Abstract
Corticosteroids have been used since the 50s as anti-inflammatory and immunosuppressive drugs for the treatment of several pathologies such as asthma, allergy, rheumatoid arthritis, and dermatological disorders. Corticosteroids have three principal mechanisms of action: 1) inhibit the synthesis of inflammatory proteins blocking NF-kB, 2) induce the expression of anti-inflammatory proteins by IkB and MAPK phosphatase I, and 3) inhibit 5-lipoxygenase and cyclooxygenase-2. The efficacy of glucocorticoids in alleviating inflammatory disorders results from the pleiotropic effects of the glucocorticoid receptors on multiple signaling pathways. However, they have adverse effects: Growth retardation in children, immunosuppression, hypertension, hyperglycemia, inhibition of wound repair, osteoporosis, metabolic disturbances, glaucoma, and cataracts. Less is known about psychiatric or side effects on central nervous system, as catatonia, decreased concentration, agitation, insomnia, and abnormal behaviors, which are also often underestimated in clinical practice. The aim of this review is to highlight the correlation between the administration of corticosteroids and CNS adverse effects, giving a useful guide for prescribers including a more careful assessment of risk factors and encourage the use of safer doses of this class of drugs.
Keywords: Adverse effects, corticosteroids, central nervous system, mood, psychosis
INTRODUCTION
Glucocorticoids (GCs) are a class of steroid hormones released from the adrenal cortex and their plasma concentration is controlled by the hypothalamic-pituitary-adrenal axis.[1] GCs are mediators of stress response and the derived drugs (also named corticosteroids) are widely used as pharmacological agents for the treatment of inflammatory disease, asthma, and immune/rheumatologic diseases.[2] However, approximately 20% of patients receiving high doses of corticosteroids develop psychiatric disorders including depression, mania, and psychosis[3] requiring pharmacological treatment, while 75% report psychiatric symptoms reversible upon discontinuation of therapy.[4]
Glucocorticoid activity: An overview
Endogenous glucocorticoids affect biological processes including growth, metabolism, development, immune function, and stress response.[5] The production of corticosteroid hormones is under the control of the hypothalamic-pituitary-adrenal axis, activated by mental and physical stimuli.[6]
They are lipophilic hormones crossing the cytoplasmic membrane and binding to specific cytosolic receptors, mineralocorticoid receptors (MR), and glucocorticoid receptors (GR) that regulate gene expression. The drug-receptor complex can trigger the transcription of anti-inflammatory genes such as NF-kB, AP-1, STAT, NFTA, c-Jun, Fos, and inhibit the production of cytokines and pro-inflammatory proteins such as chemotactic proteins and adhesion molecules.[7,8,9]
There are approximately 550 polymorphisms identified for the gene coding for the glucocorticoid receptors related to sensitivity to their effects.[10]
Glucocorticoids possess several endocrinological properties being involved in several physiological and pathological processes; they have known effects on glucose metabolism, lipid metabolism, bone and cartilage, protein metabolism, muscular function, hydro-electrolytic balance, gastric secretion, cardiovascular system, hemolymphopoietic tissue, and reproductive physiology.[11]
Endogenous glucocorticoids also control the feeling of hunger, sleep-wake cycle and affect the processes of learning and memory through interaction with specific receptors located in the prefrontal cortex, hippocampus, and basolateral amygdala.[12]
Steroid receptors are expressed in different areas of the brain and their role is related to the regulation of various neurotransmission, including serotonin and dopamine.[13] In particular, in the CNS, glucocorticoids exert their potential effects at hippocampal level, a structure intimately involved in the limbic system, which provides the processing of emotional information and memory.[14] Various studies show a correlation between high levels of endogenous cortisol and hippocampal atrophy resulting in damage and cognitive dysfunction.[15] Negative feedback ensures the activation of the hypothalamic-pituitary-adrenal axis by inducing the overproduction of cortisol and increasing the damage to brain structures.[16]
CNS adverse events
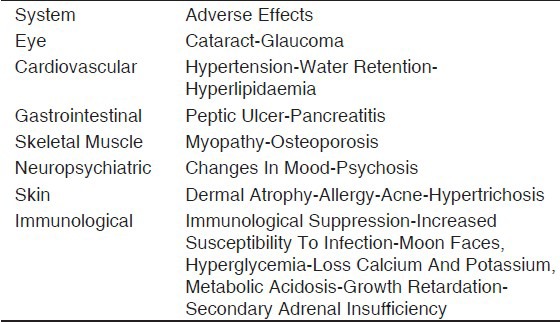
Besides their very common therapeutic use, several well-known adverse effects including weight gain, osteoporosis, and hyperglycemia are often observed.[17] Less-reported adverse events are that involving the central nervous system (CNS) such as psychiatric and cognitive disturbances [Table 1].
Table 1.
Corticosteroid dependent adverse effects
Behavioral effects
Studies showed that following the chronic intake of cortisone, 70% of patients report increased appetite with resulting increase of body weight; a 4 to 8% increase is estimated after two years of therapy.[18] Sleep disorders characterized by restlessness and insomnia were observed in 73% of cases.[19] Swinburn et al. in 1988[20] reported a study showing that patients with Chronic Obstructive Pulmonary Disease, treated with oral prednisolone, develop a sense of well-being called “steroid euphoria” characterized by a reduced sense of anxiety and depression when compared with patients receiving placebo and this occurred even in the absence of improvement in lung function. There are cases, in literature, that describe the appearance of altered behavior with states of agitation and insomnia as a result of intra-articular injection of methylprednisolone.[21]
Recently, in a set of psychiatric symptoms attributed to prolonged treatment or high-dose corticosteroids, catatonia was assessed with muscle stiffness, insomnia, and abnormal behaviors such as silence and stillness.[22]
Psychic effects
Literature reports several cases of depression related to the use of corticosteroid therapy with an incidence of 40.5%; mania, psychosis, and delirium are also very frequent with an incidence of 27.8%, 13.9%, and 10.1%, respectively.[23] Emotional lability and irritability are common symptoms sometimes accompanied by auditory hallucinations and paranoia.[22] Rarely, altered consciousness and disorientation may be observed.
The mechanism by which the corticosteroid induces symptoms such as mania, depression, and psychosis is not clear.[22] The administration of prednisone is associated with decreased levels of corticotrophin, norepinephrine, and beta-endorphin in the cerebrospinal fluid. Furthermore, corticosteroids induce an increased release of glutamate that induces neuronal toxicity due to accumulation effect.[23]
Cognitive effects
In some cases, cognitive deficits, difficulty to maintain concentration, and poor memory, especially after prolonged treatment with high doses of corticosteroids, were observed.[23] Neuroimaging studies in patients taking corticosteroids have related a decrease of hippocampal volume and brain atrophy due to a reduced blood flow in areas of the brain responsible for cognitive functions.[24]
The cognitive effects of corticosteroids appear to be occasional and include disorders, which consist of dementia or delirium. The type of deficiency coincides with the dysfunction at hippocampal level, which is rich in glucocorticoid receptors.[25]
Incidence
A small percentage (2-4%) of patients develop depression, anxiety, or becomes apathetic.[26] While another small percentage (3%) shows psychosis with hallucinations. These adverse events are dose and time dependent and remission results from the suspension of the treatment or decreasing the dose of cortisone.[27] The incidence of neuropsychiatric effects due to assumption of corticosteroid ranges from 2 to 60%, reflecting the variability of dose, the duration of administration, and risk factors identified including genetic predisposition based on polymorphisms of the GR.[28]
The incidence rate of psychiatric disorders is directly correlated to dose and time of glucocorticoids exposure. A study shows that the incidence rate was 22.2% person-years at risk for first courses, 14.0% person-years at risk for second glucocorticoid courses, and 11.7% person-years at risk for third and later courses.[29]
Onset
The beginning of the appearance of symptoms induced by corticosteroids is variable. They may arise in the first phases of treatment, during, or even at the end of therapy.[30] In most cases (86%), they occur within the first 5 days of treatment. The analysis of several studies leads to an average of 11.5 days after the beginning of corticosteroid treatment to the onset of psychiatric symptoms.[31] 89% of patients develop symptoms in the first six weeks, 62% within two weeks, and 39% in the first week. The duration of the neuropsychiatric effects is highly variable and depends on the severity, treatment discontinuation, and by other drug therapies.[31]
Risk factors
Side effects of psychiatric type have been reported following different routes of administration, e.g., intra-articular injection, epidural, topical, and systemic.
Psychiatric side effects due to corticosteroids appear to be dose dependent; they occur in 1.3% of the cases when the dose is less than 40 mg daily and reaches 18.4% for doses of 80 mg daily.[32]
It is not entirely clear whether gender affects the ability to manifest psychiatric symptoms, but some studies suggest that women are more prone.[33]
Other studies show that 73% of the pediatric population receiving steroid therapy develops hyperactivity, irritability, insomnia as well as showing deficits of attention and memory, especially those under 10 years of age and/or high doses of the drug.[32]
Treatment
Generally, symptoms related to administration of corticosteroids disappear after therapy discontinuation.[34] However, some patients require therapeutic treatment. The management of psychiatric symptoms due to administration of corticosteroids includes the reduction of the dose or treatment discontinuation.[35] The patient can be treated with medications normally used in patients with psychiatric or neurological disorders. Mood-stabilizing drugs, such as lithium and valproic acid, are able to control the symptoms caused by corticosteroids. Carbamazepine, inducing steroids metabolism, reduces their neurotoxic effects; atypical antipsychotics, such as olanzapine and fluoxetine (SSRI), are active on this symptoms.[36] The effect of anti-depressive drugs are different, i.e., tricyclic antidepressants could lead to a significant worsening of symptoms,[35] while a selective serotonin reuptake inhibitors, such as fluoxetine,[37] may improve symptoms of depression during corticosteroid therapy as well as phenytoin, lamotrigine, risperidone, quetiapine, and gabapentin.[37]
CASE REPORT
In 2012, in our Pharmacovigilance's Center (Regione Calabria, University Hospital Mater Domini of Catanzaro) a suspected adverse reaction of paraphasia, a language disorder manifested by a difficulty to order words in periods, induced by intramuscular administration of betamethasone was reported.
Regarding this case, the correlation was very high since the patient was not taking other drugs in that period and the adverse reaction appeared 2 hours after the first injection.
Unfortunately, amnesic information is limited since it is an ambulatory patient. We know that it is a middle-aged man, without family history of mental illness and under therapy with angiotensin receptor blocker for hypertension since few years. Reporting low back pain for few months and suspecting a herniated disc, the physician prescribed computerized tomography, documenting bulging lumbar, and steroid therapy was prescribed. Since the beginning of betamethasone (Bentelan®) therapy, the patient was noted to have phenomena characterized by inversion of the letters while talking. Discontinuation of treatment was advised; after treatment interruption, the phenomena disappeared.
In literature, similar cases have not been previously reported; however, many studies show a correlation between psychiatric disorders and corticosteroids use.
Psychiatric disorders secondary to corticosteroids-use are classified as mood disorders substance-induced according to the Diagnostic and Statistical Manual of Disorders Fourth Edition (DSM-IV).[38]
DISCUSSION AND CONCLUSIONS
Many scientific and literature evidences highlight how the administration of corticosteroids results in a high incidence of mood elevation, satisfaction, and optimism.[39] Less frequently, euphoria, insomnia, and increase in motor activity may occur.[40]
The use of corticosteroids is strongly associated to the development of psychiatric/neurological side effects. These effects are due to the wide expression of GR in the brain, and their long-term modulation can lead to functional and anatomical alterations, which might be responsible for the observed side-effects. The incidence and the onset of such symptoms are quite variable depending on several factors and the type of study; in any case, all healthcare professionals should be aware of such a possibility. Furthermore, such events should be early recognized and treated.
Despite of the known numerous side effects, the use of corticosteroid is widely spread considering the broad spectrum of clinical indications. Psychiatric adverse reactions are under-estimated and therefore it is not always possible to identify the effective dose and at the same time the most secure. It seems only right to recall how the spontaneous reporting of adverse reactions by health professionals and patients is the easiest way to integrate the missing information on the potential and dangers of drugs.
ACKNOWLEDGEMENT
The Italian Drug Agency (Agenzia Italiana del Farmaco, AIFA) is kindly acknowledged for its financial and technical support.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Rhen T, Cidlowski JA. Antinflammatory action of glucocorticoids Antiinflammatory Action of Glucocorticoids — New mechanisms for old drugs. N Engl J Med. 2005;353:1711–23. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 2.Rhen T, Cidlowski JA. Nuclear factor-kB and glucocorticoid receptors. In: Martini L, editor. Encyclopedia of endocrine diseases. Vol. 3. Boston: Elsevier Academic Press; 2004. pp. 391–8. [Google Scholar]
- 3.The Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13:694–8. doi: 10.1002/cpt1972135part1694. [DOI] [PubMed] [Google Scholar]
- 4.Wolkowitz OM. Prospective controlled studies of the behavioral and biological effects of exogenous corticosteroids. Psychoneuroendocrinology. 1994;19:233–55. doi: 10.1016/0306-4530(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 5.Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 6.Marques-Deak A, Cizzaand G, Sternberg E. Brain-immune interactions and disease susceptibility. Mol Psychiatry. 2005;10:239–50. doi: 10.1038/sj.mp.4001643. [DOI] [PubMed] [Google Scholar]
- 7.Duma D, Jewell CM, Cidlowski JA. Multiple glucocorticoid receptor isoforms and mechanisms of post-translational modification. J Steroid Biochem Mol Biol. 2006;102:11–21. doi: 10.1016/j.jsbmb.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Bladh LG, Liden J, Dahlman-Wright K, Reimers M, Nilsson S, Okret S. Identification of endogenous glucocorticoid repressed genes differentially regulated by a glucocorticoid receptor mutant able to separate between nuclear factor-kappaB and activator protein-1 repression. Mol Pharmacol. 2005;67:815–2. doi: 10.1124/mol.104.005801. [DOI] [PubMed] [Google Scholar]
- 9.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids - new mechanisms for old drugs. N Engl J Med. 2005;353:1711–23. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 10.Van Rossum EF, Lamberts SW. Polymorphisms in the glucocorticoid receptor gene and their associations with metabolic parameters and body composition. Recent Prog Horm Res. 2004;59:333–57. doi: 10.1210/rp.59.1.333. [DOI] [PubMed] [Google Scholar]
- 11.Lewis DA, Smith RE. Steroid-induced psychiatric syndromes. A report of 14 cases and a review of the literature. J Affect Disord. 1983;5:319–32. doi: 10.1016/0165-0327(83)90022-8. [DOI] [PubMed] [Google Scholar]
- 12.Fietta P, Fietta P, Delsante G. Central nervous system effects of natural and synthetic glucocorticoids. Psychiatry Clin Neurosci. 2005;63:613–22. doi: 10.1111/j.1440-1819.2009.02005.x. [DOI] [PubMed] [Google Scholar]
- 13.Schacke H, Docke WD, Asadullah K, Ganda JC. Mechanisms of disease 1723 Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 14.Fardet L, Petersen I, Nazareth I. Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. 2012;169:491–7. doi: 10.1176/appi.ajp.2011.11071009. [DOI] [PubMed] [Google Scholar]
- 15.Falk WE, Mahnke MW, Poskanzer DC. Lithium prophylaxis of corticotropin-induced psychosis. JAMA. 1979;241:1011–2. [PubMed] [Google Scholar]
- 16.Hall RCW, Popkin MK, Kirkpatrick B. Tricyclic exacerbation of steroid psychosis. J Nerv Ment Dis. 1978;166:738–42. [PubMed] [Google Scholar]
- 17.Halper JP. Corticosteroids and behavioral disturbances. In: Lin AN, Paget SA, editors. Principles of Corticosteroids Therapy. London: Arnold; 2002. pp. 174–201. [Google Scholar]
- 18.Da Silva JA, Jacobs JW, Kirwan JR, Boers M, Saag KG, Inês LB, et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: Published evidence and prospective trial data. Ann Rheum Dis. 2006;65:285–93. doi: 10.1136/ard.2005.038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curtis JR, Westfall AO, Allison J, Bijlsma JW, Freeman A, George V, et al. Population-based assessment of adverse events associated with long-term glucocorticoid use. Arthritis Rheum. 2006;55:420–6. doi: 10.1002/art.21984. [DOI] [PubMed] [Google Scholar]
- 20.Swinburn CR, Wakefield JM, Newman SP, Jones PW. Evidence of prednisolone induced mood change (‘steroid euphoria’) in patients with chronic obstructive airways disease. Br J Clin Pharmacol. 1988;26:709–13. doi: 10.1111/j.1365-2125.1988.tb05309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson DE, Harrison-Hansley E, Spencer RF. Steroid psychosis after an intra-articular injection. Ann Rheum Dis. 2000;59:927. doi: 10.1136/ard.59.11.926a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benyamin RM, Vallejo R, Kramer J, Rafeyan R. Corticosteroid induced psychosis in the pain management setting. Pain Physician. 2008;11:917–20. [PubMed] [Google Scholar]
- 23.Wolkowitz OM, Rubinow D, Doran AR, Breier A, Berrettini WH, Kling MA. Prednison effects on neurochemistry and behavior. Preliminary findings. Arch Gen Psychiatry. 1990;47:963–8. doi: 10.1001/archpsyc.1990.01810220079010. [DOI] [PubMed] [Google Scholar]
- 24.Ioannou N, Liapi C, Sekeris CE, Palaiologos G. Effects of dexamethasone on K(+)-evoked glutamate release from rat hippocampal slices. Neurochem Res. 2003;28:875–81. doi: 10.1023/a:1023271325728. [DOI] [PubMed] [Google Scholar]
- 25.Coluccia D, Wolf OT, Kollias S, Roozendaal B, Forster A, de Quervain DJ. Glucocorticoid therapy-induced memory deficits: Acute versus chronic effects. J Neurosci. 2008;28:3474–8. doi: 10.1523/JNEUROSCI.4893-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bolanos S, Khan D, Hanczyc M, Bauer M, Dhanani N, Brown E. Assessment of mood stat in patients receiving long-term corticosteroid therapy and in controls with patient-rated and clinician-rated scales. Ann Allergy Asthma Immunol. 2004;92:500–5. doi: 10.1016/S1081-1206(10)61756-5. [DOI] [PubMed] [Google Scholar]
- 27.Boston Collaborative Drug Surveillance Program. Acute adverse reactions to prednisone in relation to dosage. Clin Pharmacol Ther. 1972;13:694–8. doi: 10.1002/cpt1972135part1694. [DOI] [PubMed] [Google Scholar]
- 28.Lewis GP, Jusko WJ, Burke CW, Graves L. Boston collaborative drug surveillance program. Prednisolone side effects and serum protein levels. A collaborative study. Lancet. 1971;298:778–81. doi: 10.1016/s0140-6736(71)92738-3. [DOI] [PubMed] [Google Scholar]
- 29.Bender BG, Lerner JA, Kollasch E. Mood and memory changes in asthmatic children receiving corticosteroids. J Am Acad Child Adolesc Psychiatry. 1988;27:720–5. doi: 10.1097/00004583-198811000-00010. [DOI] [PubMed] [Google Scholar]
- 30.McEwen BS. Allostasis, allostatic load, and the aging nervous system: Role of excitatory amino acids and excitotoxicity. Neurochem Res. 2000;25:1219–31. doi: 10.1023/a:1007687911139. [DOI] [PubMed] [Google Scholar]
- 31.Ling MH, Perry PJ, Tsuang MT. Side effects of corticosteroid therapy. Psychiatric aspects. Arch Psychiatry. 1981;38:471–7. doi: 10.1001/archpsyc.1981.01780290105011. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein ET, Preskorn SH. Mania triggered by a steroid nasal spray in a patient with stable bipolar disorder. Am J Psychiatry. 1989;146:1076–7. doi: 10.1176/ajp.146.8.1076. [DOI] [PubMed] [Google Scholar]
- 33.George ME, Sharma V, Jacobson J, Simon S, Nopper AJ. Adverse effects of systemic glucocorticosteroid therapy ininfants with hemangiomas. Arch Dermatol. 2004;140:963–9. doi: 10.1001/archderm.140.8.963. [DOI] [PubMed] [Google Scholar]
- 34.Kahn D, Stevenson E, Douglas CJ. Effect of sodium valproate in three patients with organic brain syndromes. Am J Psychiatry. 1988;145:1010–1. doi: 10.1176/ajp.145.8.1010. [DOI] [PubMed] [Google Scholar]
- 35.Wada K, Yamada N, Sato T, Suzuki H, Miki M, Lee Y, et al. Corticosteroid-induced psychotic and mood disorders: Diagnosis defined by DSM-IV and clinical pictures. Psychosomatics. 2001;42:461–6. doi: 10.1176/appi.psy.42.6.461. [DOI] [PubMed] [Google Scholar]
- 36.Hall RC, Popkin MK, Kirkpatrick B. Tricyclic exacerbation of steroid psychosis. J Nerv Ment Dis. 1978;166:738–42. [PubMed] [Google Scholar]
- 37.Wyszynski AA, Wyszynski B. Treatment of depression with fluoxetine in corticosteroid dependent central nervous system Sjogren's syndrome. Psychosomatics. 1993;34:173–7. doi: 10.1016/S0033-3182(93)71910-6. [DOI] [PubMed] [Google Scholar]
- 38.Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Press; 2000. American Psychiatric Association. Text Revision. [Google Scholar]
- 39.Lewis DA, Smith RE. Steroid-induced psychiatric syndromes: A report of 14 cases and a review of the literature. J Affect Disord. 1983;5:319–33. doi: 10.1016/0165-0327(83)90022-8. [DOI] [PubMed] [Google Scholar]
- 40.Varney NR, Alexander B, Macindoe JH. Reversible steroid dementia in patients without steroid psychosis. Am J Psychiatry. 1984;141:369–72. doi: 10.1176/ajp.141.3.369. [DOI] [PubMed] [Google Scholar]


