Abstract
Introduction:
Alginates can be tailored chemically to improve solubility, physicochemical, and biological properties and its complexation with metal ion is useful for controlling the drug release.
Materials And Methods:
Synthesized N,O-dimethyl, N-methyl, or N-Benzyl hydroxylamine derivatives of sodium alginate were subsequently complexed with zinc to form beads. Hydroxamation of sodium alginate was confirmed by Fourier transform infra-red spectroscopy (FTIR) and differential scanning calorimetry (DSC).
Results:
The synthesized polymeric material exhibited reduced aqueous, HCl and NaOH solubility. The hydroxamated derivatives demonstrated pulsed release where change in pH of the dissolution medium stimulated the atenolol release.
Conclusion:
Atenolol loaded Zn cross-linked polymeric beads demonstrated the sustained the plasma drug levels with increased half-life. Although the synthesized derivatives greatly altered the aqueous solubility of sodium alginate, no significant differences in in vitro and in vivo atenolol release behavior amongst the N,O-dimethyl, N-methyl, or N-Benzyl hydroxylamine derivatives of sodium alginate were observed.
Keywords: Atenolol, hydroxylamine derivatives, metal-polymer cross-linked beads, pulsatile release, sodium alginate
INTRODUCTION
Typical oral controlled release system maintains the drug concentration in the therapeutic window over a period of time ensuring sustained therapeutic action. Pulsatile drug release may be an optimal approach for the treatment of several diseases including bronchial asthma, hypertension, rheumatic disease, and myocardial infarction as well for controlling the body functions like blood pressure and hormonal levels influenced by circadian rhythms.[1,2,3] Pulsatile release may also be a useful tool for the targeting the drugs that irritate the stomach or degrades in gastric environment as well for drugs developing biological tolerance or undergoes first-pass metabolism extensively.[1,2] Such pulsatile drug delivery systems (DDS) are either single unit or multiparticulate systems characterized by a rapid drug release after a predetermined lag time.[3] From such delivery devices chemical, thermal, electric, and magnetic (externally) stimuli as well as change in pH, ionic strength, and temperature (physiologically) are responsible for achieving the pulsatile release pattern.
Controlled drug release has been achieved by coating tablets with polysaccharides or formulating it to form drug containing beads.[4] Moreover, cross-linking may be used to provide effective control over drug release rate.[5] Cross-linking of polysaccharides with divalent/polyvalent cations (Pb2+, Cu2+, Ba2+, Sr2+, Cd2+, Ca2+, Zn2+, Co2+, and Ni2+) are formed coordination complexes by coordinate bonds in which a pair of electrons is transferred from an electron donor to an electron acceptor and such polymer-metal composite materials have been utilized to obtain sustained release from oral drug delivery systems.
Sodium alginate, a sodium salt of brown algae cell wall constituent, alginic acid, is an anionic unbranched polysaccharide composed of the epimers α-L-guluronic acid and β-D-mannuronic acid residues, arranged in homopolymeric and heteropolymeric blocks. Monomer composition of alginate has major impact on the drug release property. While alginates with high α-L-guluronic acid content are suitable for thickening applications, those with a high β-D-mannuronic acid are best for gelation.[6] Alginic acid has been widely evaluated as matrix material for tabletting water-soluble drug in presence of water-soluble excipients.[7] Ionically cross-linked alginate is useful in offering the control over release rate and provides sustained release. Cation-induced gelification of alginate has sustained the release of rifampicin for 72 h in physiological media from microparticles.[8] Moreover, microspheric indomethacin system has impregnation of anion-exchange with alginate demonstrated the reduced release rate.[9] Sodium alginate cross-linking with aluminum ions has been reported to decrease the release rate of theophylline with increasing cross-linker concentration.[10]
Several alginate or derivatized alginate-metal cross-links has been explored in the literature to demonstrate the controlled release rate.[11,12,13] Iron cross-linked N-methyl and N-benzyl hydroxylamine derivatives of alginic acid and hydroxamated chitosan succinate derivatives has shown the promising potential in drug loading and carriers for controlled release delivery system.[14,15,16,17] Moreover, ferric cross-links of hydroxamated alginic acid and chitosan succinate, zinc (Zn) cross-links of thiolated alginic acid and sodium lauryl sulfate exhibited the pH independent and sustained drug release profiles.[18] Iron cross-link tender stability to the formulation over the range of physiological pH as compared to calcium, but leaching of iron from the polymer composites and consequently the safety are of big concern. However, Zn being a soft metal, its ion forms covalent-like coordinate bonds with carboxylates of alginate which are more resistant to hydration.[19,20] As reported earlier, the hydroxamated alginates are comparatively stable over the physiological pH ranges,[21,22] and hence the present work was planned to synthesize N,O-dimethyl, N-methyl, and O-benzyl hydroxylamine derivatives of sodium alginate with altered lipophilicity. The synthesized polymeric material was then complexed with Zn to control the release of atenolol, a model drug used.
MATERIALS AND METHODS
Materials
Atenolol was a gift from the Vama Pharmaceuticals, Nagpur, India. All chemicals used were purchased from the corresponding supplier. Sodium alginate and sodium hydroxide (all Loba chemie); dicyclohexyl carbodiimide (DCCI), N,O-dimethyl hydroxylamine HCl, N-methyl hydroxylamine HCl, and O-benzyl hydroxylamine HCl (all Himedia, India); Zn chloride, potassium hydroxide, and potassium bromide (all Merck); and hydrochloric acid, acetone, ethanol, diethyl ether, disodium ethylenediaminetetraacetic acid (EDTA), calcium carbonate (all Rankem, India).
The protocol for preclinical study was approved by Institutional Animal Ethics Committee (IAEC no.: 853/AC/04/CPCSEA).
Synthesis of N,O-dimethyl, N-methyl, and O-benzyl hydroxamated derivatives of alginic acid
Hydroxamated alginates were synthesized as per procedure outlined by Alkhatib, et al.[16] Sodium alginate (5 g, equivalent to 0.0225 mol carboxylate) was dissolved in water (250 ml) and hydrochloric acid solution (1.0 N) was added dropwise to the stirred polymer solution till a pH of 4.0-4.5 was attained. DCCI (2.32 and 4.64 g, equivalent to 0.0112 and 0.0225 mol, respectively) was added to the stirred mixtures to activate 50 and 100% of the carboxylic acid groups, respectively. After 2 h; N,O-dimethyl hydroxylamine HCl (4.36 and 8.8 g, equivalent to 0.0448 and 0.09 mol, respectively) or N-methyl hydroxylamine HCl (3.74 and 7.51 g, equivalent to 0.0448 and 0.09 mol, respectively) or O-benzyl hydroxylamine HCl (3.59 and 7.18 g, equivalent to 0.0225 and 0.045 mol, respectively) was added to the mixture and stirred further for 1 h. pH of the reaction mixture was then raised to 6.0 with sodium hydroxide solution (1.0 N) and stirred for 2 h. Thereafter, the pH was again raised to 9.0 and 24 h at room temperature. The white precipitate of dicyclohexylurea was removed by filtration and the polymeric material was precipitated with concentrated HCl (10 ml) and acetone (250 ml), filtered and washed thoroughly with ethanol (3 × 100 ml), followed by acetone (3 ´ 100 ml) and diethyl ether (200 ml), resulting mass was left to dry at room temperature overnight and milled.
Preparation of Zn cross-linked alginate and hydroxamated alginate derivative beads
Polymeric solutions of hydroxamated alginates (0.5 g) were prepared in 15 ml of sodium hydroxide (0.1 N) and atenolol (0.5 g) was added to it under constant stirring for 1 h. The resulting suspension was slowly dropped from a height of 20 cm into a stirred (250 rpm) solution of Zn chloride (3 g in 300 ml of water). Prepared beads were left in the stirred Zn chloride solution over 30-40 min. The suspension was then filtered and washed three times with water (50 ml). The beads were dried at room temperature over 48 h and subjected for evaluation. Surface morphology of prepared beads was evaluated by scanning electron microscopy (SEM).
Solubility evaluation of sodium alginate, its hydroxamated derivatives, and drug loaded Zn cross-linked beads
Solubility of sodium alginate and its hydroxamated derivatives was determined by adding an excess amount of polymer to flat bottom test tube containing 3.0 ml of 0.1 N HCl and 0.1 N NaOH and water stirred at 250 rpm at 25 ± 2°C for 36 h. Saturated solutions were centrifuged, filtered, and undissolved material was dried overnight at room temperature and weighed accurately. The solubility of polymeric material was calculated as difference between weights.
Methods
Fourier transform infrared (FTIR) characterization
Sodium alginate, its hydroxamated derivatives and their Zn cross-linked beads were characterized using FTIR spectrophotometry (Shimadzu-8400 S, Singapore). Samples were triturated with KBr (Merck, India) and compressed less than 10 T/cm2 for 10 min to prepared thin pellets; those were scanned in IR range from 400-4,000 cm-1.
Thermal characterization
Thermal behavior of the prepared hydroxamated sodium alginate was assessed by differential scanning calorimetry (DSC). The dried samples were weighed (3-5 mg) in aluminum pans and scanned between 0 and 300C at a heating rate of 10C/min under nitrogen (Shimadzu DSC TA60 WS).
Drug content from Zn cross-linked beads
Accurately weighed atenolol loaded beads were dissolved in the 100 ml of 0.1 N NaOH and sonicated for 1 h. Solution was then filtered and absorbance of solution was determined at 225 nm (UV-Vis spectrophotometer, Shimadzu-1700S).
Determination of cross-linked Zn from polymeric beads
Zinc cross-linked per gram of polymeric beads was calculated by dissolving accurately weighed atenolol loaded beads in 100 ml of 0.1 N NaOH sonicated for 2 h and these solutions were titrated with 0.01 M EDTA using modern black indicator.
In vitro atenolol release from Zn cross-linked polymeric beads
Rotating basket apparatus (dissolution system, model DRS-14) fitted with a 0.125 mm stainless steel basket was used. In each case, dried beads were placed in the basket. Three dissolution media were used over three subsequent stages: 0.1 N HCl media (pH 1.2) for 2 h, phosphate buffer media of pH 7.4 for 3 h, and then phosphate buffer media pH 6.8 for remaining hours. The dissolution media (900 ml) were maintained at 37°C. The baskets were rotated at 100 rpm and samples (5 ml) were withdrawn at predetermined time intervals for the analysis of released drug. The withdrawn volume was immediately replaced with an equivalent volume of the fresh medium maintained at the same temperature. The absorbance of undiluted samples was determined at 225 nm for atenolol release using UV-visible spectrophotometer (Shimadzu-1700S).
Determination of released Zn
Following the completion of dissolution experiment, 5 ml of dissolution medium was withdrawn and collected samples were diluted with 10 ml of distilled water with subsequent addition of 1 ml of ammonium buffer (pH 10) and resulting solutions were then titrated with 0.01 M EDTA solution using modern black indicator.[23]
In vivo studies
Plasma sample preparations
All experimental protocols were approved by IAEC and carried out under strict compliance with ethical principles and guidelines of Committee for Purpose of Control and Supervision of Experimental Animals (CPCSEA), Ministry of Environment and Forests; Government of India; New Delhi. Adult Sprague-Dawley rats (weight, 210 ± 10 g) purchased from National Institute of Nutrition, Hyderabad, India were housed under a controlled environment of temperature (25 ± 2°C) maintained on 12 h light/dark cycle (lights on 07.00 am) with free access to water and standard pelleted diet for 7 days. Sprague-Dawley rats (n = 6, total 42) were fasted for 12 h prior to experimentation and randomly divided into seven groups. Rats were administered orally with bead powder suspended in normal saline just before the administration in the dose equivalent to 16 mg/kg of atenolol. Under ether anesthesia, 0.5 ml blood from three animals from a group at one time point was withdrawn from retro-orbit sinus through heparinized capillary tubes in heparinized Eppendorff tubes at 1, 3, 6, 10, and 12 h after dosing. The blood from a rat was taken at alternative time point to reduce the animal sufferings. Plasma was separated by centrifugation at 1,500 rpm for 5 min and stored at -20°C until analysis.
Sample extraction and analytical procedures
Atenolol from the stored plasma specimens was extracted by earlier reported method.[24] Frozen plasma samples were liquefied at ambient temperature and 100 μl rat plasma was mixed with 10 ml of metronidazole (50 μg/ml) (internal standard). This mixture was then made alkaline by adding 150 μl of sodium hydroxide solution (1 mol) and vortexed vigorously for 3 min at ambient temperature. Two milliliter of dichloromethane was then added and vortex mixed for 5 min. The samples were then centrifuged at 4,000 rpm for 10 min and organic layer was separated and evaporated to dryness under nitrogen flow at 42C. The extract was resuspended in 100 μl of methanol, vortex-mixed and 20 μl of this solution was injected into the high-performance liquid chromatography (HPLC; Jasco, Quaternary Gradient system) equipped with MD-2010 multiwavelength detector and Develosil ODS-HG-5 (150 mm × 4.6 mm) column. Acetonitrile, distilled water, and triethylamine (5:94.8:0.02, v/v/v) was used as mobile phase with flow rate of 0.7 ml/min and column temperature of 40°C. The detection was carried out at 274 nm.
Calibration curve
Stock solution of atenolol (100 μg/ml) was prepared in methanol and stored at 4C. Working dilutions (0.02, 0.05, 0.1, 0.2, 0.5, 1.0, and 2.0 μg/ml) were prepared by diluting the stock solution in methanol. One hundred microliter of these dilutions were transferred into Eppendorf tubes (1.5 ml) and evaporated to dryness under nitrogen. The dried residue was then used to spike the blank rat plasma (100 μl) and analyzed as per procedure described in the above section to generate calibration plot in rat plasma. Pharmacokinetic analysis of plasma atenolol determinations were done by PKSolver add-in program.[25]
RESULT AND DISCUSSION
Synthesis of hydroxamated derivatives of sodium alginate
Free hydroxyl and carboxyl groups in alginate backbone make it an ideal entrant for chemical modification with altered solubility, hydrophobicity, and physicochemical and biological characteristics. Alginate consists of (1→4) linked β-D-mannuronic acid and α-L-guluronic acid residues in varying composition and sequence.[26] Alkylated hydroxyl amines can be covalently linked through amide bonds formed between the primary amino group of hydroxylamine and a carboxylic acid group of the polymer.[16,27] The conjugation reaction was accomplished by using theoretically calculated amount of coupling agent, DCCI, for activating 50 and 100% of the available carboxylic acid moieties under acidic conditions (pH 4-4.5) to enhance the reactivity of DCCI via imino-nitrogen protonation. This reaction mixture was then agitated with N,O-dimethyl, N-methyl, or N-benzyl hydroxylamine hydrochloride added in excess to ensure the forward reaction which may otherwise proceed in reverse direction due to steric hindrance in alginate. The pH was then raised to 9.0 for complete emancipation of hydroxylamine nucleophile from hydrochloride salt. Dicyclohexylurea formed in the reaction separates as white precipitate due to low solubility. The reaction mixture neutralized by adding concentrated HCl converts highly hydrated carboxylate anions into the less hydrated carboxylic acid form with reduction of aqueous solubility and terminate the reaction. The derivatized hydroxamated alginates were precipitated by the addition of acetone, filtered, and purified to determine the yield. As shown in Table 1, % yield for all the semisynthetic polymers was in the range of 32-52. Highest yield was calculated for 100% O-benzyl hydroxamated derivative (52.49 ± 1.78%) [Table 1].
Table 1.
Yield, solubility details of N,O-drug-loaded zinc cross-linked sodium alginate and its N,O-dimethyl, N-methyl, or N-benzyl hydroxylamine derivatives
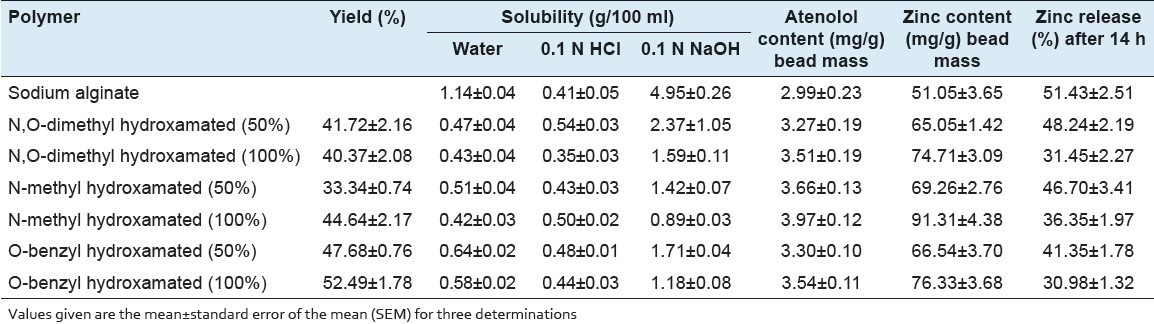
Infrared characterization of hydroxamated alginate derivatives
FTIR spectrum of sodium alginate shows the absorption around 3,050-3,650; 1,625; 1,420; and 1,035 cm−1 due to -OH, -COO- (asymmetric), and -COO- (symmetric) stretching [Figure 1]. Infrared spectrum comparison between sodium alginate and its hydroxamated derivatives showed the introduction of hydroxamic amidic groups reflected by an intense stretching vibration band within the region of (1,626-1,638 cm-1) along with the reduction in the intensity of carboxylic acid carbonyl bands (1,736-1,746 cm-1). The hydroxamation of sodium alginate altered the shape of stretching hydroxyl vibrations (at 3,500-3,300 cm-1) with pronounced sharp bands around 3,610 cm-1 corresponding to non-hydrogen-bonded hydroxyl (free) groups in N,O-dimethyl, O-benzyl, and N-methyl hydroxamated derivatives. Steric hindrance due to N,O-dimethyl, N-methyl, and O-benzyl substitution may reduce the hydrogen bonding in carboxylic acid dimers.
Figure 1.
Fourier transform infrared spectroscopy (FTIR) spectra of synthesized hydroxylamine derivatives. A = Sodium alginate, B = N,Odimethyl hydroxamated alginate, C = N-methyl hydroxamated alginate, D = O-benzyl hydroxamated alginate
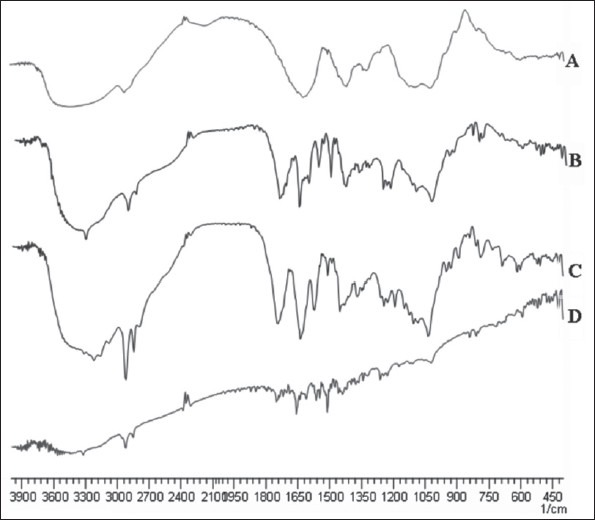
Thermal evaluation of hydroxamated derivatives
Figure 2 shows DSC thermograms of sodium alginate and its N,O-dimethyl, N-methyl, and O-benzyl hydroxamated derivatives. DSC thermogram of sodium alginate [Figure 2] exhibited an endotherm (90-100C) corresponding to the heat of evaporation of the associated water of hydration, while other peaks signify heat required for dissociation of intra- and intermolecular hydrogen bonding within the polymeric matrix and polymeric degradation. As depicted in Figure 2, the appearance of endotherm around 140-150C in all the derivatized polymers indicate the formation of hydroxamated alginates along with inter- and intramolecular hydrogen bonds.
Figure 2.
Differential scanning calorimetry (DSC) thermograms of synthesized hydroxylamine derivatives. A = Sodium alginate, B = N,Odimethyl hydroxamated alginate, C = N-methyl hydroxamated alginate, D = O-benzyl hydroxamated alginate
Solubility evaluation of sodium alginate and its hydroxamated derivatives
Carboxylic acid moieties of alginate polymers ionize under alkaline conditions facilitating the hydration and consequently dissolution of polymer in aqueous conditions. Different alginate-based polymers demonstrated to have maximum solubility in 0.1 N NaOH solutions while less soluble in water and in 0.1 N HCl. Chemical modification of sodium alginate showed three-, five-, and fourfold reductions in NaOH solubility for N,O-dimethyl, O-benzyl, and N-methyl hydroxamated derivatives (100%); respectively [Table 1]. Increased hydrophobicity upon hydroxamation may decrease the water uptake. While solubility of synthesized alginate derivates in water was substantially reduced, it remained least influenced in 0.1 N HCl.
Preparation of Zn cross-linked beads
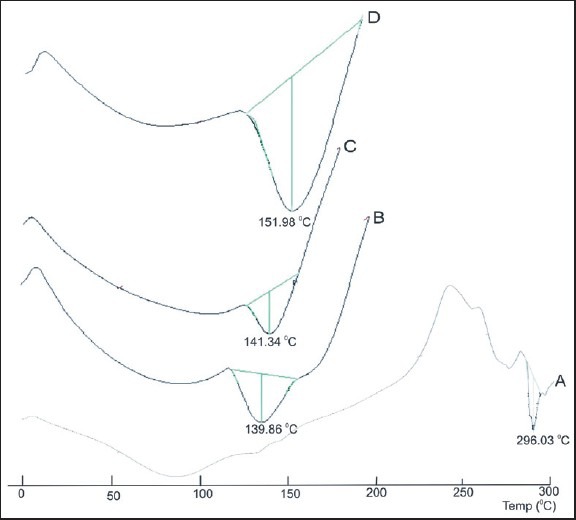
Viscous drug polymer suspensions dropped into a Zn chloride solution were cured in solution for 30-40 min under constant stirring to avoid sticking and agglomeration of beads. The resulting beads were almost spherical in shape and brown colored which shriveled on drying. Figure 3 shows the surface morphology (SEM) of atenolol containing Zn cross-linked hydroxamated alginate beads. Surface morphology showed the presence of fractured surface with comparative brittleness in all the 50% hydroxamated alginate Zn cross-linked beads which became even and smooth with high degree of substitution (100%). The surface of the Zn cross-linked bead was smooth with low brittleness and high elasticity. Increase in the degree of hydroxamation of alginate seems to decrease the fractures on the bead surface.
Figure 3.
Scanning electron microscopy (SEM) photographs of atenolol loaded zinc cross-linked beads prepared from: (a) Sodium alginate, (b) N,O-dimethyl hydroxamated alginate (50%), (c) N,O-dimethyl hydroxamated alginate (100%), (d) N-methyl hydroxamated alginate (50%), (e) N-methyl hydroxamated alginate (100%), (f) O-benzyl hydroxamated alginate (50%), (g) O-benzyl hydroxamated alginate (100%)
Infrared characterization atenolol loaded Zn cross-linked beads of hydroxamated alginate derivatives
FTIR spectra for the Zn cross-linked beads of sodium alginate and its hydroxamated derivative containing atenolol are depicted in Figure 4. It is clearly evident from the FTIR absorption bands at 1,600,-1,700 cm-1 corresponding to carboxylic has been modified. The major difference in the infrared spectra of Zn cross-linked hydroxamated alginate is the shift in the carbonyl stretching vibrations from 1,560 to 1,444 cm−1 which indicates the formation of coordinate bonds between carboxylate moieties and Zn ions.
Figure 4.
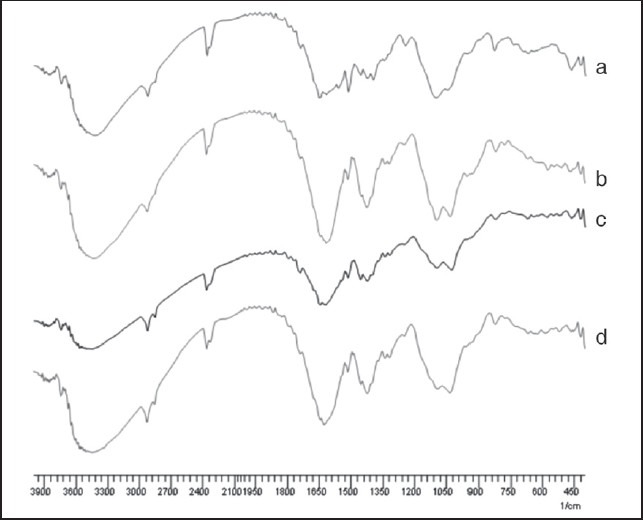
FTIR spectra of atenolol loaded zinc cross-linked beads prepared from: (a) Sodium alginate. (b) N,O-dimethyl hydroxamated alginate. (c) N-methyl hydroxamated alginate. (d) O-benzyl hydroxamated alginate
Atenolol and Zn content in cross-linked beads
As depicted in Table 1, hydroxamated alginates showed high degree of atenolol entrapment and Zn content than sodium alginate alone. Moreover, atenolol loading was proportional to the degree of substitution as well as Zn content. The beads that contain higher Zn content demonstrated greater atenolol loading. Amount of Zn complexed with alginate and its hydroxamated derivatives is dependent on the availability of carboxylic and hydroxamic moieties and accessibility of Zn ions to these groups which in turn depends upon hydrophobicity of polymer and its hydration. O-benzyl derivatized alginates contained highest atenolol followed by N-methyl hydroxamated, while lowest atenolol entrapment was evident with N,O-dimethyl hydroxamated derivatives of sodium alginate. This may be probably due to the steric hindrance that increases with bulkier substitution and reduces the drug entrapment.
In vitro atenolol release
Zinc cross-linked beads of alginate and its hydroxamated derivatives viz. N,O-dimethyl, N-methyl, and O-benzyl were evaluated for drug release carried out under sink conditions for first 2 h in hydrochloric acid pH 1.2 (stomach pH), followed by 3 h in phosphate buffer pH 7.4 (duodenal pH), and subsequently for 9 h in phosphate buffer pH 6.8 (colonic pH) which is in close correlation with pH and transit time of gastrointestinal tract (GIT) [Table 2].
Table 2.
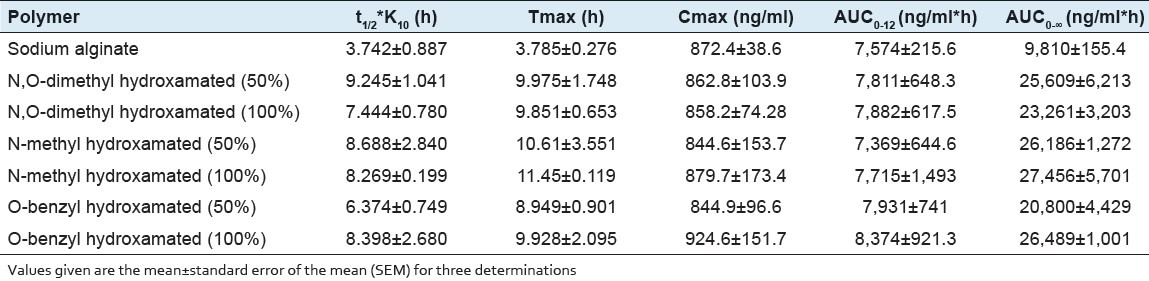
Pharmacokinetic parameters calculated from the plasma atenolol profile following single oral administration of atenolol loaded zinc cross-linked sodium alginate and its N,O-dimethyl, N-methyl, or N-benzyl hydroxylamine derivatives
Atenolol loaded Zn cross-linked beads of sodium alginate released almost about 96% of its atenolol after 6 h, whereas beads prepared from hydroxamated substitution released about 80%. It seems that hydroxamation of alginic acid forms the stable matrices with Zn having capacity to sustained or controlled released more than 12 h. Figure 5 shows the atenolol release per hour over the period of 14 h under the dissolution conditions of physiological pH and transit.
Figure 5.
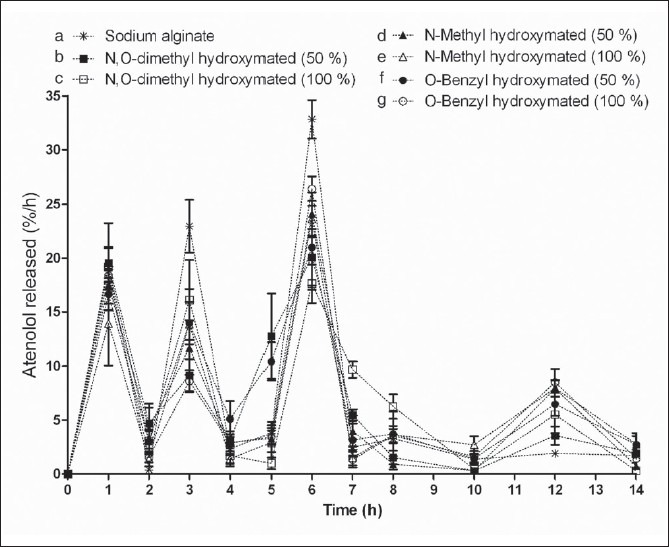
In vitro atenolol release zinc cross-linked beads prepared from (a) Sodium alginate; (b) N,O-dimethyl hydroxamated alginate (50%); (c) N,O-dimethyl hydroxamated alginate (100%); (d) N-methyl hydroxamated alginate (50%), (e) N-methyl hydroxamated alginate (100%), (f) O-benzyl hydroxamated alginate (50%), and (g) O-benzyl hydroxamated alginate (100%)
Atenolol release from the Zn cross-linked alginate and derivatized alginate was almost similar (14-19%) in the first 2 h; however, Zn cross-linked beads prepared from sodium alginate demonstrated higher release in phosphate buffer pH 7.4 (23%) as compare to hydroxamated alginates (8-15%) at 3rd h. Matrices of alginate under basic conditions diffused high amount of atenolol might be due to high solubility of sodium alginate and reverse may be true for the hydroxamated alginates. At 6th h, shifting of pH from 7.4 to 6.8 released almost 33% atenolol while it remained almost 18-24% for derivatized alginate. It is interesting to mention here that the higher release of atenolol was calculated at the time point of switch over of the dissolution medium, while no significant release was observed after the initial hour. The plot drug release per hour and time shows the three pulses of atenolol release from the beads prepared with sodium alginate, while additional pulse at 10th h was observed for hydroxamated alginates. The pulsed release seems to the function of solubility of the polymer under different pH condition and the hydrophobicity of the synthesized polymer conferred by the hydroxamated substitution.
Drug release from formulation is a complex function of the degree and nature of hydroxamation. However, no profound differences in the drug release was found in the Zn cross-linked hydroxamated alginate derivatives. All the Zn cross-linked matrices exhibited sigmoidal pattern of atenolol release demonstrating radical drug release with change in dissolution media. Hence the prepared Zn cross-linked beads of hydroxamated alginates may be suitable for the formulation of pulsatile drug delivery system because of its stability to control the drug delivery in all the pH media. Ionization of the amine group may also influence the release of the atenolol as a function of the pH of the medium which may influence the drug release by interactions with the carboxylic groups of the alginate derivatives. Furthermore, the possible hydrophobic interactions of the drug through its methyl groups with hydrophobic clusters of the hydroxamated alginate beads can also influence atenolol release.
Zinc leaching from Zn cross-linked alginate beads
Metal cross-linked polymeric complexes are usually associated with the problem metal ion leaching, which limits the suitability of such systems. Earlier studies demonstrated that Zn content in animal body remains constant even after its ten-fold dietary intake. Adjustments in gastrointestinal Zn absorption and endogenous fecal Zn excretion are the major phenomenon that controls the Zn content.[28] Daily adult Zn intake ranges from 15 to 22 mg/day and oral administration of 237-623 mg/kg in rats and 86-605 mg/kg of Zn in mice produces acute toxicity.[29]
Zinc release from the alginate and hydroxamated alginates cross-linked beads is presented in Table 1. A drift of decrease in the Zn leaching was determined with increased degree of substitution. Increased hydroxamation is expected to increase the degree of Zn cross-linking as well as the hydrophobicity of the polymer, thus restraining the penetration of water molecules; and therefore, the dissociation of the complexes and Zn leaching. It has been observed that Zn release from prepared cross-links of hydroxymated alginates is quite below the levels that are tolerated and prescribed for human beings.
Plasma atenolol estimation
The plot of mean plasma atenolol concentration and time is shown in Figure 6 and pharmacokinetic parameters calculated are listed in Table 2. Cmax and area under the curve (AUC)0-12 were not significantly different in the animals administered with Zn cross-linked beads prepared either from sodium alginate or its hydroxamated derivatives. Although the AUC0-12 h values were almost similar with drastic increment in AUC0-∞ values was found between alginate Zn cross-link and hydroxamated alginate Zn cross-link. On another hand, t½ *K10 of atenolol from Zn cross-linked hydroxamated alginates was significantly extended. Decreased atenolol bioavailability from sustained release formulation has been reported in healthy male volunteers.[30] However, significant differences in AUC0-∞ values between Zn cross-linked beads prepared with hydroxamated alginates indicated the increased bioavailability of atenolol from all the hydroxamated polymeric Zn complexes. Whereas, almost similar AUC0-12 h values for the metal complexes demonstrate the lack of the effect of alginate derivatization (hydroxamation) on the atenolol bioavailability. Although these results seem to be unusual; however, there are similar reports available in the literature that showed the lack of correlation between AUC0-∞ and AUC0-12 h from the same formulation. The pharmacokinetic analysis of the data suggests the extension of t½ *K10 and increased bioavailability with the hydroxamated alginates as compare to sodium alginate; however, differences in type and the degree of hydroxamation found to have least or no effect. Presence of free hydroxyl and carboxyl groups facilitate the chemical modification of alginate that improves solubility, hydrophobicity, and physicochemical and biological characteristics of alginate.[27] Hydroxamation of alginate and its subsequent complexation with metal ion has shown to be a better approach for controlling the delivery.[31] Early diffusion of the drug as well as complexes from the calcium cross-linked polymers failed to prolong the release, while iron leaching was the problem associated with iron cross-linked derivatives of alginate.[16,32] Moreover, aqueous solubility and hydrophilicity of alginate is another controlling factor that plays critical role in controlling the release rate.[31]
Figure 6.
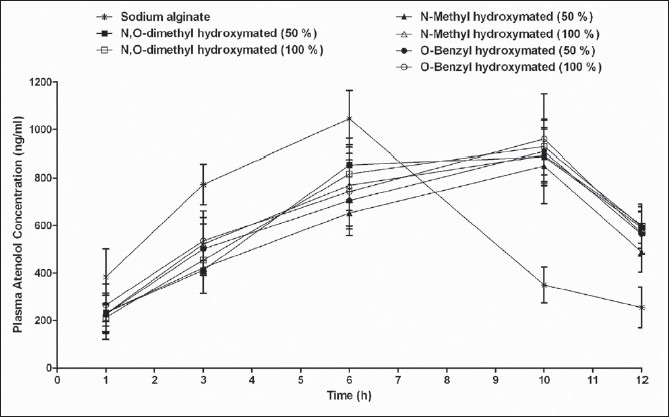
In vivo plasma atenolol concentrations following single oral administration of atenolol loaded zinc cross-linked sodium alginate and its N,O-dimethyl, N-methyl, or N-benzyl hydroxylamine derivatives
Besides most of the polysaccharides, alginate is reported to be useful for bead formation. Complexation with metal ions offers protection to the polysaccharide against the degradation by proteases and amylases in the upper GIT.[4,33] Apart from several applications in drug delivery, calcium alginate cross-link has shown to produce pulsed release of dextran.[34] Zn, a stronger cross-linker than other metal ions used, interacts strongly and provides the stability to the beads in dissolution medium[35] and drug release from Zn cross-linked polymeric beads depend on the medium. Use of Zn in the cross-linking of pectin has shown to be suitable for pulsatile delivery.[36] Moreover, calcium cross-linked of alginate has demonstrated the pulsatile release, however the lag time offered was depended upon the thickness of the coating employed.[34] The present study demonstrated the single unit Zn cross-linked beads prepared from the hydroxamated alginates releases the drug in four different pulses at 1st, 3rd, 6th, and 10th h with maximum Zn leaching of about 35 mg at 14 h. This pulsed release from the derivatized alginate seems to be stimulated as result of change in the pH of dissolution medium. In vivo studies demonstrated the extended peak of drug absorption of around 10 h as compare to 3.75 h with alginate cross-links. The data obtained from In vivo and In vivo studies may be projected for the usefulness of these polymeric complexes for sustained delivery with pulsatile release in physiological or simulated conditions. Moreover, this study also proposes the application of hydroxamated alginate Zn cross-links for chronopharmacotherapy.
CONCLUSION
Substitution with hydrophobic groups during hydroxamation of alginate alters the solubility and their cross-linking with Zn results into the preparation of beads which controls the release rate. Programmed discharge of drug from formulation after lag phases is suitable to meet chronopharmaceutical demands. Such a release as observed with the atenolol loaded Zn cross-linked hydroxamated alginate beads is referred as pulsatile delivery which gives a prompt and quantitative, repeated or prolonged release after the lag phase/s depending on formulation characteristics. Formulation using pH dependent polymer can be prepared in such a manner that change in pH during gastric transit will trigger drug release. Therefore, this investigation suggests that hydroxamated derivatives of alginate can be developed, which upon cross-linking with Zn may be useful for the formulation of the chronopharmacotherapy preparations and controlling the drug delivery.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Ritschel WA, Forusz H. Chronopharmacology: A review of drugs studies. Methods Find Exp Clin Pharmacol. 1994;16:57–75. [PubMed] [Google Scholar]
- 2.Lemmer B. Chronopharmacokinetics: Implications for drug treatment. J Pharm Pharmacol. 1999;51:887–90. doi: 10.1211/0022357991773294. [DOI] [PubMed] [Google Scholar]
- 3.Bussemer T, Otto I, Bodmeier R. Pulsatile drug-delivery systems. Crit Rev Ther Drug. 2001;18:433–58. [PubMed] [Google Scholar]
- 4.Liu L, Fishman ML, Kost J, Hicks KB. Pectin-based systems for colon specific drug delivery via oral route. Biomaterials. 2003;24:3333–43. doi: 10.1016/s0142-9612(03)00213-8. [DOI] [PubMed] [Google Scholar]
- 5.Bourgeois S, Gernet M, Pradeau D, Andremont A, Fattal E. Evaluation of critical formulation parameters influencing the bioactivity of [beta]-lactamases entrapped in pectin beads. Int J Pharm. 2006;324:2–9. doi: 10.1016/j.ijpharm.2006.05.059. [DOI] [PubMed] [Google Scholar]
- 6.Pandey R, Khuller GK. Kentucky, USA: Taylor and Francis Group, LLC; 2005. Alginate as a Drug Delivery Carrier. Handbook of Carbohydrate Engineering; pp. 799–814. [Google Scholar]
- 7.Liew CV, Chan LW, Ching AL, Heng PW. Evaluation of sodium alginate as drug release modifier in matrix tablets. Int J Pharm. 2006;309:25–37. doi: 10.1016/j.ijpharm.2005.10.040. [DOI] [PubMed] [Google Scholar]
- 8.Desai JV, Patil JS, Kulkarni RV, Marapur SC, Dalavi VV. Alginate-based microparticulate oral drug delivery system for rifampicin. Res J Pharm Tech. 2009;2:301–3. [Google Scholar]
- 9.Chretien C, Boudy V, Allain P, Chaumeil JC. Indomethacin release from ion-exchange microspheres: Impregnation with alginate reduces release rate. J Control Release. 2004;96:369–78. doi: 10.1016/j.jconrel.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 10.Nokhodchi A, Tailor A. In situ cross-linking of sodium alginate with calcium and aluminum ions to sustain the release of theophylline from polymeric matrices. Farmaco. 2004;59:999–1004. doi: 10.1016/j.farmac.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Aslani P, Kennedy RA. Studies on diffusion in alginate gels. I. Effect of crosslinking with calcium or zinc ions on diffusion of acetaminophen. J Controlled Release. 1996;42:75–82. [Google Scholar]
- 12.Park SB, Kang HW, Haam S, Park HY, Kim WS. Ca-alginate microspheres encapsulated in chitosan beads. J Microencapsul. 2004;21:485–97. doi: 10.1080/02652040410001729269. [DOI] [PubMed] [Google Scholar]
- 13.Setty CM, Sahoo SS, Sa B. Alginate-coated alginate– polyethyleneimine beads for prolonged release of furosemide in simulated intestinal fluid. Drug Dev Ind Pharm. 2005;31:435–46. doi: 10.1080/03639040500214647. [DOI] [PubMed] [Google Scholar]
- 14.Aiedeh K, Taha MO. Synthesis of iron-crosslinked chitosan succinate and iron-crosslinked hydroxamated chitosan succinate and their in vitro evaluation as potential matrix materials for oral theophylline sustained-release bead. Eur J Pharm Sci. 2001;13:159–68. doi: 10.1016/s0928-0987(00)00217-7. [DOI] [PubMed] [Google Scholar]
- 15.Taha MO, Aiedeh KM, Al-Hiari Y, Al-Khatib H. Synthesis of zinc-crosslinked thiolated alginic acid beads and their in vitro evaluation as potential enteric delivery system with folic acid as model drug. Pharmazie. 2005;60:736–42. [PubMed] [Google Scholar]
- 16.Alkhatib HS, Taha MO, Aiedeh KM, Bustanji Y, Sweileh B. Synthesis and in vitro behavior of iron-crosslinked N-methyl and N benzyl hydroxamated derivative of alginic acid as controlled release carriers. Eur Polym J. 2006;42:2464–74. [Google Scholar]
- 17.Ostberg T, Lund EM, Graffner C. Calcium alginate matrixes for oral multiple unit administration: IV. Release characteristics in different media. Int J Pharm. 1994;112:241–8. [Google Scholar]
- 18.Taha MO, Nasser W, Ardakani A, AlKhatib HS. Sodium lauryl sulfate impedes drug release from zinc crosslinked alginate beads: Switching from enteric coating release into biphasic profiles. Int J Pharm. 2008;350:291–300. doi: 10.1016/j.ijpharm.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 19.Kim CK, Lee EJ. The controlled release of blue dextran from alginate beads. Int J Pharm. 1992;79:11–9. [Google Scholar]
- 20.Aiedeh K, Taha MO, Al-Hiari Y, Bustanji Y, Alkhatib HS. Effect of ionic crosslinking on the drug release properties of chitosan diacetate matrices. J Pharm Sci. 2007;96:38–43. doi: 10.1002/jps.20764. [DOI] [PubMed] [Google Scholar]
- 21.Feng M, Van Der Does L, Bantjes A. Iron (III) chelating resins. V. Crosslinked copolymers of 1-(b-acrylamidoethyl)- 3-hydroxy-2- methyl-4(1H)-pyridinone (AHMP) and N, N dimethylacrylamide (DMAA) for iron (III) chelation studies. J Appl Polym Sci. 1994;52:21–8. [Google Scholar]
- 22.Lee TS, Hong SI. Synthesis and metal binding properties of poly (hydroxamic acid) resins from poly (ethyl acrylate-codivinylbenzene) beads. J Appl Polym Sci. 1995;57:311–7. [Google Scholar]
- 23.Skoog DA, West DM, Holler FJ, Crouch SR, editors. Analytical Chemistry: An Introduction. 2000;15:345–81. [Google Scholar]
- 24.Jia J, Dong C, Zhang W, Cui Y, Liu J. Evaluation of pharmacokinetic and pharmacodynamic relationship for oral sustained-release atenolol pellets in rats. J Pharm Biomed Anal. 2011;55:342–8. doi: 10.1016/j.jpba.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Huo M, Zhou J, Xie S. PKSolver: An add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed. 2010;99:306–14. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 26.Coviello T, Matricardi P, Marianecci C, Alhaique F. Polysaccharide hydrogels for modified release formulations. J Control Release. 2007;119:5–24. doi: 10.1016/j.jconrel.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Yang JS, Xie YJ, He W. Research progress on chemical modification of alginate: A review. Carbohyd Polym. 2011;84:33–9. [Google Scholar]
- 28.King JC, Shames DM, Woodhouse LR. Zinc Homeostatsis in Humans. J Nutr. 2000;130:1360S–6. doi: 10.1093/jn/130.5.1360S. [DOI] [PubMed] [Google Scholar]
- 29.Domingo JL, Llobet JM, Patermain JL, Corbella J. Acute zinc intoxication: Comparison of the antidotal efficacy of several chelating agents. Vet Hum Toxicol. 1988;30:224–8. [PubMed] [Google Scholar]
- 30.Rouge N, Allemann E, Gex-Fabry M, Balant L, Cole ET, Buri P, et al. Comparative pharmacokinetic study of a floating multiple-unit capsule, a high-density multiple-unit capsule and an immediate-release tablet containing 25 mg atenolol. Pharm Acta Helv. 1998;73:81–7. doi: 10.1016/s0031-6865(97)00050-2. [DOI] [PubMed] [Google Scholar]
- 31.Li Z, Ni C, Xiong C, Li Q. Preparation and drug release of hydrophobically modified alginate. Chemistry. 2009;1:93–6. [Google Scholar]
- 32.Fundueanu G, Nastruzzi C, Carpov A, Desbrieres J, Rinaudo M. Physico-chemical characterization of Ca-alginate microparticles produced with different methods. Biomaterials. 1999;20:1427–35. doi: 10.1016/s0142-9612(99)00050-2. [DOI] [PubMed] [Google Scholar]
- 33.Das S, Ng KY. Resveratrol-loaded calcium-pectinate beads: Effects of formulation parameters on drug release and bead characteristics. J Pharm Sci. 2010;99:840–60. doi: 10.1002/jps.21880. [DOI] [PubMed] [Google Scholar]
- 34.Kikuchi A, Kawabuchi M, Sugihara M, Sakurai Y, Okano T. Pulsed dextran release from calcium–alginate gel beads. J Control Release. 1997;47:21–8. doi: 10.1016/s0168-3659(98)00141-2. [DOI] [PubMed] [Google Scholar]
- 35.Atyabi F, Majzoob S, Iman M, Salehi M, Dorkoosh F. In vitro evaluation and modification of pectinate gel beads containing trimethyl chitosan, as a multi-particulate system for delivery of water-soluble macromolecules to colon. Carbohydr Polym. 2005;61:39–51. [Google Scholar]
- 36.Dhalleine C, Assifaoui A, Moulari B, Pellequer Y, Cayot P, Lamprecht A, et al. Zinc-pectinate beads as an in-vivo self-assembling system for pulsatile drug delivery. Int J Pharm. 2011;414:28–34. doi: 10.1016/j.ijpharm.2011.04.059. [DOI] [PubMed] [Google Scholar]


