and treatment strategies improved skin symptoms and patient's compliance with therapy.
DISCUSSION
EGFR is over-expressed in many solid tumors, including CRC, squamous cell cancer of the head and neck and non-small-cell lung cancer. Interestingly, its over-expression is associated with disease progression, higher likelihood of metastases, poor prognosis, resistance to chemotherapy and radiotherapy, and decreased survival rates.[7,10]
Based on these advances, EGFR represents an important target in tumor therapy.[5] Currently, EGFR inhibition is well-established as an effective treatment for aforementioned malignancies: EGFRIs may be used as first-line through third-line treatments, alone or in combination with other agents.[11]
For many decades, fluoropyrimidine-based chemotherapy was the only treatment option available to patients with mCRC. Subsequently, introduction of irinotecan and oxaliplatin gave additional first-line therapy choices.[12] More recently, it has been estimated that between 60% and 80% of CRCs over-express EGFR, which may be also associated with an advanced disease stage. This discovery has resulted in the development and use of EGFRIs in CRC.[2,6] One class of agents that is currently used to target EGFR in the treatment of mCRC is the mAbs, including cetuximab and panitumumab.[7] Both antibodies have been approved as monotherapy in patients with EGFR-positive, KRAS wild-type mCRC after failure of oxaliplatin and irinotecan-containing chemotherapy regimens. They are also indicated for use in combination with chemotherapy in mCRC patients.[2,13]
Both cetuximab and panitumumab bind with high affinity to EGFR, thereby preventing endogenous ligands from activating it. They also induce receptor internalization and consequent down-regulation.[2,7]
Patients with KRAS wild-type tumor are significantly more likely to benefit from treatment with EGFRIs mAbs, whereas patients with KRAS mutations do not respond to both cetuximab and panitumumab. Therefore, KRAS mutational status is now considered as the most relevant biomarker for lack of response to EGFRIs.[14] We underline that both our patients had KRAS wild-type tumors.
The EGFRIs mAbs lack of many severe systemic side-effects commonly observed with cytotoxic chemotherapy, resulting in a more tolerable treatment.[2,7] However, they are associated with a set of unique and class-specific dermatologic toxicities, which represent the most common side-effects. The majority of patients treated with an anti-EGFR mAb will experience cutaneous side-effects, most notably papulopustular skin rash, as common on-target toxicities.[6,7,15]
In fact, EGFR is normally expressed in the basal and suprabasal layers of the epidermis and contributes to epithelial maintenance by causing epidermal growth, differentiation, taking part in wound healing and keratinocyte migration. Hence, EGFR inhibition causes abnormal upkeep of the epithelium, altered growth and migration of keratinocytes and inflammatory chemokine expression by these cells. The resulting neutrophilic suppurative infiltrate in the dermis and subsequent loss of the skin's protective barrier function account for most of dermatologic symptoms related to EGFRIs therapy. Moreover, compromising this barrier, they may also predispose to bacterial superinfection, further exacerbating cutaneous injury.[7,11]
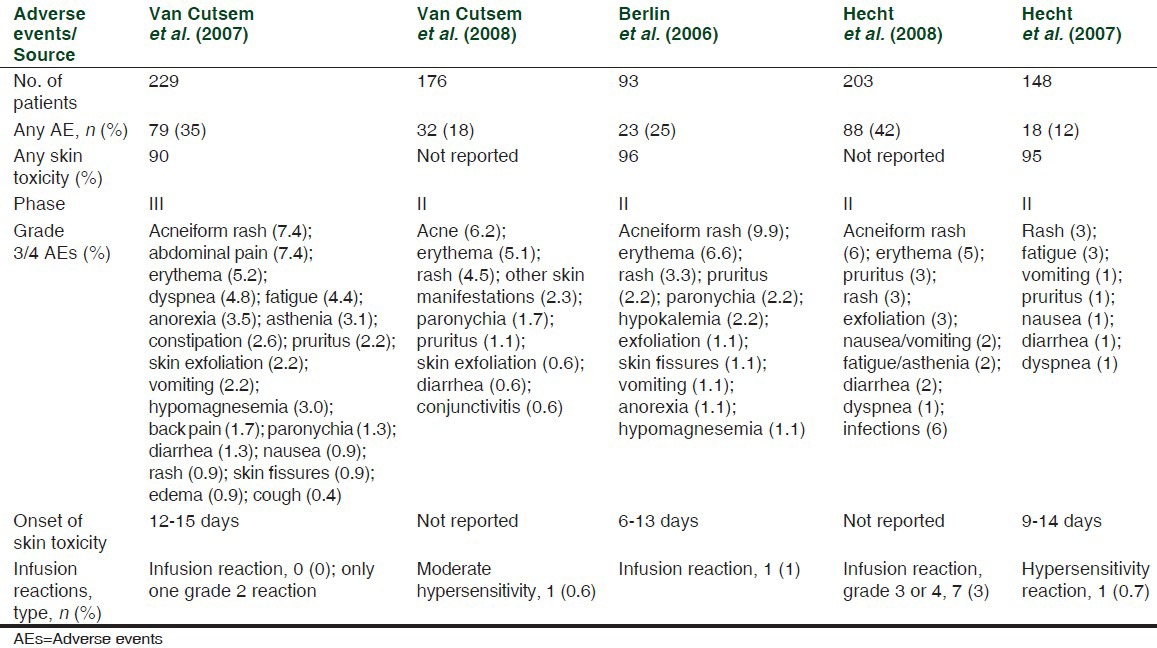
Skin toxicities have been reported in 80-95% of patients with mCRC treated with cetuximab (80-88%) and panitumumab (90-96%) as monotherapy;[16,17] [Tables 1 and 2] similar frequencies were observed in trials of both antibodies in combination with chemotherapy.[7,18]
Table 1.
AEs in cetuximab monotherapy trials, adapted from Peeters et al.[17]
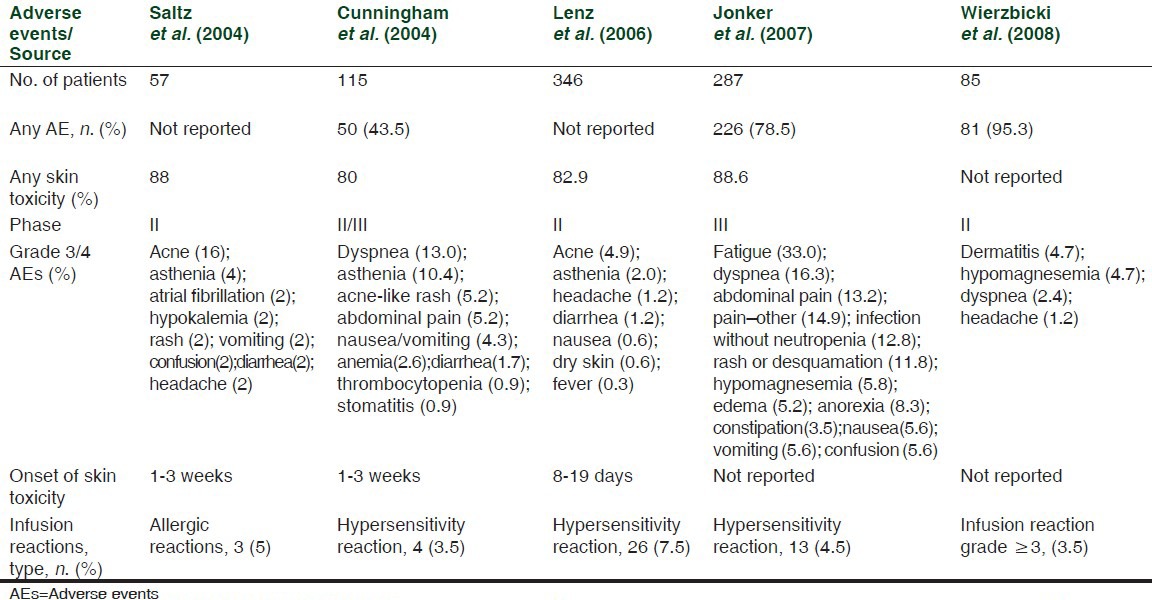
Table 2.
AEs in panitumumab monotherapy trials, adapted from Peeters et al.[17]
Dermatologic toxicities associated with cetuximab and panitumumab fall into three categories:[19] Skin rash; xerosis/fissures and pruritus; paronychia.
The severity of these toxicities is defined according to NCI-CTCAE v4.0.
Dermatologic toxicities are rarely life-threatening; however, they impair quality-of-life and compliance with therapy. When severe, in addition to pharmacological measures, they also may lead to dose reduction or discontinuation of EGFRIs, affecting therapy outcomes. Therefore, a management strategy of them is crucial.[6,11]
The most common (90% of patients) and clinically significant skin toxicity associated with cetuximab and panitumumab is the papulopustular rash, also called acneiform rash. It is characterized by erythematous inter- and intrafollicular papulopustules, which usually develop in cosmetically sensitive, sun-exposed areas of the body such as the face, scalp, neck and upper chest.[6,7]
Although clinical presentation can be similar to acne vulgaris, rash is clinically and histologically different, without the presence of comedones. Regarding differential diagnosis, in very few cases the administration of other EGFRIs such as erlotinib or lapatinib has been related to another particular pustulosis named acute generalized exanthematous pustulosis (AGEP).[20,21] However, as we could observe in other cases,[22] AGEP is a more generalized pustular rash with more interfollicular pustules, less involvement of sebaceous regions and some clinic-pathological features similar to the ones of pustular psoriasis.
On the contrary, the EGFR inhibitor related papulopustular rash typically presents with initial involvement of sebaceous regions. It develops through a series of phases: sensory disturbance, erythema and edema (within the 1st week of treatment); papulopustular eruption (from week 1-3); crusting of lesions (at week 4).[2]
Based on this timeline, our first case describes a papulopustular eruption characterized by an early timing because crusting of lesions was already evident at week 3. Moreover, our first case presented a more severe papulopustular skin eruption, initially Grade 3B, while the second patient presented a less severe papulopustular skin eruption, Grade 2B.
Rash can negatively impact psychosocial well-being, quality-of-life, related costs and EGFRIs dose intensity, resulting in EGFRIs dose modification and discontinuation by 76% and 32%, respectively.[23,24] Since dose reductions and therapy delays or discontinuations can negatively affect clinical outcomes, a proactive management of skin rash should be optimized in order to promote adherence to therapy[2] as underscored by our case report 1: papulopustular eruption is an usually manageable adverse effect of panitumumab therapy, necessitating treatment discontinuation only as a last option.
Rash management should be individualized based on the type, severity and location.[7]
As with other toxicities, management can follow two ways: preventive/prophylactic or treatment/reactive.[25]
In general, at the start of EGFRIs treatment, patients should be informed of potential treatment-related skin toxicities and should be properly educated to appreciate them. To reduce the risk of rash, the oncologists should counsel patients to take appropriate sun-protective measures because exposure can exacerbate rash severity in uncovered areas of the body. Patients should be instructed to apply sunscreen with at least sun protection factor (SPF) ≥15 several times a day. Other general measures include the use of moisturizing cream (twice daily). In addition, patients should be advised to avoid activities and products drying the skin: long hot showers, alcohol-based products, soaps, and common anti-acne medications.[2,11]
According to recent guidelines established by the Multinational Association for Supportive Care in Cancer (MASCC) skin toxicity study group, preventive management is recommended unless contraindications. Based on randomized data,[6] hydrocortisone 1% cream combined with skin moisturizer, sunscreen (SPF ≥15) and oral doxycycline (100 mg twice daily) for the first 6 weeks is recommended. This randomized prospective study has shown that pre-emptive treatment reduced the incidence of panitumumab-related Grade 2 or greater rash by more than 50% compared with reactive treatment, without affecting drug antitumor activity. In another study, Scope et al.[26] revealed that prophylactic minocycline (100 mg daily) is effective in reducing the number of cetuximab-related facial lesions during the first 4 weeks of treatment. Doxycycline is a preferred agent in patients with renal impairment, whereas minocycline is preferable in geographic locations with a high ultraviolet index, being less photosensitizing[11,25] [Table 3].
Table 3.
Papulopustular rash management recommendations, adapted from Lacouture et al.[25]

Although the rash severity decreases after 6-8 weeks, post-inflammatory skin alterations can last for months or years. Therefore, an appropriate preventive strategy should be considered throughout treatment and follow-up to reduce these late long lasting effects.[25]
Confirming the importance of prophylactic strategy, cetuximab manufacturer delivers a free protective and moisturizing, vitamin K1-based cream to reduce/prevent rash associated with anti-EGFR therapy. However, in case report 2, the clinicians did not use this product.
Topical steroids and antibiotics have shown benefit for rash treatment, preventing superinfection. After in vitro studies showing release of inflammatory chemokines following EGFRIs therapy, reactive use of topical corticosteroids has been recommended [Table 3]. The topical antibiotics commonly used are clindamycin, erythromycin and metronidazole.[11]
Several studies have reported favorable outcomes from the reactive use of oral tetracycline-based antibiotics. MASCC guidelines[25] have graded these agents based on the level of evidence available for use in EGFRIs-associated rash: doxycycline 100 mg twice daily and minocycline 100 mg daily have been advised for systemic use.
The published reports supporting the use of vitamin K1 are based on studies without control groups; therefore, it is not recommended.
Probably, in case report 2, the patient was not advised to use vitamin K1 cream based on these findings.
On the contrary, consistent reports of isotretinoin at low doses (20-30 mg/day) support the recommendation for its use when other strategies have failed.[25]
Interestingly, data from clinical trials for cetuximab and panitumumab suggest a positive correlation between the occurrence and severity of skin rash and response/survival benefits.[27,28,29] This important association could offer patients an “evidence-based incentive” to cope and overcome EGFRIs-related rash in order to optimize treatment response. However, results from a dose-escalation study demonstrate that rash may represent a surrogate marker of efficacy only in patients with KRAS wild-type tumors: KRAS and rash are independent predictors of outcomes.[30]
The rash and survival relationship also suggests a possible immune pathway underlying the rash and tumor response: possibly, an increase in systemic cytokines results in tumor immunomodulation and better response. Based on this speculation, pharmaceutical companies are developing biochemical compounds able to augment this immune response for use in combination with anti-EGFR therapy.[31,32]
Lastly, the well-established correlation between rash and clinical outcome imposes that management strategies of skin toxicities do not interfere with antitumor activity of EGFRIs. In this regard, two randomized studies[6,26] have demonstrated that preventive use of systemic oral doxycycline or minocycline decreases the risk of Grade 2 and higher skin toxicity, without reducing anti-EGFR efficacy.
CONCLUSIONS
Agents targeting EGFR, such as cetuximab and panitumumab, offer patients with wild-type KRAS mCRC a valid treatment alternative when used as monotherapy or in combination with chemotherapy. However, the majority of patients (90%) treated with EGFRIs mAbs experienced dermatologic toxicities, the most common of which is the papulopustular skin rash. It occurs early during treatment, impairing patients’ quality-of-life and interfering with their therapeutical compliance. Since a positive correlation between rash severity and efficacy of treatment has been established, it is essential to adopt a proactive and early management strategy of skin rash in order to ensure adherence to therapy and maintain quality-of-life. It is also crucial that anti-toxicity measures do not affect the action of EGFRIs.
At the start of treatment, the proper patient education about the rash from EGFRIs is critical for its management because it may allow patients to cope and also overcome the significant impairment of quality-of-life posed by the toxicity. The clinicians should encourage them to use preventive measures that may enhance their comfort.
Based on randomized data, MASCC guidelines recommend a pre-emptive treatment including oral doxycycline or minocycline. This treatment strategy is a potential tool to minimize the need for dose modifications and improve clinical outcomes because it does not affect EGFRIs efficacy.
These findings highlight the importance of establishing a pre-emptive, comprehensive skin toxicity treatment/management program in patients treated with cetuximab and panitumumab in order to optimize EGFRIs therapies.
ACKNOWLEDGMENT
The Italian Drug Agency (Agenzia Italiana del Farmaco, AIFA) is kindly acknowledged for its financial and technical support.
Footnotes
Source of Support: The Italian Drug Agency (Agenzia Italiana del Farmaco, AIFA) is kindly acknowledged for its financial and technical support
Conflict of Interest: Nil.
REFERENCES
- 1.Gemmete JJ, Mukherji SK. Panitumumab (vectibix) AJNR Am J Neuroradiol. 2011;32:1002–3. doi: 10.3174/ajnr.A2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouwerkerk J, Boers-Doets C. Best practices in the management of toxicities related to anti-EGFR agents for metastatic colorectal cancer. Eur J Oncol Nurs. 2010;14:337–49. doi: 10.1016/j.ejon.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Ritter CA, Arteaga CL. The epidermal growth factor receptor-tyrosine kinase: A promising therapeutic target in solid tumors. Semin Oncol. 2003;30(1 Suppl 1):3–11. doi: 10.1053/sonc.2003.50027. [DOI] [PubMed] [Google Scholar]
- 4.Capdevila J, Saura C, Macarulla T, Casado E, Ramos FJ, Tabernero J. Monoclonal antibodies in the treatment of advanced colorectal cancer. Eur J Surg Oncol. 2007;33(Suppl 2):S24–34. doi: 10.1016/j.ejso.2007.09.025. [DOI] [PubMed] [Google Scholar]
- 5.Eames T, Kroth J, Flaig MJ, Ruzicka T, Wollenberg A. Perifollicular xanthomas associated with epidermal growth factor receptor inhibitor therapy. Acta Derm Venereol. 2010;90:202–3. doi: 10.2340/00015555-0792. [DOI] [PubMed] [Google Scholar]
- 6.Lacouture ME, Mitchell EP, Piperdi B, Pillai MV, Shearer H, Iannotti N, et al. Skin toxicity evaluation protocol with panitumumab (STEPP), a phase II, open-label, randomized trial evaluating the impact of a pre-emptive skin treatment regimen on skin toxicities and quality of life in patients with metastatic colorectal cancer. J Clin Oncol. 2010;28:1351–7. doi: 10.1200/JCO.2008.21.7828. [DOI] [PubMed] [Google Scholar]
- 7.Fakih M, Vincent M. Adverse events associated with anti-EGFR therapies for the treatment of metastatic colorectal cancer. Curr Oncol. 2010;17(Suppl 1):S18–30. doi: 10.3747/co.v17is1.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacouture ME, Maitland ML, Segaert S, Setser A, Baran R, Fox LP, et al. A proposed EGFR inhibitor dermatologic adverse event-specific grading scale from the MASCC skin toxicity study group. Support Care Cancer. 2010;18:509–22. doi: 10.1007/s00520-009-0744-x. [DOI] [PubMed] [Google Scholar]
- 9.Giofrè C, Scicchitano F, Palleria C, Mazzitello C, Ciriaco M, Gallelli L, et al. Pharmacovigilance and drug safety 2012 in Calabria (Italy): Adverse events analysis. J Pharmacol Pharmacother. 2013 doi: 10.4103/0976-500X.120963. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodarte CM, Abdallah OA, Barbosa NF, Koch Lde O, Resende UM. Cutaneous reactions due to the use of epidermal growth factor receptor inhibitors: Two case reports. An Bras Dermatol. 2009;84:667–70. doi: 10.1590/s0365-05962009000600015. [DOI] [PubMed] [Google Scholar]
- 11.Abdullah SE, Haigentz M, Jr, Piperdi B. Dermatologic toxicities from monoclonal antibodies and tyrosine kinase inhibitors against EGFR: Pathophysiology and management. Chemother Res Pract 2012. 2012 doi: 10.1155/2012/351210. 351210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bauer KA, Hammerman S, Rapoport B, Lacouture ME. Completeness in the reporting of dermatologic adverse drug reactions associated with monoclonal antibody epidermal growth factor receptor inhibitors in phase II and III colorectal cancer clinical trials. Clin Colorectal Cancer. 2008;7:309–14. doi: 10.3816/CCC.2008.n.040. [DOI] [PubMed] [Google Scholar]
- 13.Benay S, Fanciullino R, Mercier C, Iliadis A, Ciccolini J, Lacarelle B. 100% human monoclonal antibodies in oncology: Hype or breakthrough? Curr Top Med Chem. 2012;12:1643–8. doi: 10.2174/156802612803531351. [DOI] [PubMed] [Google Scholar]
- 14.Petrelli F, Borgonovo K, Barni S. The predictive role of skin rash with cetuximab and panitumumab in colorectal cancer patients: A systematic review and meta-analysis of published trials. Target Oncol. 2013;8:173–81. doi: 10.1007/s11523-013-0257-x. [DOI] [PubMed] [Google Scholar]
- 15.Balagula Y, Garbe C, Myskowski PL, Hauschild A, Rapoport BL, Boers-Doets CB, et al. Clinical presentation and management of dermatological toxicities of epidermal growth factor receptor inhibitors. Int J Dermatol. 2011;50:129–46. doi: 10.1111/j.1365-4632.2010.04791.x. [DOI] [PubMed] [Google Scholar]
- 16.Van Cutsem E, Peeters M, Siena S, Humblet Y, Hendlisz A, Neyns B, et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J Clin Oncol. 2007;25:1658–64. doi: 10.1200/JCO.2006.08.1620. [DOI] [PubMed] [Google Scholar]
- 17.Peeters M, Price T, Van Laethern JL. Anti-epidermal growth factor receptor monotherapy in the treatment of metastatic colorectal cancer: Where are we today? Oncologist. 2009;14:29–39. doi: 10.1634/theoncologist.2008-0167. [DOI] [PubMed] [Google Scholar]
- 18.Vectibix (Panitumumab) Summary of Product Characteristics. Amgen Europe BV, Breda, The Netherlands. 2013. [Last accessed on 30th of April 2013]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_Product_Information/human(000741/WC500047710.pdf .
- 19.Burtness B, Anadkat M, Basti S, Hughes M, Lacouture ME, McClure JS, et al. NCCN task force report: Management of dermatologic and other toxicities associated with EGFR inhibition in patients with cancer. J Natl Compr Canc Netw. 2009;7(Suppl 1):S5–21. doi: 10.6004/jnccn.2009.0074. [DOI] [PubMed] [Google Scholar]
- 20.Liquete E, Ali S, Kammo R, Ali M, Alali F, Challa H, et al. Acute generalized exanthematous pustulosis induced by erlotinib (Tarceva) with superimposed Staphylococcus aureus skin infection in a pancreatic cancer patient: A case report. Case Rep Oncol. 2012;5:253–9. doi: 10.1159/000338806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lakshmi C, Pillai S, Srinivas CR. Lapatinib-induced acute generalized exanthematous pustulosis. Indian Dermatol Online J. 2010;1:14–7. doi: 10.4103/2229-5178.73251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grieco T, Cantisani C, Innocenzi D, Bottoni U, Calvieri S. Acute generalized exanthematous pustulosis caused by piperacillin/tazobactam. J Am Acad Dermatol. 2005;52:732–3. doi: 10.1016/j.jaad.2004.08.030. [DOI] [PubMed] [Google Scholar]
- 23.Boone SL, Rademaker A, Liu D, Pfeiffer C, Mauro DJ, Lacouture ME. Impact and management of skin toxicity associated with anti-epidermal growth factor receptor therapy: Survey results. Oncology. 2007;72:152–9. doi: 10.1159/000112795. [DOI] [PubMed] [Google Scholar]
- 24.Hassel JC, Kripp M, Al-Batran S, Hofheinz RD. Treatment of epidermal growth factor receptor antagonist-induced skin rash: Results of a survey among German oncologists. Onkologie. 2010;33:94–8. doi: 10.1159/000277656. [DOI] [PubMed] [Google Scholar]
- 25.Lacouture ME, Anadkat MJ, Bensadoun RJ, Bryce J, Chan A, Epstein JB, et al. Clinical practice guidelines for the prevention and treatment of EGFR inhibitor-associated dermatologic toxicities. Support Care Cancer. 2011;19:1079–95. doi: 10.1007/s00520-011-1197-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scope A, Agero AL, Dusza SW, Myskowski PL, Lieb JA, Saltz L, et al. Randomized double-blind trial of prophylactic oral minocycline and topical tazarotene for cetuximab-associated acne-like eruption. J Clin Oncol. 2007;25:5390–6. doi: 10.1200/JCO.2007.12.6987. [DOI] [PubMed] [Google Scholar]
- 27.Saltz LB, Meropol NJ, Loehrer PJ, Sr, Needle MN, Kopit J, Mayer RJ. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–8. doi: 10.1200/JCO.2004.10.182. [DOI] [PubMed] [Google Scholar]
- 28.Vincenzi B, Santini D, Rabitti C, Coppola R, Beomonte Zobel B, Trodella L, et al. Cetuximab and irinotecan as third-line therapy in advanced colorectal cancer patients: A single centre phase II trial. Br J Cancer. 2006;94:792–7. doi: 10.1038/sj.bjc.6603018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hecht JR, Patnaik A, Berlin J, Venook A, Malik I, Tchekmedyian S, et al. Panitumumab monotherapy in patients with previously treated metastatic colorectal cancer. Cancer. 2007;110:980–8. doi: 10.1002/cncr.22915. [DOI] [PubMed] [Google Scholar]
- 30.Van Cutsem E, Tejpar S, Vanbeckevoort D, Peeters M, Humblet Y, Gelderblom H, et al. Intrapatient cetuximab dose escalation in metastatic colorectal cancer according to the grade of early skin reactions: The randomized EVEREST study. J Clin Oncol. 2012;30:2861–8. doi: 10.1200/JCO.2011.40.9243. [DOI] [PubMed] [Google Scholar]
- 31.Tamayo ME, Bautista JB, Flores ML, Kurman MR, Paul MM, Gargano MA, et al. A phase Ib/2, dose-escalating, safety, and efficacy study of imprime PGG, cetuximab and irinotecan in patients with advanced colorectal cancer (CRC) J Clin Oncol. 2010:28. abstract e14103, ASCO Annual Meeting Proceedings. [Google Scholar]
- 32.Segal NH, Gada P, Marsh LM, Gargano MA, Patchen ML. Imprime PGG improves survival in KRAS mutant colorectal cancer patients. European Society for Medical Oncology (ESMO) 13th World Congress on Gastrointestinal Cancer. 2011 [Google Scholar]


