Abstract
Introduction:
The aim of present work was to develop intestinal-targeted pellets of Budesonide, a potent glucocorticoid, used for the treatment of ulcerative colitis and Crohn's disease by extrusion and spheronization method. Current available oral formulations of Budesonide have low efficacy because of the premature drug release in the upper part of the gastrointestinal tract. In this study, a pH-controlled intestinal-targeted pellet of budesonide was established using 32 full factorial design by giving an enteric coating with Eudragit S100.
Materials and Methods:
Budesonide-sustained release pellets were prepared by extruder and spheronization technique using a combination of water-soluble and permeable polymers by applying 32 full factorial design. The pellets were coated by spray coating technique using Eudragit S100 as an enteric polymer. The pellets were characterized for its flowability, sphericity, friability, and in vitro drug release. Release behaviour was studied in different pH media. The release profile was studied for the mechanism of drug release.
Result:
The optimized formulation showed negligible drug release in the stomach followed by release for 12 h in the intestinal pH. Differential scanning calorimetry and Fourier Transform Infrared Spectroscopy studies indicated no interaction between drug and polymer. Scanning Electron Microscopy image of coated pellets suggested a uniform and smooth coat over the surface of pellets. Accelerated stability studies showed a stable nature of drug in the formulation. All evaluation parameter showed that pellets were good in spherocity and flowability.
Conclusion:
Sustained release pellets of Budesonide could be prepared by extrusion and spheronization which released the drug in intestinal pH for an intestine to treat inflammatory bowel disease. A ratio of polymer combination could be decided using a full factorial design.
Keywords: Budesonide, eudragit S100, extruder spheronizer, inflammatory bowel disease, spray coating, sustained release
INTRODUCTION
Most drugs require multiple daily dosing to achieve desired blood concentration to produce therapeutic activity. To overcome these types of problems, sustained release and controlled release delivery systems have got a considerable attention in pharmaceutical industries.[1]
Pellets are defined as spherical, free-flowing granules with a narrow size distribution, typically varying between 500 and 1500 mm for pharmaceutical applications. Their multiparticulate nature offers some important pharmacological as well as technological advantages over conventional single-unit solid dosage forms, like smaller size of pellets can rapidly empty the stomach, reduction in intra and inter-subject variability in gastric emptying times, uniform drug dispersion in gastrointestinal tract (GIT) can reduce the risk of side effect due to high drug concentration, maximise drug absorption and reduce the peak plasma fluctuations, spherical shape exhibits a good flow property with narrow size distribution.[2,3] The interest in pellets as dosage forms (filled into hard gelatine capsules or compressed into disintegrating tablets) has been increasing continuously.
Budesonide is a mixture of C-22S (epimer A) and C-22R (epimer B) epimers of 16α, 17-[(1RS)-butylidenebis (oxy)]-11β, 21-dihydroxypregna-1, 4-diene-3, 20-dione used in inflammatory bowel disease. One of the most common uses of Budesonide is in ulcerative colitis and Crohn's disease. The use of Budesonide is associated with side effects like nose irritation or burning, bleeding, stomach upset, etc. To achieve maximum therapeutic effect with a low risk of adverse effects, sustained released preparations are preferred.[4,6] Moreover the drug is unstable in gastric pH. Coating with pH-dependent polymers approach is one of the simplest and easiest approaches available for intestinal drug delivery.
The appropriate delivery of a drug for the local treatment of inflammatory bowel diseases, such as Crohn's disease and ulcerative colitis is highly challenging, because the release needs to be suppressed in the upper GIT.[7] The most critical challenge in such drug delivery approach is to preserve the formulation during its passage through the stomach.[8] In these conditions, formulation of delivery systems capable of reaching the specific site of drug activity is required.[9] It also offers a significant advantage in terms of cost and ease of manufacture.[10]
In the present study, a novel extrusion-spheronization method was employed to prepare pellets of Budesonide using water soluble and water permeable [Hydroxy propyl methyl cellulose (HPMC) and Ethyl cellulose (EC)] carriers’ material and non-toxic solvents to load the drug into pellets. Microcrystalline cellulose was incorporated in formulation via extruder-spheronization to enhance the rheological properties of the wetted mass, resulted in good sphericity, low friability, high density, and smooth surface for successful extrusion and spheronization.[11,12,13] Eudragit S100 has commonly been used as pH-dependent polymer for coating pellets for intestinal drug release.[14,15]
MATERIALS AND METHODS
Budesonide was procured as a gift sample from Avik Pharma Pvt. Ltd., Vapi, Gujarat. Eudragit S100 was procured as a gift sample from Evonik Degussa Pvt. Ltd., Mumbai, HPMC E3 LV, EC, Polyvinyl pyrollidone (PVP), Xanthan gum, Guar gum, Pectin, Sodium alginate were commercially purchased from Loba Chem laboratories, Nasik, India. All other reagents and chemicals used were of analytical grade.
Preparation of Budesonide pellets
The pellets of Budesonide were prepared by extrusion and spheronization technique.[16,17] Budesonide, HPMC, EC, and MCC were passed through sieve no. 40 prior to pelletization and mixed uniformly. A solution of PVP K30 (2% w/v) was added in sufficient quantity to the powder blend and mixed properly. The obtained dough mass was extruded using a piston extruder (1.5 mm orifice). The extrudates were immediately spheronized in 80 mm diameter friction plate with groove spaces of 3 mm for 5 min at a rotation speed of 1200 rpm. The pellets were dried overnight at room temperature.
Composition and preparation of coating solution
Eudragit S100 (6% w/v) was dissolved in quantity sufficient organic solvent (acetone) and then it was stirred until a uniform mixture was formed. Glycerol (1% w/v) was added as a plasticizer in it and stirred until a uniform mixture was formed.
Preparation of enteric-coated pellets
The enteric-coated pellets were prepared by using spray-coating technique. Coating solution was prepared by dissolving coating polymer in to the acetone and uniform dispersion of coating solution was spray on the pellets under the condition indicated in the Table 1.
Table 1.
Coating procedure specification

Study of micromeritic properties of prepared pellets
The flow property was ensured by angle of repose of drug loaded pellets using fixed base cone method.[18]
Pellets were allowed to fall freely through a funnel fixed at 1 cm above the horizontal flat surface until the apex of the conical pile just touched to the tip of the funnel. The height and diameter of the cone were measured and angle of repose was calculated using following formula. Each experiment was carried out in triplicate.

The bulk density was the quotient of weight to the volume of the sample at zero tap. Tapped density was determined as the quotient of weight of the sample to the volume after tapping a measuring cylinder for 1000 times from a height of 2 inch. The Carr's index (percentage compressibility CI) was calculated as 100 times the ratio of the difference between tapped density and bulk density to the tapped density.

Hausner's ratio (HR) was calculated using measured values of bulk density and tapped density as follows:

Friability study
The friability study was performed on the pellets to ensure their mechanical strength. Pellets of known weight were placed in a Roche Friability tester and subjected to impact at 25 rpm for 4 min. The friability was calculated using the following equation;
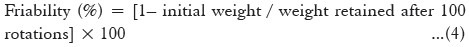
Pellet sphericity
Pellets sphericity was determined by measuring the size of pellets by vernier calliper. From that mean Feret diameter (FD), Aspect ratio (AR) (ratio of longest FD and its longest perpendicular diameter) and two-dimensional shape factor (eR) was determined.

Where, r is the radius, Pm is the perimeter, l is the length (longest FD) and b is the width (longest perpendicular diameter to the longest FD) of a pellet.
Shape factor for the prepared sample were obtained from the area (A) and perimeter (P) of a pellet.
Differential scanning calorimetry (DSC)
Samples were accurately weighed and heated in sealed aluminium pans at a rate of 10°C/min between 25 and 350°C temperature rang under nitrogen atmosphere. Empty aluminium pan was used as a reference.
Fourier transform- infrared spectroscopic analysis (FT-IR)
Spectra of pure drug and prepared sample were recorded using infrared spectrophotometer (Thermo scientific, Nicolet IS 10). The samples were dispersed in KBr and compressed into disc/pellet by application of pressure. The pellets were placed in the light path for recording the IR spectra. The scanning range was 400-4000 cm-1 and the resolution was 1 cm-1.
Scanning electron microscopy (SEM)
The surface and shape characteristics of pellets were determined by scanning electron microscopy (model-ZEISS Evo 18 Special edition). Photographs were taken and recorded at suitable magnification.
Determination of drug content
Budesonide content of the prepared pellets was determined spectrophotometrically (UV-1800 Spectrophotometer) at 247 nm. Budesonide-loaded pellets were crushed in a mortar and an amount an equivalent to 9 mg of Budesonide was dispersed in 100-ml volumetric flask containing methanol. It was further diluted with phosphate buffer (pH 7.4) and volume was made upto 100 ml. The solution was filtered and Budesonide amount was measured spectrophotometrically at 247 nm after appropriate dilution.
In vitro dissolution study
The release measurements were performed using USP dissolution apparatus I (Basket type) at 50 rpm. The test was performed using 250 ml of simulated gastric fluid (pH 1.2) at 37 ± 0.5 °C. An accurately weighed amount (equivalent to 9 mg) of the prepared pellets (filled in hard gelatine capsule) was placed in basket and immersed in to the dissolution flask at 50 rpm for first 2 hours and then dissolution medium was replaced with phosphate buffer 7.4 for 4 hrs. Finally the dissolution of pellets was done in phosphate buffer pH 6.8 for 6 hrs. Aliquotes of 5 ml was withdrawn at specific time interval (1 hr) and replaced with the same amount of fresh dissolution medium. The sample was analysed spectrophotometrically at 247 nm and cumulative percentage drug release was calculated.
Full factorial design
A 32 randomized full factorial design was adopted to optimize the variables. In this design three factors were evaluated each at three levels, and experimental trials were performed for all nine possible combinations. The amounts of water-soluble polymer, HPMC E 3LV(X1) and the amount of water-permeable polymer, EC (X2), were selected as independent variables. The amount of drug release at third hour, time required for 50% drug release (t50%) and time required for 85% drug release (t85%) was selected as dependent variables.
A statistical model incorporating interactive and polynomial terms was utilized to evaluate the response.
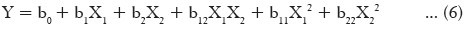
Where Y is dependent variable, b0 is the arithmetic mean response of the nine runs, b1 and b2 are the estimated coefficients for factor X1 and X2, respectively. The main effects (X1 and X2) represent the average result of changing one factor at a time from its low to high value. The interaction term (X1 X2) show how the response changes when two factors are changed simultaneously. The polynomial terms (X12 and X22) are included to investigate the nonlinearity. The statistical analysis of the factorial design batches was performed by multiple linear regression analysis using Microsoft Excel.
Kinetic analysis of dissolution data
The drug release data were analyzed for different kinetic models (zero order, first order, Higuchi's, and Korsmeyer Peppas model) to understand the release kinetics and release mechanism of pellets. This kinetic analysis of dissolution data was done by using FORTRAN software.
Stability study
Stability study of optimized batch was carried out by packing the pellets into a suitable packaging and subjected for an accelerated stability study at 40°C, 75% RH conditions as per ICH guidelines for a period of 6 months. The pellets were then analysed for different evaluation parameters (e.g., flowability, strength, shape factor, and drug release).
RESULTS
Optimization of binder
An attempt was made to prepare pellets by using HPMC, EC, and MCC in different ratio using various types of binders like PVP K30, pectin, guar gum, sodium alginate, and xanthan gum in 2% w/v. On the basis of physical appearance roundness, smoothness, and strength of the pellets, PVP K30 was selected as a binder for further pellet preparation.
Optimization of polymer
Different types of polymers like HPMC, EC, Eudragit RS, PEG 4000 were taken for the pellet preparation using PVP K30 as a binder. Different batches of pellets were prepared in combination of (1) HPMC, EC, and MCC, (2) HPMC, Eudragit RS, and MCC, and another (3) PEG 4000, EC, and MCC by taking different amount of each polymer. Among this, pellets with Eudragit RS and PEG 4000 showed larger size of pellets as well as more breaking. While in pellets prepared by HPMC, EC, and MCC, uniformity in size and good surface was observed. On the basis of appearance of pellets HPMC, EC, and MCC was selected as polymer for further pellets preparation.
For the pellets preparation by PEG 4000, hot melt extrusion method was also tried but PEG 4000 did not melt properly in that owing to which there was no proper extrudes, hence pellets could not form. While in another method PEG 4000 was melted outside and then mixed with EC and MCC. But in this method, spherical-shaped pellets did not generate. Thus, HPMC, EC, and MCC were selected as a polymer combination for further pellets preparation with Budesonide.
Optimization of polymer ratio
Pellets of Budesonide were prepared using HPMC: EC in the ratio of 1:8 by adding MCC as sphericity-enhancing agent. Drug: total excipients weight ratio of 1:2 and 1:3 was prepared in the form of pellets for optimization.
In vitro drug release in case of 1:2 and 1:3 ratios of Eudragit-coated pellets showed 35.85% and 55.70% drug release after 12 hours. Dissolution data showed that, in both the batches drug release was poor in pH 1.2 due to polymer coating, whereas release was increased in phosphate buffer 6.8. But the overall drug release was very poor. Drug: total excipients weight ratio of 1:1 was finally studied and optimized. Figure 1 shows the release profile of preliminary batches of pellets.
Figure 1.
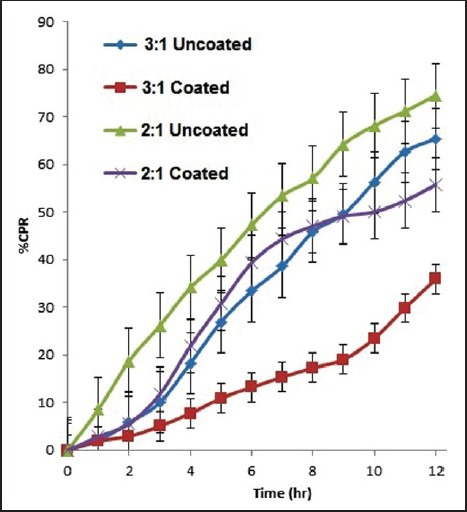
Dissolution profiles of preliminary batch
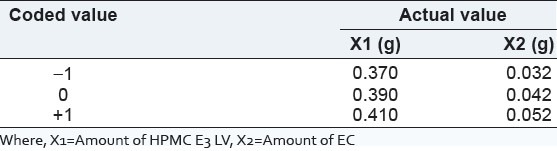
On the basis of the drug release in preliminary batch, a 1:1 ratio of drug to total amount of excipients was selected where HPMC: EC ratio was 8:1. On the basis of this ratio, levels were selected as shown in Table 2.
Table 2.
Different level of polymer
From the preliminary batches it was observed that HPMC and EC affected on the drug release whereas MCC did not show any effect on drug release profile. Therefore, HPMC and EC were selected as dependent variables.
In vitro dissolution study
Dissolution of final factorial design batches F1 to F9 in different media [Figure 2] was carried for 12 hrs. There was very less amount of drug release in first 2 hours. Later on in phosphate buffer drug release was observed.
Figure 2.
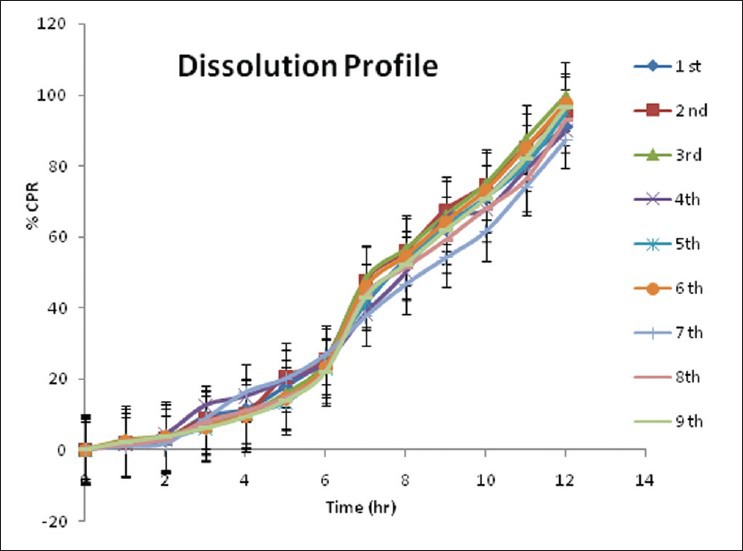
Dissolution study of F1 to F9 batches for 12 hrs drug release
Factorial design
The effects of independent variables viz., amount of water soluble polymer, (HPMC E 3 LV, X1) and the amount of water-permeable polymer, (EC, X2) were studied on dependent variables Y1 (amount of drug release at 3rd hours), Y2 (time required for 50% drug release), and Y3 (time required for 85% drug release). The response layout and release profile of 32 full-factorial design batches are shown in Table 3 and Figure 2, respectively along with the regression summery and validation of the model [Tables 4 and 5].
Table 3.
Formulation and evaluation of full factorial batches
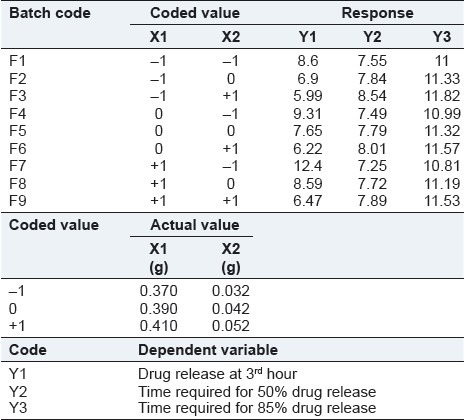
Table 4.
Summary of result of regression analysis
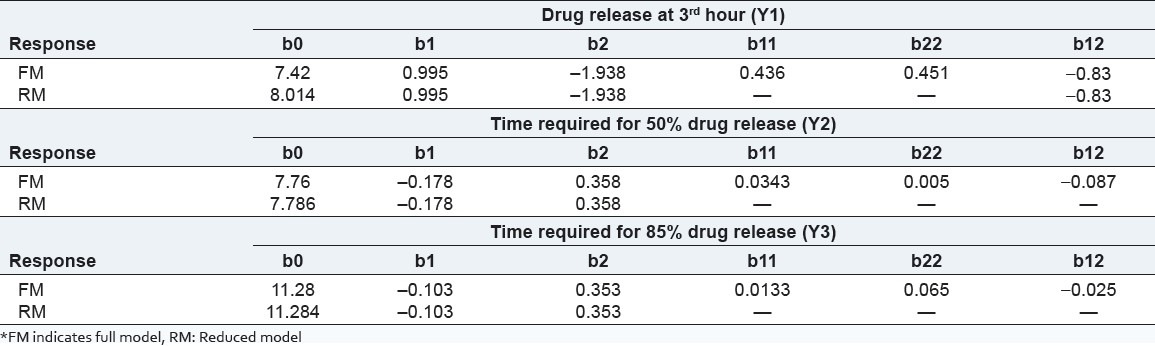
Table 5.
Calculation for testing the model
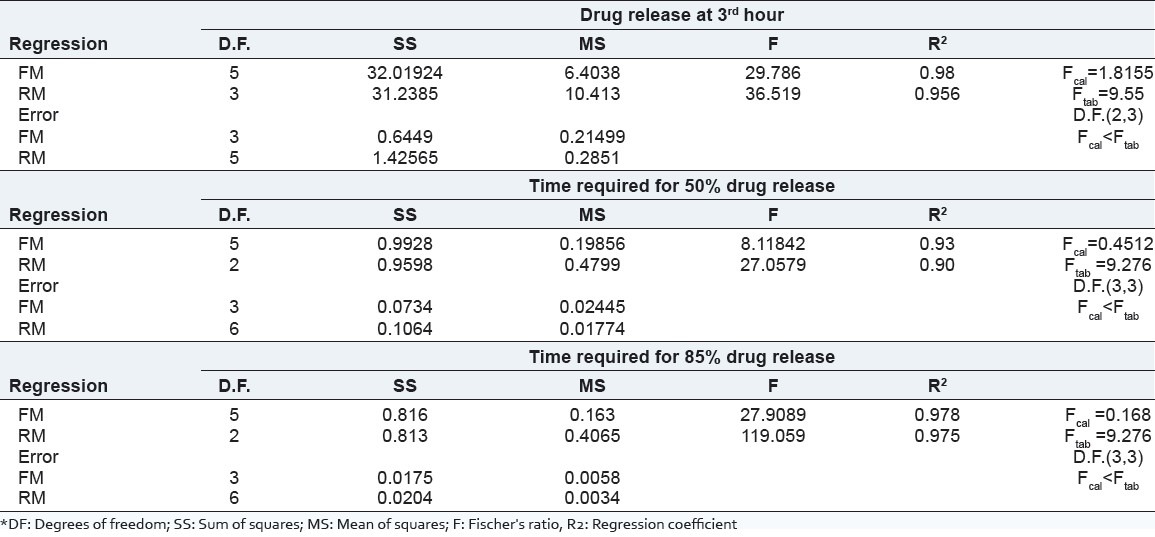
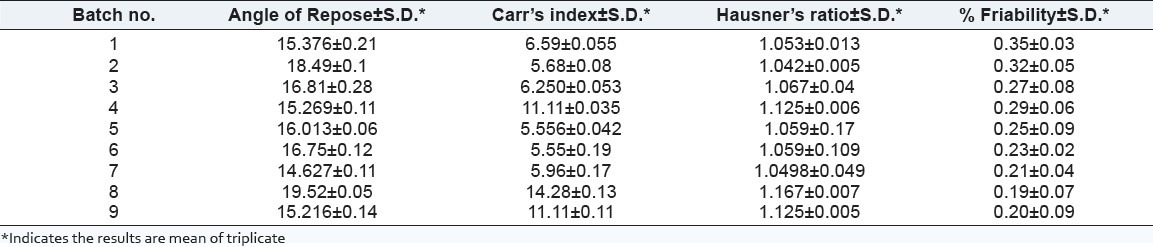
Budesonide pellets were characterized for flow parameter and friability. Results are shown in Table 6.
Table 6.
Flow parameter for Budesonide pellets
Shape parameter study
All the batches of pellets showed aspect ratio in the range of 1.029-1.131, two-dimensional shape factor in range of 0.6549-0.7785, and FDs in range of 1813-1962. The result indicated spherical shape of pellets.[16]
Differential scanning calorimeter
Thermogram of Budesonide, excipients, physical mixture, and final formulation were recorded as described above. Thermal properties of drug and pellets were studied using DSC shown in Figure 3. Melting point of drug was observed at 258.06°C (ΔHf = –103.76 mJ). Melting point peak of drug was slightly broadened in thermogram of physical mixture as well as pellets. This might be due to the change in crystallinity of drug and not because of the pharmaceutical incompatibility with EC and HPMC. Endothermic peaks of drug remained almost unchanged in physical mixture (258.73°C, ΔHf = –129.36 mJ) and pellets (259.74°C, ΔHf = –189.49 mJ).
Figure 3.
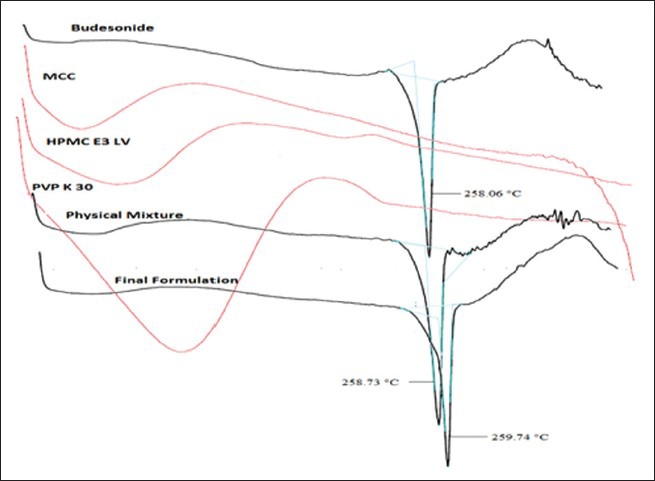
DSC of pure drug, excipients, physical mixture and final formulation
FT-IR spectra
FT-IR spectra of Budesonide, excipients, and prepared samples were recorded as described above. FT-IR spectra were shown in Figure 4. FT-IR spectrum of pure Budesonide showed characteristic peaks at 3488.71 cm−1 due to –OH group, 1602.41 cm−1 due to C=O ring stretching, 1720.98 cm−1 due to C = O ketone stretching, 1438.17 cm−1 due to C-C ring stretching, 1013.91 cm−1 due to C-O stretching. All the peaks of drug appeared in the physical mixture as well as pellets, which showed that there was no interaction between drug and excipients used.
Figure 4.
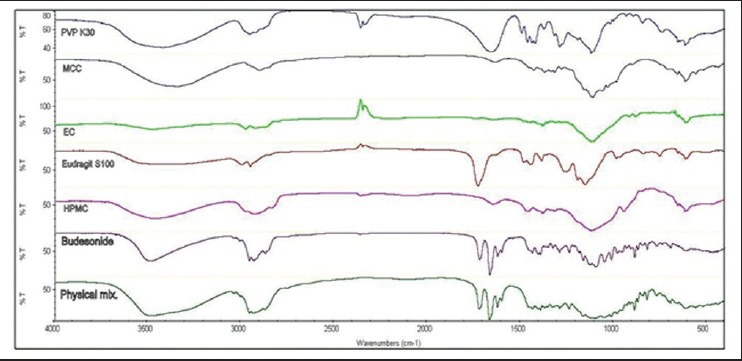
FT-IR of pure drug and physical mixture
Scanning electron microscopy
SEM photomicrographs in Figure 5 showed pellets without coating and with Eudragit S100 coating.
Figure 5.
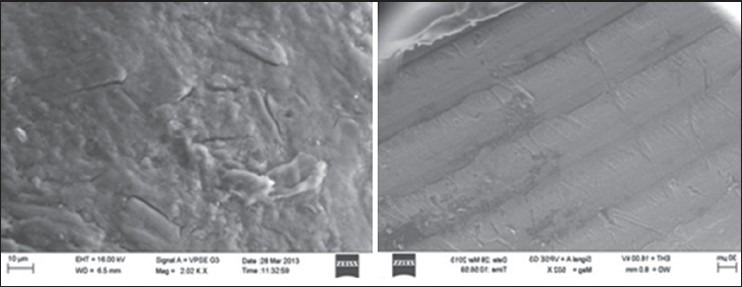
SEM photographs of pellets without coating and pellets with coating
Kinetic analysis of optimized batch
Model fitting was applied to best batch (F7) that was done using FORTRAN software. Zero order, First order, Higuchi, Korsmeyer-Peppas, Hixon crowell, and Weibull model were tested. The results are shown in Table 7. The best-fitted kinetic model for the drug release was determined by studying the parameter like sum of square root of error (SSR), R2 value, and F-value (Fischer ratio) for given models. Higher value of R2 as well as lower value of F and SSR gives the best-fitted model for drug release [Table 7].[19]
Table 7.
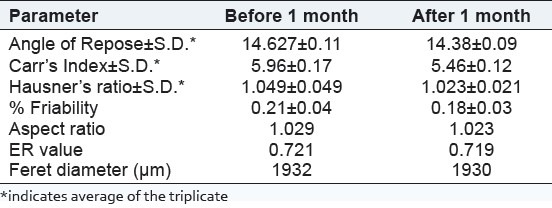
Comparison of evaluation parameters optimized batch before and after stability study
According to kinetic data, drug release was best fitted to Korsmeyer-Peppas model. From the above data, mechanism of drug release was found to be super case II transport.
Stability study of optimized batch
Stability study was carried out for the optimized batch accordingly accelerated stability study. The dissolution profile after stability study [Figure 6] was similar compared to samples soon after the preparation. The statistical analysis also proved sameness in dissolution profile.
Figure 6.
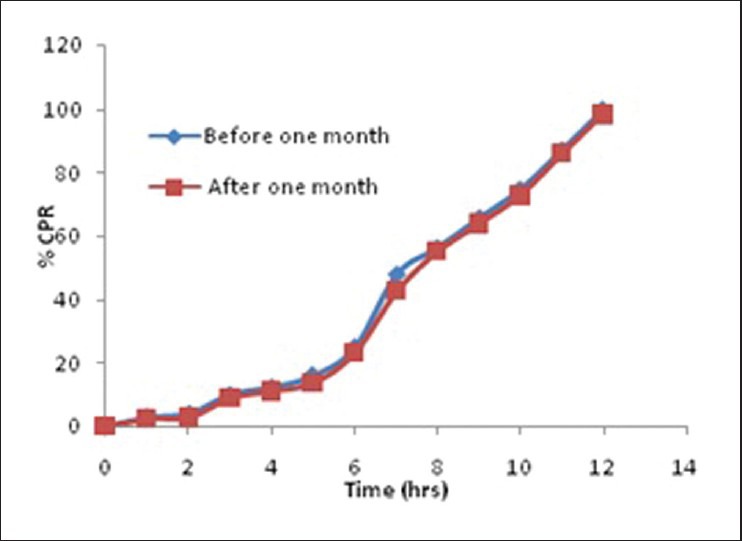
Comparison of in vitro dissolution profile before and after stability Study
DISCUSSION
The coated pellets were subjected to in vitro drug release study at different pH conditions [Figure 2]. The results have shown that enteric coating protects the pellets from acidic condition. Drug release was minimal in the simulated gastric fluid in case of formulation coated with Eudragit S100 due to its insolubility in acidic pH. In the basic pH, Eudragit coating was dissolved followed by exposing the pellets directly to the dissolution media, and hence drug release was observed in this media. More amount of drug was released from batches F7 (99.5%), F4 (97.5%) and F1 (96.68%) as compared to the other batches. Drug release data clearly indicated the satisfactory drug release from pellets with low amount of EC in its formulation. Since, HPMC is water soluble and EC is water permeable, pellets containing high amount of EC showed retardation in the drug release.
The ANOVA results (P-value) of the effects of variables on % drug release from pellets are shown in Table 5. The significant factors in the equation were selected using a stepwise forward and backward elimination for the calculation of regression analysis. The terms of full model had non-significant P-value (P > 0.05) and negligible contribution in obtaining dependent variables and thus were neglected.[20]
The equations representing are quantitative effect of the formulation variables on the % drug release are shown below:
Amount of drug released at 3rd hour,

Coefficients with one factor representing effect of that particular factor, while coefficients with more than one factor and those with second-order terms represent interaction between those factors and quadratic nature of that phenomena, respectively. Positive sign in front of the terms indicates a synergistic effect and negative sign in front of the factor indicates antagonistic effect of that factors. The above equations indicated synergistic effect of HPMC on Y1, while it showed negative effect on Y2 and Y3. In the same way, EC showed positive effect on Y2 and Y3 but negative effect on Y1.
Figure 7 indicates surface plots of amount of HPMC (X1) and amount of EC (X2) versus Y1, Y2 and Y3. The plot was drawn using Design-Expert® Software 7.1 Trial Program (Stat-Ease, Inc., Minneapolis, MN). The data demonstrates that both (X1 and X2) affected dependent variables. It may be concluded from Figure 7 that higher level of X1 and low level of X2 increased the value of Y1. At the same time, if the amount of X1 increased and/or amount of X2 decreased, value of Y2 as well as Y3 decreased. It means EC worked as a release retardant. It can be concluded that, the drug-release pattern may change by appropriate selection of the X1 and X2 levels.
Figure 7.
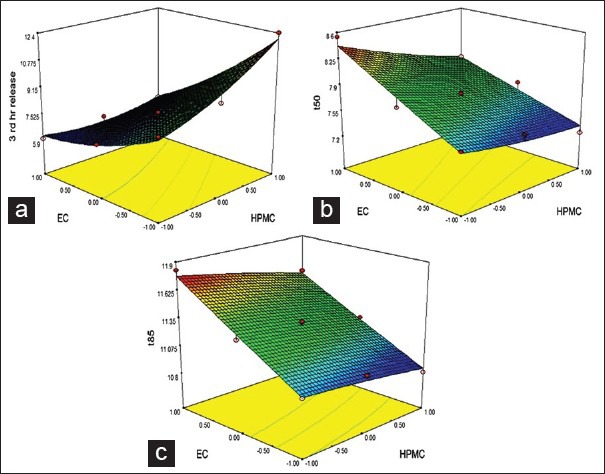
Response surface plot for (a) 3rd hr release, (b) t50 and (c) t85
As per the data shows in Table 6, the value of angle of repose for the pellets was in the range of 14.62-19.52, which indicated good flow properties of pellets. The measured range of % Carr's Index (5.55-14.28%) and HR (1.053-1.167) also indicated good flow property for the prepared pellets.[16]
The friability of the pellets was found to be in the range of 0.20-0.35%, which indicated good mechanical strength of pellets in the term of fragmenting or powdering during filling operation into capsule shell.[16]
All the batches of pellets showed aspect ratio in the range of 1.029-1.131, two-dimensional shape factor in the range of 0.6549–0.7785, and FDs in range of 1813-1962. Aspect ratio and two-dimensional shape factor value equal to unity indicate spherical shape of pellets. The result indicated that both the values were near to 1 hence, indicative of the spherical shape of pellets.[19]
From the DSC thermogram [Figure 3], there were no more changes observed in melting point peak in pure drug, physical mixture, and final formulation. So, it indicated no any type of interaction created between the pure drug and excipients.
FTIR spectra showed that the characteristic peaks were not altering in physical mixture, indicating no chemical interaction between the drug and excipients [Figure 4].
SEM image of outer surface of pellets in Figure 5 indicated that surface without coating was uneven, whereas the coated surface was smooth. Figure 5 indicated a uniform coating on the surface of the pellets.
Zero order, First order, Higuchi, Korsmeyer-Peppas, Hixon Crowell, and Weibull model were tested. The results are shown in Table 8.
Table 8.
Kinetic study of dissolution data
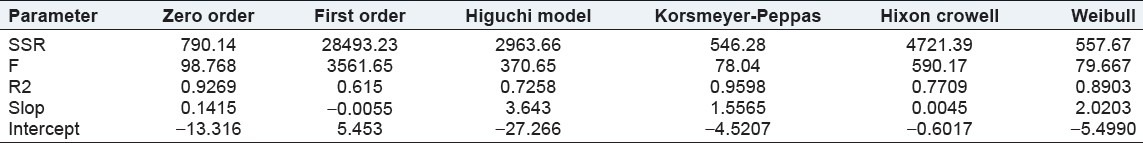
Accordingly, the kinetic data for drug release was best fitted to Korsmeyer-Peppas model. Also, the n value (release coefficient or x-variable) in Korsmeyer-Peppas model was found to be 1.5565. So mechanism of drug release was found to be super case II transport. The mechanism of release of Budesonide from the formulated batch was by anamolous non- Fickian diffusion i.e., diffusion coupled with erosion.
Stability study of pellets was done for 6 months at 40 ± 2°C/75 ± 5% RH. The dissolution profile of pellets was found to be similar and the statistical analysis of pellets after stability study also proved sameness (f2 = 86.92) with the pellets before stability study. The data of all the evaluation parameters related to the pellets were performed and are shown in Table 7. Stability study results were compared and found that there were no significant changes in the respective data of before stability study.
CONCLUSIONS
The method employed to prepare Budesonide pellets were simple, rapid and economical without any use of toxic solvents. The result of Micromeritic properties, HR and friability of the pellets were within the limits which indicated good flow potential for the prepared pellets. Drug-loaded pellets exhibited spherical shape with uniform and smooth coating as evidenced by SEM photomicrographs and spherocity studies. From the FTIR and DSC studies, it was observed that there was no chemical interaction between the drug and polymers used, indicating that drug was in stable form. Based on the result, 6% coating level formulations are suitable for the successful delivery of the drug into the lower part of intestine and colon. From the present work, it can be concluded that the prepared sustained drug delivery system can be used for many water-insoluble drugs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Vogelson CT. Modern Drug Discovery. 4th ed. 2001. Advances in drug delivery systems; pp. 49–52. [Google Scholar]
- 2.Hamdani J, Moës AJ, Amighi K. Physical and thermal characterization of Precirol® and Compritol® as lipophilic glycerides used for the preparation of controlled-release matrix pellets. Int J Pharm. 2003;260:47–57. doi: 10.1016/s0378-5173(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 3.Trivedi NR, Rajan MG, Johnson JR, Shukla AJ. Pharmaceutical approaches to preparing pelletized dosage forms using the extrusion–spheronization process. Crit Rev Ther Drug Carrier Syst. 2007;24:1–40. doi: 10.1615/critrevtherdrugcarriersyst.v24.i1.10. [DOI] [PubMed] [Google Scholar]
- 4.Bidah D, Vernaud JM. Kinetics of in vivo release of sodium salicylate dispersed in Gelucire. Int J Pharm. 1990;58:215–20. [Google Scholar]
- 5.Meltzer HY, Bobo WV, Nuamah IF, Lane R, Hough D, Kramer M, Ewrdekens M. Efficacy and tolerability of oral paliperidone extended release tablets in the treatment of acute schizophrenia: Pooled data from three 6 week, placebo-controlled studies. J Clin Psychiatry. 2008;69:817–29. doi: 10.4088/jcp.v69n0515. [DOI] [PubMed] [Google Scholar]
- 6.Karrouta Y, Neutb C, Wilsc D, Siepmanna F, Deremauxc L, Desreumauxd P, et al. Novel polymeric film coatings for colon targeting: How to adjust desired membrane properties. Int J Pharm. 2009;371:64–70. doi: 10.1016/j.ijpharm.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Kshirsagar SJ, Bhalekar MR, Umap RR. In Vitro In Vivo Comparison of Two pH sensitive eudragit polymers for colon specific drug delivery. J Pharm Sci Res. 2009;1:61–70. [Google Scholar]
- 8.Mura C, Valenti D, Floris C, Sanna R, De Luca MA, Fadda AM, et al. Metronidazole Prodrugs: Synthesis, physicochemical properties, stability, and ex vivo release studies. Eur J Med Chem. 2011;46:4142–50. doi: 10.1016/j.ejmech.2011.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Patel JM, Brahmbhatt MR, Patel VB, Muley SV, Yeole PG. Colon targeted oral delivery of ornidazole using combination of ph and time dependent drug delivery system. Int J Pharm Res. 2010;2:28–35. [Google Scholar]
- 10.Kleinebudde P. The crystallite gel model for microcrystalline cellulose in wet granulation, extrusion and spheronization. Pharm Res. 1997;14:804–9. doi: 10.1023/a:1012166809583. [DOI] [PubMed] [Google Scholar]
- 11.Ek R, Newton JM. Microcrystalline cellulose as a sponge as an alternative concept to the crystalline-gel model for extrusion and spheronization. Pharm Res. 1998;15:509–12. doi: 10.1023/a:1011905222168. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich H, Nada A, Bodmeierr N. Solid state and dissolution rate characterization of nifedipine and hydrophilic carrier. Drug Dev Ind Pharm. 2005;31:719–28. doi: 10.1080/03639040500216097. [DOI] [PubMed] [Google Scholar]
- 13.Chauhan CS, Naruka PS, Rathore RS, Badadwal V. Formulation and evaluation of prednisolone tablet for colon targeted drug delivery system. J Chem Pharm Res. 2010;2:993–8. [Google Scholar]
- 14.Patel SN, Prajapati PH, Patel CN, Patel CM, Patel TD. Formulation and development of enteric coated tablets of prednisolone as a colon targeted drug delivery. J Global Pharm Technol. 2010;2:128–32. [Google Scholar]
- 15.Das B, Srujankumar M, Ramaraju SV. Formulation and evaluation of multiunit pellets system of Venlafaxine hydrochloride. J Pharm Biomed Sci. 2012:18. [Google Scholar]
- 16.Gowda DV, Venkatesh MP, Khan MS. Development and evaluation of clozapin pellets for controlled release. Int J Res Ayur Pharm. 2012;3:611–8. [Google Scholar]
- 17.Lachman L, Liberman HA, Kanig JL. 3rd ed. Bombay: Varghese publishing House; The Theory and practice of industrial pharmacy; pp. 171–95. [Google Scholar]
- 18.Masazumi K, Hiroaki N. Development of controlled release matrix pellets by annealing with micronized water-insoluble or enteric polymers. J Control Release. 2002;82:335–43. doi: 10.1016/s0168-3659(02)00143-8. [DOI] [PubMed] [Google Scholar]
- 19.Costa P, Sousa Lobo JM. Modeling and comparison of dissolution profiles. Eur J Pharm Sci. 2000;13:123–33. doi: 10.1016/s0928-0987(01)00095-1. [DOI] [PubMed] [Google Scholar]
- 20.Dave BS, Amin AF, Patel MM. Gastro retentive drug delivery system of ranitidine hydrochloride: Formulation and in vitro evaluation. AAPS PharmSciTech. 2004;5:e34. doi: 10.1208/pt050234. [DOI] [PMC free article] [PubMed] [Google Scholar]


