Abstract
The aim of this study is to evaluate the efficacy of a Unani formulation in hypertension. A total of 90 patients with total cholesterol level of more than 220 mg/dl with associated conditions were included in this study. A total of 30 patients having a mean systolic blood pressure (BP) of 133.86 mmHg comprising Group A received Unani formulation Safoof-e-Muhazzil (SM) in its classical powder form in the dose of 5 g twice a day orally. Group B comprising of 30 patients with a mean systolic BP of 133.13 mmHg received same drug, but in compressed tablet form in the same dosage, whereas, 30 patients comprising Group C with a mean systolic BP of 129.45 mmHg, received Atorvastatin 10 mg as a standard control. Patients were evaluated on each follow-up at 2nd, 4th and 6th week. The mean systolic BP in Group A and B before treatment was 133.86 ± 3.028 mmHg and 133.13 ± 2.852 mmHg, which significantly decreased to 119.33 ± 1.922 mmHg (P < 0.001) and 119 ± 1.760 mmHg (P < 0.001) respectively. In the control Group C before treatment BP was 129.45 ± 2.499 mmHg and after treatment it significantly decreased to 124.34 ± 1.794 mmHg (P < 0.01). The percentage change after treatment was 10.85%, 10.61% and 3.94% respectively in each group. Mean diastolic BP in Group A and B before treatment was 85.06 ± 2.11 mmHg and 84.56 ± 1.5 mmHg, which significantly decreased to 79.06 ± 1.56 mmHg (P < 0.001) and 79.96 ± 1.15 mmHg (P < 0.001) respectively, BP before treatment in Group C was 83.23 ± 1.588 mmHg, which was decreased to 124.34 ± 1.794 mmHg (P < 0.01). The study results indicate that the test drug was quite effective in reducing both systolic as well as diastolic BP.
Keywords: Diastolic blood pressure, obesity, Safoof-e-Muhazzil, systolic blood pressure, Unani formulation
INTRODUCTION
In present times, for the human race, the prime challenge is the cardiovascular disease (CVD) which account for approximately 50% of all deaths world-wide and is the leading cause of death in western and developing countries.[1] According to National Commission on Macroeconomics and Health (NCMH-2008), a Government of India Undertaking, there would be around 62 million patients with CVDs by 2015 in India alone and of these, 23 million patients would be younger than 40 years of age.[2] An estimated 17.5 million people died from CVDs in 2005, representing 30% of all global deaths.[3]
Amongst, CVDs, hypertension is the leading cause of death world-wide and accounts for 13.5% of all deaths.[4] Hypertension is also emerging as major health problem in India. It accounts for two-third of all strokes and one half of all ischemic heart diseases in India.[5]
The association between hypertension, obesity and hyperlipidemia is well-established. Undoubtedly, one of the most important risk factors for hypertension is obesity[6] and all overweight and obese persons are at risk for hypertension, high blood cholesterol, type 2 diabetes, and coronary heart disease.[7] Hyperlipidemia is strongly associated with hypertension and plays a crucial role in the development of CVD, which has become the leading cause of death in most developed countries as well as in developing countries.[8]
The test drug Safoof-e-Muhazzil (SM) is a classical Unani pharmacopeial formulation indicated for obesity[9] and also proven to be anti hyperlipidemic.[10] The relation between weight loss and corresponding decrease in hypertension has already been established.[11] The test drug in each 5 g contains five herbal ingredients Tukhme badyan (Foeniculum vulgare) −1 g, Nankhwa (Trachyspermum ammi) −1 g, Zeera Siyah (Carum carvi) −1 g, Sudab (Ruta graveolens) −1 g, Marzanjosh (Origanum vulgare) −0.25 g, one animal origin drug Luc Maghsool (purified stick lac) −0.5 g and a mineral origin drug Bura Armani (Borax) −0.25 g. Many of the constituents of the test drug are known to possess diuretic and antihypertensive properties individually.[12,13,14,15]
MATERIALS AND METHODS
The study was conducted at a Hospital in New Delhi after obtaining written informed consent from patients and approval from the institutional ethics committee under No: DM/FOM/JH/Ethics Committee/09 prior to enrollment of patients in the study. During the trial Helsinki declaration, GCP and ICMR guidelines were adhered to strictly. The study was randomized, open-label, comparative, standard controlled. Out of total screened patients, 128 were enrolled in this study fulfilling the inclusion criteria and had total cholesterol above 220 mg/dl. Randomization was done on the bases of pre-assigned case numbers as per computer generated chart.
Patients were randomized into Test Group A, Test Group B and a Control Group C. Out of 124 patients 90 completed the trial with 30 in each group. The Test Group A was given SM in its conventional powdered form, 5 g twice a day orally with water. The Test Group B was put on compressed tablets of equivalent dose of SM 5 tablets orally twice a day with water. The control group was prescribed Atorvastatin 10 mg orally once daily.
The test drug SM of a single batch manufactured by GMP compliant Company was procured from the market of which half of the drug was compressed into tablets. The composition of this particular formulation is as per the Qarabadeen e Jadeeedi and Bayaz e Kabir; Vol-II.[9]
During the protocol therapy subjects in all the groups were asked to adhere to the diet prescribed diet of 1600 calories and exercise, which was defined as half an hour brisk walk daily. Duration of protocol therapy was 6 weeks with follow-up at 2nd, 4th and 6th week.
Assessment of efficacy and safety was done on clinical and biochemical parameters before, at each follow-up and after the completion of protocol therapy as per the approved protocol. Hemogram, liver function test and kidney function test were conducted before and after the study to record the safety and tolerability of the drug.
Adverse effects
Adverse effects, if any, either reported or observed by the patient were recorded in the clinical research file at each follow-up with information about severity, date of onset, duration and action taken.
Statistical analysis
Assessment of results was performed as per the protocol using Graphpad Instat 3.10 32 for windows [Graphpad softwares Inc., San Diego, California, USA] created July 10, 2009 by using paired t-test, repeated measures analysis of variance and Tukey-Kramer multiple comparisons test. Test results were ranked as:
ns - Non significant, *P < 0.05 significant, **P < 0.01 very significant, ***P < 0.001 extremely significant
RESULTS
Out of total patients, 5.55% belonged to 20-29 years age group, 23.33% belonged to 30-39 years, 30% belonged to 40-49 years age group, 24.44% belonged to 50-59 years age group, and 16.66% belonged to 60-70 years age group.
Group A consisted of 18 men and 12 women with an average age 45.2 ± 1.91 years, Group B also had men and women in the same ratio with an average age 45.86 ± 2.01 and Group C consisted of 12 men and 18 women with an average age of 46.63 ± 2.27. Out of total study subjects, 17 (18.88%) patients were vegetarian, whereas 73 (81.11%) were non-vegetarian. Only 12.22% of total patients had significant/relevant past history such as ischemic heart disease whereas, 87.77% did not have any significant history [Table 1, Graph 1]. In all three groups out of total subjects 40% had a body mass index of ≥25 kg/m2 [Table 2, Graph 2] which decreased by around 3% in the test groups and by less than 1% in the control group [Table 3, Graph 3].
Table 1.
Distribution of patients according to significant personal history

Graph 1.

Distribution of patients according to significant personal history
Table 2.
Distribution of patients according to BMI

Graph 2.

Distribution of patients according to body mass index
Table 3.
Group wise effect on BMI

Graph 3.

Group wise effect on body mass index
Systolic blood pressure (BP)
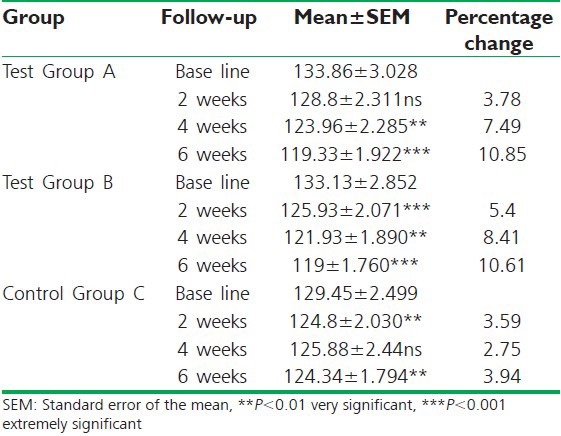
In Group A, mean systolic BP was recorded to be 133.86 ± 3.028 mmHg before treatment and decreased to 128.8 ± 2.311 mmHg which is non-significant at first follow-up. With a significant reduction recorded at 123.96 ± 2.285 mmHg (P < 0.01) at second follow-up. After treatment it significantly decreased to 119.33 ± 1.922 mmHg (P < 0.001). The percentage change was 3.78%, 7.49% and 10.85% respectively [Table 4, Graph 4].
Table 4.
Group wise effect on systolic blood pressure
Graph 4.

Group wise effect on systolic blood pressure
In Group B mean systolic BP before treatment was recorded at 133.13 ± 2.852 mmHg. Subsequently, it significantly decreased to 125.93 ± 2.071 mmHg (P < 0.001) and 121.93 ± 1.890 mmHg (P < 0.01) at second and third follow-ups. After treatment significant reduction was recorded at 119 ± 1.760 mmHg (P < 0.001). The percentage change was 5.4%, 8.41% and 10.61% respectively.
In Group C mean systolic BP was 129.45 ± 2.499 mmHg before treatment, which significantly decreased to 124.8 ± 2.030 mmHg (P < 0.01) at first follow-up, and non-significantly to 125.88 ± 2.44 mmHg at second follow-up. After treatment, it decreased to 124.34 ± 1.794 mmHg (P < 0.01). The percentage change was 3.59%, 2.75% and 3.94% respectively.
Diastolic BP
Patients in Group A before treatment recorded a mean diastolic BP of 85.06 ± 2.11 mmHg. On first follow-up a non-significant decrease to 82.33 ± 1.75 mmHg was recorded. Subsequently, it decreased significantly to 80.93 ± 1.69 mmHg (P < 0.01) and 79.06 ± 1.56 mmHg (P < 0.001) at next follow-up and at the completion of therapy respectively. The percentage change at each follow-up was 3.20%, 4.85% and 7.05% respectively [Table 5, Graph 5].
Table 5.
Group wise effect on diastolic blood pressure

Graph 5.

Group wise effect on diastolic blood pressure
In Group B mean diastolic BP was recorded 84.56 ± 1.5 mmHg before treatment and significantly decreased to 82.2 ± 1.12 mmHg (P < 0.01), 81.26 ± 1.29 mmHg (P < 0.001) and 79.96 ± 1.15 mmHg (P < 0.001) at subsequent follow-ups until completion of therapy respectively. The percentage change was 2.79%, 3.90% and 5.47% at each interval.
Group C patients recorded a mean diastolic BP of 83.23 ± 1.588 mmHg at base line, It initially decreased to 82.7 ± 1.605 mmHg non-significantly and subsequently decreased significantly to 81.13 ± 1.45 mmHg (P < 0.01) and 81.5 ± 1.394 mmHg (P < 0.05) at further follow-ups respectively. The percentage change was 0.63%, 2.52% and 2.07% respectively.
DISCUSSION
The high prevalence of hypertension condition continues despite the introduction of increasingly effective numbers of anti-hypertensive agents. It is thought that stress of everyday life with a change in environmental factors, living, eating habits and lack of exercise have led to this increase. Previously hypertension which is 90-95% essential in origin, was predominant only in industrialized and developed countries. Of late, a sudden increase in the number of such cases in the developing countries has also been reported.
In this study, we evaluated the effect of the test drug SM, which is mainly an antiobesity formulation as mentioned in the ancient Unani pharmacopeias, on systolic and diastolic BP. Further, the powdered drug was compressed into tablets to make it more palatable. The test drug was evaluated in both its traditional powder form in one group and compressed tablet form in the other group in equivalent dose of 5 g twice daily orally against Atorvastatin 10 mg once daily orally as standard control in a third group.
Overall picture of all three groups indicates significant changes in pre- and post-treatment systolic and diastolic BP reading. This can firstly be attributed to the prescribed exercise patients had adhered to during the study.[16]
But secondly, when we compare the difference of percentage change in study groups and the control group the difference in BP (systolic as well as diastolic) is around 3 times more in study groups. This clearly indicates the antihypertensive activity of the test drugs which is both understandable as well as explainable because many of the constituents of the test drug are already known to have diuretic, antihyperlipidemic, antiobesity, vasorelaxant and even antihypertensive properties individually as proven through different studies.[12,13,14,15,17,18,19,20,21,22]
It was also observed that in both test groups serum potassium levels were slightly raised after treatment, which in most likelihood could be due to the presence of potassium in some of the ingredients of the test formulation as already known. Dietary supplementation of potassium can lower BP in normal and some hypertensive patients.[23] Hence the potassium in the test drug also might have contributed in lowering BP [Table 6, Graph 6].
Table 6.
Group wise effect on serum electrolytes

Graph 6.

Group wise effect on serum electrolytes
On the safety parameters, the test dug did not exhibit any side effects [Tables 7-9, Graphs 7-9]
Table 7.
Group wise effect on blood counts

Table 9.
Group wise effect on KFT

Graph 7a.

Group wise effect on blood counts
Graph 9.

Group wise effect on KFT
Table 8.
Group wise effect on LFT

Graph 7b.

Group wise effect on Total Leucocytes Count
Graph 8a.

Group wise effect on liver function test
Graph 8b.

Group wise effect on liver function test (Serum Alkaline Phosphatase)
CONCLUSION
Overall the results in all three groups are indicative of around two and a half times decrease in systolic as well as diastolic BP levels along with certain other parameters in both test groups when compared with the test group. The ingredients of the test drug with its diuretic, antiobesity and hypolipidimic effect have a played a significant role in controlling the BP levels.
Findings of this study are proposed to lead to better understanding of obesity and hypertension and their relation with each other. Unani system of medicine coupled with the scientific information available can amalgate toward the development of a new and comprehensive treatment regime.
It will pave the way for managing the cardiovascular risk factors including hypertension and obesity in a more efficient manner without or least adverse effects of drugs in the future.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Kampoli AM, Tousoulis D, Antoniades C, Siasos G, Stefanadis C. Biomarkers of premature atherosclerosis. Trends Mol Med. 2009;15:323–32. doi: 10.1016/j.molmed.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 2.Sawant AM, Shetty D, Mankeshwar R, Ashavaid TF. Prevalence of dyslipidemia in young adult Indian population. J Assoc Physicians India. 2008;56:99–102. [PubMed] [Google Scholar]
- 3.Alam B, Ahasan H, Islam Z, Islam N, Mohammed F, Nur Z, et al. Pattern of lipid profile and obesity among secretariat employees of Bangladesh. J Med. 2009;10:3–6. [Google Scholar]
- 4.Victor RG. Arterial hypertension. In: Ausiello G, editor. Cecil Medicine. 23rd ed. Philadelphia: W B Saunders Company; 2007. [Google Scholar]
- 5.Kaur P, Rao SR, Radhakrishnan E, Rajasekar D, Gupte MD. Prevalence, awareness, treatment, control and risk factors for hypertension in a rural population in South India. Int J Public Health. 2012;57:87–94. doi: 10.1007/s00038-011-0303-3. [DOI] [PubMed] [Google Scholar]
- 6.Jousilahti P, Tuomilehto J, Vartiainen E, Valle T, Nissinen A. Body mass index, blood pressure, diabetes and the risk of anti-hypertensive drug treatment: 12-year follow-up of middle-aged people in eastern Finland. J Hum Hypertens. 1995;9:847–54. [PubMed] [Google Scholar]
- 7.Rana TF, Mahmood S, Ahmad M, Akhtar RM. Assessment of body mass index (BMI) in medical students (a cross sectional survey) Ann King Edward Med Coll. 2006;12:545–6. [Google Scholar]
- 8.Zhang X, Sun Z, Zheng L, Li J, Liu S, Xu C, et al. Prevalence of dyslipidemia and associated factors among the hypertensive rural Chinese population. Arch Med Res. 2007;38:432–9. doi: 10.1016/j.arcmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 9.Hifz A. New Delhi: CCRUM; 2005. Qarabadeen e Jadeedi. Reprint. [Google Scholar]
- 10.Jahangir U, Urooj S, Khan AA. 1st ed. Saarbrucken: Scholars Press; 2013. Metabolic Syndrome and Alternative Medicine. [Google Scholar]
- 11.Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure: A meta-analysis of randomized controlled trials. Hypertension. 2003;42:878–84. doi: 10.1161/01.HYP.0000094221.86888.AE. [DOI] [PubMed] [Google Scholar]
- 12.Lahlou S, Tahraoui A, Israili Z, Lyoussi B. Diuretic activity of the aqueous extracts of Carum carvi and Tanacetum vulgare in normal rats. J Ethnopharmacol. 2007;110:458–63. doi: 10.1016/j.jep.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 13.Gilani AH, Jabeen Q, Ghayur MN, Janbaz KH, Akhtar MS. Studies on the antihypertensive, antispasmodic, bronchodilator and hepatoprotective activities of the Carum copticum seed extract. J Ethnopharmacol. 2005;98:127–35. doi: 10.1016/j.jep.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 14.El Bardai S, Lyoussi B, Wibo M, Morel N. Pharmacological evidence of hypotensive activity of Marrubium vulgare and Foeniculum vulgare in spontaneously hypertensive rat. Clin Exp Hypertens. 2001;23:329–43. doi: 10.1081/ceh-100102671. [DOI] [PubMed] [Google Scholar]
- 15.Wright CI, Van-Buren L, Kroner CI, Koning MM. Herbal medicines as diuretics: A review of the scientific evidence. J Ethnopharmacol. 2007;114:1–31. doi: 10.1016/j.jep.2007.07.023. [DOI] [PubMed] [Google Scholar]
- 16.Murphy MH, Nevill AM, Murtagh EM, Holder RL. The effect of walking on fitness, fatness and resting blood pressure: A meta-analysis of randomised, controlled trials. Prev Med. 2007;44:377–85. doi: 10.1016/j.ypmed.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 17.Tognolini M, Ballabeni V, Bertoni S, Bruni R, Impicciatore M, Barocelli E. Protective effect of Foeniculum vulgare essential oil and anethole in an experimental model of thrombosis. Pharmacol Res. 2007;56:254–60. doi: 10.1016/j.phrs.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 18.Mueller M, Lukas B, Novak J, Simoncini T, Genazzani AR, Jungbauer A. Oregano: A source for peroxisome proliferator-activated receptor gamma antagonists. J Agric Food Chem. 2008;56:11621–30. doi: 10.1021/jf802298w. [DOI] [PubMed] [Google Scholar]
- 19.Lemhadri A, Hajji L, Michel JB, Eddouks M. Cholesterol and triglycerides lowering activities of caraway fruits in normal and streptozotocin diabetic rats. J Ethnopharmacol. 2006;106:321–6. doi: 10.1016/j.jep.2006.01.033. [DOI] [PubMed] [Google Scholar]
- 20.Ijaz J, Rahman ZU, Khan MZ, Muhammad F, Aslam B, Iqbal Z, et al. Antihyperlipidaemic efficacy of Trachyspermum ammi in albino rabbits. Acta Vet BRNO. 2009;78:229–36. [Google Scholar]
- 21.Aristatile B, Al-Numair KS, Veeramani C, Pugalendi KV. Antihyperlipidemic effect of carvacrol on D-galactosamine-induced hepatotoxic rats. J Basic Clin Physiol Pharmacol. 2009;20:15–27. doi: 10.1515/jbcpp.2009.20.1.15. [DOI] [PubMed] [Google Scholar]
- 22.Ratheesh M, Shyni GL, Sindhu G, Helen A. Inhibitory effect of Ruta graveolens L. on oxidative damage, inflammation and aortic pathology in hypercholesteromic rats. Exp Toxicol Pathol. 2011;63:285–90. doi: 10.1016/j.etp.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Haddy FJ, Vanhoutte PM, Feletou M. Role of potassium in regulating blood flow and blood pressure. Am J Physiol Regul Integr Comp Physiol. 2006;290:R546–52. doi: 10.1152/ajpregu.00491.2005. [DOI] [PubMed] [Google Scholar]


