Abstract
Introduction:
Zinc oxide nanoparticles (ZnOn) have been used as carriers and sun-protecting agents for Teucrium polium L. extract to enhance sun protection. ZnOn was synthesized by hydrolyzing zinc acetate using sodium hydroxide with mean particle diameter less than 500 nm.
Materials and Methods:
Top flowerings of T. polium L. were extracted by percolation method with petroleum ether, chloroform, and 80% methanol consecutively. Methanolic extract was lyophilized and used as a flavonoid-rich fraction. Sunscreen was prepared by the reconstitution of 0.5 g of the lyophilized extract in water and mixing with 0.5 to 2 g zinc-oxide (ZnO). Sun protection factor (SPF) of the aqueous extract of T. polium, the prepared gel, as well as the zinc oxide suspension alone and in combination with each other was determined spectrophotometrically based on a modified Transpore® tape method.
Results and Conclusion:
Obtained results showed that the T. polium extract has a wide band of ultraviolet radiation (UV) spectrum absorption ranging from 250 nm to 380 nm. SPF of the combination product in the ultraviolet B (UVB) area was greater than 80, revealing a synergistic action between ZnO and T. polium. The adsorption of flavonoids of T. polium on Zinc-oxide nanoparticles (ZnOn) slowed down their release thereby lengthening their persistence on the skin and contributing to further duration of action.
Keywords: Nanomedicines, Sun protection factor, Teucrium polium
INTRODUCTION
The unprotected exposure to ultraviolet radiation (UV) is a major contributing factor in the development of skin cancer. The ultraviolet B (UVB) as the most active terrestrial waveband region causes direct photochemical damage to DNA, leading to gene mutations. Indirect evidence suggests that ultraviolet A (UVA) plays a greater role in the long-term sun damage than it does in acute cases, such as sunburn or vitamin D synthesis, which are overwhelmingly attributable to UVB.[1,2] Due to the depletion in the ozone layer, carrying out research in the field of sun protection has become a major concern.[3] Sun-protecting substances are capable of protecting human beings against the harmful solar radiation effects, such as aging and skin cancers.[4] Since these preparations are often applied on large skin areas, even low penetration rates can cause systemic toxicity,[5] therefore, sun preparations should have a controlled release[6] like liposomes, microsponges, microspheres, nanocapsules, and inclusion complexes.[7,8,9] In addition, sunscreen preparations are required to have a high degree of resistance to photodecomposition during exposure to the sunlight. T. polium is a plant which has been widely distributed in Iran and has many medicinal applications. This plant is most commonly used as antioxidant and as a protection against UV-radiation. Nowadays, due to the common belief that natural products have less adverse effects, there exist tremendous interests in using various plants for health purposes through standardized preparations. The studies on the chemical composition of T. polium[10] show a wide range of compounds. Apigenine, luteolin-7-glycoside, and salvigenine were the most abundant flavonoids in the plant.[11] Flavonoids have antioxidant and prooxidant activities.[12,13,14] In this study, the antisolar activity was considered and attempts have been made to prepare the nanoparticles of the flavonoid-rich fraction from T. polium and Zinc-oxide nanoparticles (ZnOn). Since sunscreens containing simple Zinc-oxide (ZnO) have some failures,[15] we decided to use a combination of T. polium extract and ZnO. The application of nanoparticle technology is proposed to increase the penetration of the UV absorbents from skin, to control their release and to improve the photo stability of the formulation.[16] To the best of our knowledge it is the first time that a combination of a natural sunscreen and ZnOn has been designed for sunscreen purposes.
MATERIALS AND METHODS
Chemicals
ZnOn was kindly donated by Catalyst Noavaran Co., Tehran, Iran; T. polium was collected from Shahrbabak city in Kerman province (30°16’ latitude and 55°25’ longitude) in June 2011. Hydroxypropylmethyl cellulose (HPMC), polyethylene glycol 400 (PEG400), propylene glycol (PG), methanol, ZnO, HCL, AlCl3, ethyl acetate, Methenamine, chloroform, and acetone were purchased from Merck Co., Darmsdtat, Germany. The deionized water (DIW) prepared by Milli-Q (Millipore, USA) was used in all experiments. All chemicals were of analytical grade.
Plant materials
T. polium (Lamiaceae) was collected in June, 2011 from Shahrbabak in Kerman province, Iran. The plant was identified in the Pharmacognosy Department of the Faculty of Pharmacy, Kerman University of medical sciences, Kerman, Iran and a voucher specimen (KF1249) was deposited at the herbarium of the Department. The top flowerings of the plant were dried in shade and milled.
Extraction
The plant (500 g) was milled and sieved to a mesh size of 60 and then extracted using the percolation method with petroleum ether and chloroform, respectively, for discoloration. The residue was re-extracted with methanol and concentrated in a rotary evaporator (Heidolph, Germany) to give a viscous mass, and then it was dissolved in distilled water completely and freeze-dried (Eyla, Japan) to give a dry powder. The dried extract was stored in a tight container in the desiccators in a refrigerator until use.
Assay of the total flavonoids of the extract
Rutin was used as a standard for the determination of the plant flavonoids on the basis of previous study.[11] An amount of 0.1g of the dried extract was added to 20 ml acetone. A volume of 1 ml of the methenamine solution and 2 ml of HCl (25%) were added, respectively. The mixture was boiled for 30 minutes and filtered through cotton. Filtrate was decanted with 20 ml distilled water and 15 ml ethyl acetate. 1 ml of the AlCl3 solution was added to 10 ml of extract and diluted to 25 ml with acetic acid 5% in methanol. After 30 minutes, the absorbance of the samples was determined at 360 nm using a UV-Visible spectrophotometer (Lambda 25, Perkin Elmer, and USA). The percentage of flavonol-o-glycoside was computed based on rutin as a reference.[17]
Preparation of T. polium standard solution and construction of calibration curve
In this method, 20 mg of the extract was dissolved in 100 ml of DIW and regarded as a stock solution (200 mg/l). Consecutive dilutions were prepared by the dilution of the stock standard solution with DIW as 40, 60, 80, 100, and 120 mg/l. All measurements were made at room temperature. The UV spectrum of the solutions was recorded in the wavelength range of 200 to 400 nm. The calibration curve was prepared by plotting the absorbance of each standard solution at 269.5 nm versus concentration.
Assay validation
Validation parameters such as linearity, precision, and accuracy were determined. Linearity was measured by the correlation between absorbance and concentration. The intra-day and inter-day precision was determined by measuring the absorbance in the replicate standard samples at three concentration levels (40, 80 and 120.0 mg/l). For the intra-day assay precision, five replicates of the samples at each concentration were assayed simultaneously within a day. Relative standard deviation percentage (RSD%) <5 was regarded as a criterion for acceptable precision. Accuracy was measured by the calculation of the error percent.
Determination of the percentage of the plant extract adsorbed on ZnOn (FZnE)
100 mg of the extract was dissolved in 100 ml of DIW (1000 mg/l), added to 20 mg of the ZnOn, and stirred at 400 rpm for 10 minutes. After the centrifugation of the suspension in 5000 rpm for 20 minutes, the UV spectrum of the upper phase was recorded, and the total extract concentration was determined according to the calibration curve. The results were also compared to the extract solution without the nanoparticles. The percentage of the extract adsorbed on the surface of nanoparticles was calculated by means of the following equation:
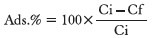
Where Ci and Cf represent the initial and final concentrations, respectively.
The UV spectrum of ZnOn and the fortified ZnOn with FznE
Formulations containing 20% of ZnO and 20% of ZnOn plus 1 mg/ml of plant extract (FZnE) were prepared separately. Six mg of each suspension was mounted as a thin film on the surface of the Transporeâ tape (3M Company, USA) and considering the formula base (all components except T. polium extract, ZnO, ZnOn, and FZnE) as a reference; their UV spectra were recorded as blank in the range of 200 to 400 nm.
Preparation of the sunscreen formulation
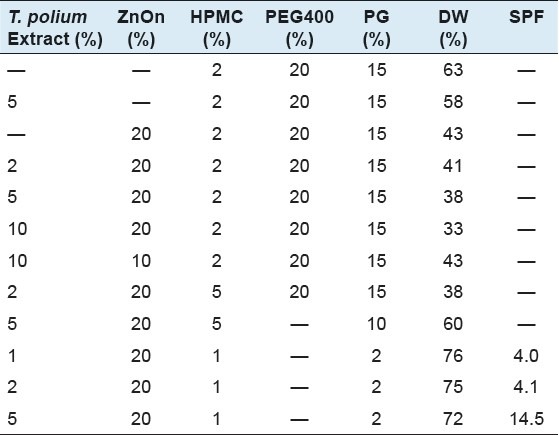
Different formulations with various concentrations of HPMC, FZnE, ZnOn, PEG400 and PG were prepared [Table 1]. All formulations were made to 100% by adding DIW.
Table 1.
Percentage of different ingredients in prepared formulations and calculated sun protecting factor (SPF) for roughly stable formulations (Formulations 10-12)
Characterization of the ZnOn
The average particle size and the polydispersity index of each batch of nanoparticles were measured using a Laser Light Scattering method (photon correlation Spectroscopy-SEMATECH) equipped with a laser light source operating at a scattering of 90° and at a temperature of 26°C. Before counting, the samples were diluted with DIW by the ratio of 1:5 to 1:10 v/v. A sample for scanning electron microcope (SEM) analysis was also prepared.
Stability of the formulations
Each formulation was held at 4˚C, ambient temperature and 37°C, separately. All samples were inspected visually for physical variations, such as phase separation and color changes at 0, 2, 4, and 8 weeks.[18,19]
SPF determination
SPF of prepared formulations was determined using the Transpore® tape with some modifications.[20,21,22,23,24] The Transpore® tape from a part, excluding the first or last 60 cm of a roll, was used. Transpore® 3M, a surgical tape manufactured by the 3M Company, was used as a substrate for its reported similar topography and sunscreen distribution to the human skin.[24] The test samples were applied in the amount of 2 mg/cm2 of the tape placed on a quartz slide, which were distributed over the surface by rubbing the surface of the slide with a single finger-cot-coated for 20 seconds, and were allowed to be dried for a few minutes. The scanning of the samples was done from 200-400 nm.[25,26,27]
The in vitro SPF is calculated as follows:
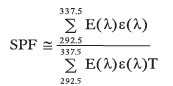
where:
E (λ) is the intensity of the solar light at the surface of the earth
ε (λ) is the erythemal effect of the UV- light, and
T is the measured sunscreen transmittance at wavelength (λ)
In vitro release study
The release of the flavonoids (rutin as a major component) from the stable formulation, which had the greatest physical stability in the refrigerator, at room temperature, and at 37°C, was measured using a Franz diffusion cell on a 0.2 μm cellulose acetate membrane.[28] 200 mg of the sample was applied on the membrane in the donor compartment. DIW was used as a receptor phase. The temperature was kept at 37°C and the receiver solution was stirred by a Teflon-coated magnet at 500 rpm. 3 ml of each sample was collected at specified times and the amount of released and penetrated extract were determined by UV spectroscopy.
RESULTS AND DISCUSSION
Extraction and assay of the total flavonoids
The yield of extraction was about 15.6% (w/w). The assay of the total flavonoids of the methanolic extract of T. polium was conducted using the method described in Iranian Plants Pharmacopeia.[17] Based upon rutin, the amount of flavonoids was estimated to be 1.8% (g/g).
Determination of T. polium extract loaded by ZnOn
Results showed that about 99.5% of the extract was adsorbed by the ZnOn, as illustrated in Figure 1.
Figure 1.
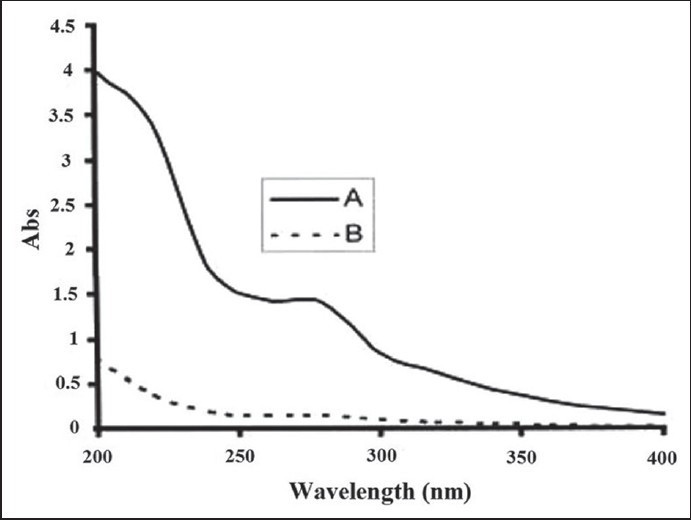
UV spectrum of T. polium methanolic extract (0.1 mg/ml) (a) extract before addition of ZnOn (b) extract after addition of ZnOn
Effect of PEG as humectant and co-solvent on in vitro SPF efficacy
The effect of PEG on the adsorption at the Zn/polymer solution interface was determined. In the present study, the formulations with PEG have shown much lower UV absorption than those without PEG [Figure 2].
Figure 2.
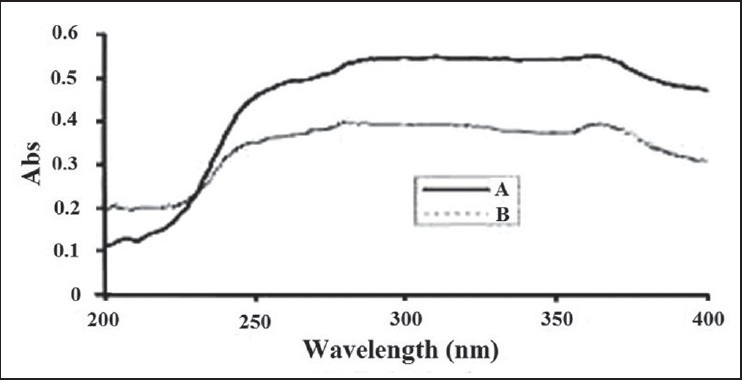
UV spectrum of prepared formulation (a) without PEG and (b) with PEG 400
The OH-group of the ZnOn surface may interact with PEG macromolecules through hydrogen binding.[29] This interaction decreases the binding sites for the adsorption of the other polar molecules, such as those which are present in the extract. Therefore, the SPF of the formulation containing PEG and the extract may differ from those without PEG.
Characterization of the nanoparticles
By formulating ZnOn with HPMC the size of the ZnOn increased from 534 ± 297 nm to 599 ± 314 nm and polydispersity index changed from 0.1 ± 0.06 to 0.07 ± 0.04. This can be due to the consequence of adding HPMC to the formulation. Further, it can be shown that the addition of HPMC narrowed the distribution of the particle size, average count number, and polydispersity index, as compared with the sample made of ZnOn. SEM of the ZnOn has been shown in the Figure 3.
Figure 3.

SEM image of ZnO nanoparticles
It seems that the particles are loosely adhered, contributing to much porosity that may increase the adsorption of the extract.
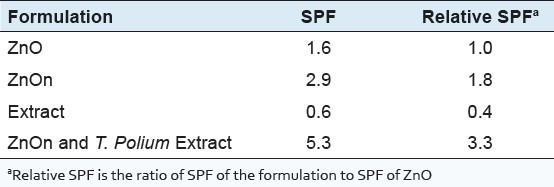
Formulation SPF determination
The relative SPF of the formulations have been summarized in Table 2. These results indicate that ZnOn with extract has the highest SPF. Moreover, it can be seen that the presence of the extract increased the SPF more than the expected sum of the SPFs of the components.
Table 2.
SPF and relative SPF calculated for ZnO ordinary particles, ZnOn, T. Polium extract, and ZnOn and T. polium extract combination in the gel base
Results of the SPF determination of the roughly stable formulation have been shown in Table 1.
The Linear relationship of SPF versus lnC resulted in the equation of SPF = 6.8 LnC + 21.4. The intercept, thereof (21.4) is an SPF of 1 mg of the formulation applied per 1 cm2 of the area. Results show that the formulation containing ZnOn and T. Polium extract may be applied for the protection of skin against harmful rays, which are in the wide range of spectra.
Release studies
The spectra of different concentrations of the extracts of T. polium in the range of 200 to 400 nm are shown in Figure 4. The desired correlation (r > 0.999) and repeatability (RSD% < 3%) were shown.
Figure 4.
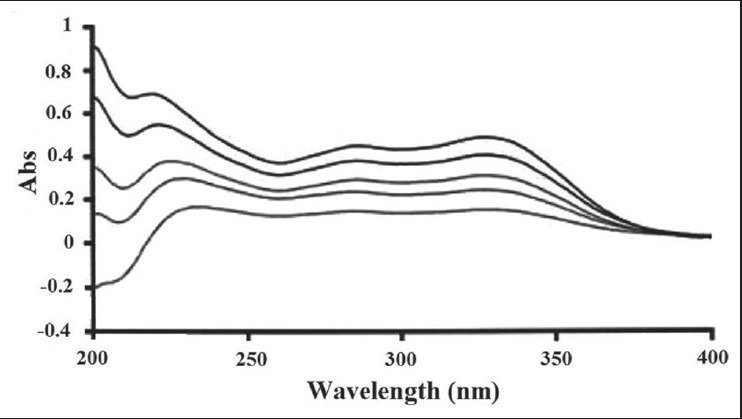
UV spectrum of different concentrations of methanolic extract of T. polium (40, 60, 80, 100, and 120 mg/l)
This method can be used reliably for the determination of the FZnE release and partly its assay in various preparations; further, some pretreatments are required. Accuracy greater than 98% indicates that the optimum results for each run during a day can be obtained using the standard solutions and the calibration curve in that run.
After conducting stability studies and determining the most stable formulations, respectively, the release studies were carried out. The physically stable formulation (No. 11) was selected for the release study. To determine the release mechanism, data were fitted on the Peppas equation as given below[30,31]:
Mt/M∞ = Ktn
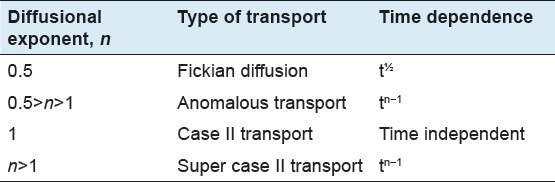
Where, Mt and M∞ are the absolute cumulative amount of drug released at time ‘t’ and infinite time ‘∞’, respectively. In this equation, ‘K’ is a constant incorporating the geometric characteristics of the device and ‘n’ is the release exponent, indicative of the mechanism of drug release. Table 3[31] shows to what release mechanism each calculated may correspond.
Table 3.
Drug transport mechanisms and diffusional exponents
Results of the formulation release have been shown in Figure 5 and shows that the diffusion of flavonoids from ZnOn surfaces occurs at two steps.
Figure 5.
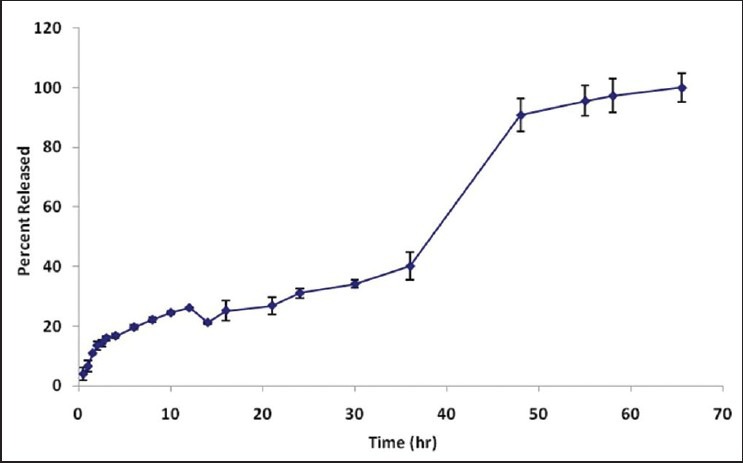
Release profile of T. polium methanolic extract on the basis of rutin from formulation No 11
At the first step (up to 36 h), data were fitted on the Peppas equation and the slope of the line was computed i.e., n = 0.5. According to the Table 3, it can be concluded that data were fitted by Fickian diffusion. This step of release probably belongs to the release of free flavonoids of the extract not adsorbed on the ZnOn surfaces or the flavonoids which have weak physical adhesion to the upper layer of flavonoids on the ZnOn. At this step, about 40 percent of the flavonoids content was released by the average rate of 1.1% per hour. At the next stage, the slope of the line changed, and under the conditions where the data were between 48 to 65.5 hours fitted on Peppas[31] equation with an average release rate of 0.5 percent per hour, the computed value of n was 0.31. This stage is also best fitted on Fickian diffusion according to the Table 3. This shows that the mechanism of the flavonoids release did not change but the rate of release decreased because most of the content had been released at the previous steps. At the medial step, during the interval of 36 to 48 hr, the burst effect was observed with an average rate of 4.2 percent per hour. This was probably due to desorption of the flavonoids adsorbed on ZnOn, and approximately 50% of the content of the flavonoids of the formulation was released. Considering the duration of sun-protecting effects of the sunscreens that usually continues for 12 hours per day, this release profile can make this product suitable for use as a long lasting sunscreen preparation.
CONCLUSION
The aim of this study was to prepare a natural sunscreen formulation of ZnOn and T. polium extract to produce a fortified sunscreen. The formulation was designed on a combination of a flavonoid rich extract of T. polium as a natural sunscreen with ZnOn, which is physical sunscreen, for protection in a wide range of UV radiation (UVA and UVB). The basis of the sunscreen is oil-free and it is more suitable for greasy skins. Because of anti-inflammatory and anti-oxidant activities of flavonoids, this formulation is more efficient for sensitive skins, especially in children. The most significant advantages of this formulation were the higher release rate, longer effects of SPF, and suitable appearance of applying ZnO on skin. Finally, formulation No. 11 is recommended as a choice sunscreen product after this study.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Lautenschlager S, Wulf HC, Pittelkow MR. Photoprotection. Lancet. 2007;370:528–37. doi: 10.1016/S0140-6736(07)60638-2. [DOI] [PubMed] [Google Scholar]
- 2.Gordon M. Dietary antioxidants in disease prevention. Nat Prod Rep. 1996;13:265–73. doi: 10.1039/np9961300265. [DOI] [PubMed] [Google Scholar]
- 3.Yener G, Incegül T, Yener N. Importance of using solid lipid microspheres as carriers for UV filters on the example octyl methoxy cinnamate. Int J Pharm. 2003;258:203–7. doi: 10.1016/s0378-5173(03)00203-5. [DOI] [PubMed] [Google Scholar]
- 4.Ngo MA, Maibach HI. Dermatotoxicology: Historical perspective and advances. Toxicol Appl Pharmacol. 2010;243:225–38. doi: 10.1016/j.taap.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 5.Watkinson AC, Brain KR, Walters KA, Hadgraft J. Prediction of the percutaneous penetration of ultra-violet filters used in sunscreen formulations. Int J Cosmet Sci. 1992;14:265–75. doi: 10.1111/j.1467-2494.1992.tb00059.x. [DOI] [PubMed] [Google Scholar]
- 6.Jain SK, Jain NK. Multiparticulate carriers for sun-screening agents. Int J Cosmet Sci. 2010;32:89–98. doi: 10.1111/j.1468-2494.2010.00547.x. [DOI] [PubMed] [Google Scholar]
- 7.Gaspar LR, Maia Campos PM. Evaluation of the photostability of different UV filter combinations in a sunscreen. Int J Pharm. 2006;307:123–8. doi: 10.1016/j.ijpharm.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 8.Scalia S, Villani S, Casolari A. Inclusion complexation of the sunscreen agent 2-Ethylhexyl-p-dimethylaminobenzoate with Hydroxypropyl-β-cyclodextrin: Effect on Photostability. J Pharm Pharmacol. 1999;51:1367–74. doi: 10.1211/0022357991777182. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez-Román R, Barré G, Guy RH, Fessi H. Biodegradable polymer nanocapsules containing a sunscreen agent: Preparation and photoprotection. Eur J Pharm Biopharm. 2001;52:191–5. doi: 10.1016/s0939-6411(01)00188-6. [DOI] [PubMed] [Google Scholar]
- 10.Kazemizadeh Z, Basiri A, Habibi Z. Chemical composition of the essential oil of Teucrium hyrcanicum and T. chamaedrys L. SUBSP. chamaedrys from Iran. Chem Nat Compd. 2008;44:651–3. [Google Scholar]
- 11.Sharififar F, Dehghn-Nudeh G, Mirtajaldini M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem. 2009;112:885–8. [Google Scholar]
- 12.Tsutomu N, Munetaka Y, Toshihiko O, Shunro K. Suppression of active oxygen-induced cytotoricity by flavonoids. Biochem Pharmacol. 1993;45:265–7. doi: 10.1016/0006-2952(93)90402-i. [DOI] [PubMed] [Google Scholar]
- 13.Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997;2:152–9. [Google Scholar]
- 14.Takamatsu S, Galal AM, Ross SA, Ferreira D, ElSohly MA, Ibrahim AR, et al. Antioxidant effect of flavonoids on DCF production in HL-60 cells. Phytother Res. 2003;17:963–6. doi: 10.1002/ptr.1289. [DOI] [PubMed] [Google Scholar]
- 15.Draelos ZD. Compliance and sunscreens. Dermatol Clin. 2006;24:101–4. doi: 10.1016/j.det.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Cross SE, Innes B, Roberts MS, Tsuzuki T, Robertson TA, McCormick P. Human skin penetration of sunscreen nanoparticles: In-vitro assessment of a novel micronized zinc oxide formulation. Skin Pharmacol Physiol. 2007;20:148–54. doi: 10.1159/000098701. [DOI] [PubMed] [Google Scholar]
- 17.Tehran: Ministry of Health, Treatment and Medical Education; 2002. IHP-Commision Iranian Herbal Pharmacopoeia (IHP) p. 272. [Google Scholar]
- 18.Kuntic V, Pejic N, Ivkovic B, Vujic Z, Ilic K, Micic S, et al. Isocratic RP-HPLC method for rutin determination in solid oral dosage forms. J Pharm Biomed Anal. 2007;43:718–21. doi: 10.1016/j.jpba.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 19.Montaña MP, Massad WA, Criado S, Biasutti A, García NA. Stability of flavonoids in the presence of riboflavin-photogenerated reactive oxygen species: A kinetic and mechanistic study on quercetin, morin and rutin. Photochem Photobiol. 2010;86:827–34. doi: 10.1111/j.1751-1097.2010.00754.x. [DOI] [PubMed] [Google Scholar]
- 20.Nesseem D. Formulation of sunscreens with enhancement sun protection factor response based on solid lipid nanoparticles. Int J Cosmet Sci. 2011;33:70–9. doi: 10.1111/j.1468-2494.2010.00598.x. [DOI] [PubMed] [Google Scholar]
- 21.Garoli D, Pelizzo MG, Nicolosi P, Peserico A, Tonin E, Alaibac M. Effectiveness of different substrate materials for in vitro sunscreen tests. J Dermatol Sci. 2009;56:89–98. doi: 10.1016/j.jdermsci.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 22.Bleasel MD, Aldous S. In vitro evaluation of sun protection factors of sunscreen agents using a novel UV spectrophotometric technique. Int J Cosmet Sci. 2008;30:259–70. doi: 10.1111/j.1468-2494.2008.00453.x. [DOI] [PubMed] [Google Scholar]
- 23.Diffey BL, Tanner PR, Matts PJ, Nash JF. In vitro assessment of the broad-spectrum ultraviolet protection of sunscreen products. J Am Acad Dermatol. 2000;43:1024–35. doi: 10.1067/mjd.2000.109291. [DOI] [PubMed] [Google Scholar]
- 24.Diffey BL, Robson J. A new substrate to measure sunscreen protection factors throughout the ultraviolet spectrum. J Soc Cosmet Chem. 1989;40:127–33. [Google Scholar]
- 25.Kaur CD, Saraf S. In vitro sun protection factor determination of herbal oils used in cosmetics. Pharmacognosy Res. 2010;2:22–5. doi: 10.4103/0974-8490.60586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.da Silva VV, Ropke CD, de Almeida RL, Miranda DV, Kera CZ, Rivelli DP, et al. Chemical stability and SPF determination of Pothomorphe umbellata extract gel and photostability of 4-nerolidylcathecol. Int J Pharm. 2005;303:125–31. doi: 10.1016/j.ijpharm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Ferrero L, Pissavini M, Marguerie S, Zastrow L. Sunscreen in vitro spectroscopy: Application to UVA protection assessment and correlation with in vivo persistent pigment darkening. Int J Cosmet Sci. 2002;24:63–70. doi: 10.1046/j.1467-2494.2002.00130.x. [DOI] [PubMed] [Google Scholar]
- 28.Wissing SA, Müller RH. Solid lipid nanoparticles as carrier for sunscreens: In vitro release and in vivo skin penetration. J Control Release. 2002;81:225–33. doi: 10.1016/s0168-3659(02)00056-1. [DOI] [PubMed] [Google Scholar]
- 29.Liufu S, Xiao H, Li Y. Investigation of PEG adsorption on the surface of zinc oxide nanoparticles. Powder Technol. 2004;145:20–4. [Google Scholar]
- 30.Siepmann J, Peppas NA. Modeling of drug release from delivery systems based on hydroxypropyl methylcellulose (HPMC) Adv Drug Deliv Rev. 2001;48:139–57. doi: 10.1016/s0169-409x(01)00112-0. [DOI] [PubMed] [Google Scholar]
- 31.Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-fickian release from non-swellable devices in the form of slabs, spheres, cylinders or discs. J Control Release. 1987;5:23–36. [PubMed] [Google Scholar]


