Abstract
Background:
The aim of the study was to develop piroxicam-Aloe vera gel (PAG) formulation and make a pharmacodynamic evaluation of the formulation.
Materials and Methods:
The gel was prepared by using carbopol 934 as gelling agent and methyl paraben as a preservative in an Aloe vera gel base. The formulated gel was also evaluated for physicochemical parameters like pH, viscosity, drug content, and in vitro diffusion assessment. Pharmacodynamic activity of the formulation was evaluated in Wistar albino rats. The formulated gel was compared with that of similar marketed gel (commercial piroxicam gel (CPG)) against the same parameters.
Results:
From in vitro studies, an effective drug release from PAG was observed to be 68.17% when compared with that of the CPG (62.71%) at 180 min indicating better drug release from the gel formulated in this study. Percentage inhibition of edema was greater for the preparation of PAG (29.57 mean percent inhibition after 60 min) compared to marketed gel which exhibited 18.3% after 60 min.
Conclusion:
It was concluded from the results that the Aloe vera gel acts as an effective gel base to prepare piroxicam gel with high drug loading capacity and improved anti-inflammatory effect. From the statistical analysis the formulation of PAG showed better release than the CPG at p < 0.05 level of significance.
Keywords: Aloe vera, carbopol 934, edema, flux, in vitro diffusion, pharmacodynamic activity, piroxicam, % inhibition
INTRODUCTION
Piroxicam (4-hydroxy-2-methyl-2-H-1,2-benzothiazine-1-(N-(2-pyridinyl) carboxamide)-1, 1-dioxide) is a nonsteroidal anti-inflammatory drug (NSAID), used in the treatment of many inflammatory conditions including rheumatoid arthritis and osteoarthritis.[1] It is a nonselective cyclooxygenase (COX) inhibitor possessing anti-inflammatory, analgesic, and antipyretic properties. Since approximately 30% of patients receiving daily doses of 20 mg of piroxicam experience side effects such as upper abdominal pain and ulceration of the gastrointestinal mucosa, there is a restricted indication for the oral use of the drug. Transdermal delivery of NSAIDs is an effective strategy for avoiding their adverse effects on the gastrointestinal tract, while improving patient compliance.[2,3] Piroxicam is a lipophilic drug with a log P of 1.2466 having good transdermal penetration.[4] Hence, in the present investigation, piroxicam was formulated into a transdermal gel.
Aloe vera (Aloe barbadensis Miller) is a tropical and subtropical plant of the Liliaceae family. It was reported that Aloe vera has immunomodulatory, anti-inflammatory, antimicrobial, antifungal, UV protective, wound healing properties.[5,6] In the present research work the central parenchymal portion of Aloe vera was formulated into a gel using carbopol 934. Piroxicam was incorporated into the prepared Aloe vera gel and evaluated for viscosity, pH, in vitro drug release, and in vivo studies. Its anti-inflammatory effect was evaluated in rats to assess the synergistic effect of piroxicam with Aloe vera. The results were compared with that of a commercial piroxicam gel.[7]
A continuous intravenous infusion of piroxicam is a superior route to oral route of administration. But patient compliance is less as it is not suitable for self-medication. To overcome this problem, transdermal delivery of piroxicam is preferential. Delivery through transdermal is a convenient and safe route for administration of piroxicam.[8,9] The positive aspect of this delivery system is the delivery of drugs across skin to achieve systemic effects with less adverse effects by avoidance of first pass metabolism and gastrointestinal irritation. Transdermal drug delivery offers platform for the drugs with short biological half-lives as well as narrow therapeutic window and thereby improving physiological and pharmacological response. This system avoids the fluctuation in drug levels with maintenance of sustained plasma concentration of potent drugs and proves suitable for self-administration with enhanced therapeutic efficacy.[10,11]
The main objective of the present study was to develop a transdermal piroxicam-Aloe vera gel (PAG). PAG was prepared by incorporating carbopol 934 into the Aloe vera gel base. The formulated gel was compared with that of plain Aloe gel (without piroxicam) and commercial piroxicam gel (CPG).
MATERIALS AND METHODS
Piroxicam was obtained as a gift sample from Sun Pharma Pvt. Ltd., Mumbai. Carbopol 934, methyl paraben, and carrageenan were obtained from HiMedia Labs Pvt. Ltd., Mumbai. All other chemicals were of analytical grade and were used without any chemical modifications.
Methods
Preparation of medicated gel from aloe juice
The central parenchymatous pulp was scooped out with a spatula from the aloe leaves and the pulp was washed repeatedly with water and finally washed with 0.1N sodium hydroxide (NaOH) solution to elevate the pH of Aloe pulp, since it is highly acidic due to the presence of acemannan.[12,13] The treated pulp was placed in a blender to obtain the juice. The obtained juice was prefiltered using a cotton bed to remove the leftover rind particles. Then the juice was subjected to repeated vacuum filtration until a clear liquid was obtained. To the clear liquid obtained, 1% w/w carbopol 934 was added and dispersed uniformly ensuring that no lumps of carbopol were left.[14,15] 0.5% w/w methyl paraben was added to the liquid while dispersing the carbopol. Carbopol gellifies under alkaline conditions. A solution of 0.5 N NaOH solution was added dropwise until a gel was formed. The prepared Aloe vera gel was weighed and stored in air tight containers in a dark room to prevent photooxidation.[16] Medicated aloe gel with carbomer was prepared by dispersing the carbopol 934 (1%) in aloe juice to form a lump-free mixture, which is allowed to stand to free entrapped air. Piroxicam (0.5%) was also dispersed along with the carbopol 934. A small portion of 0.1N NaOH was added using moderate agitation to form the gel.
Evaluation studies
Physical appearance
The prepared gel formulation was evaluated for color, appearance, and consistence visually.[17]
Weight of the gel
The obtained juice was weighed to measure the amount of gel formed from the volume of juice. The prepared gel juice was also weighed.
Percentage practical yield
Percentage yield was calculated by knowing the practical yield and theoretical yield. And it was calculated by using the formula:
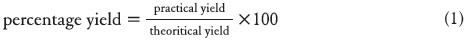
Measurement of pH
The pH of the gels was determined by a digital pH meter (Elico LI 120). One gram of the sample was placed on a watch glass and the surface pH was noted. The pH of each formulation was measured thrice and the average values used for the data.[18]
Viscosity
The flow of the prepared formulations was studied in a Brookfield Viscometer (LVDV-E Model) using spindle #63. The shear stress in % torque applied was as follows: 11.9% torque for Aloe vera gel, 6.9% torque for PAG, and 10.4% torque for CPG.[19]
Drug content
Piroxicam content in PAG and CPG was measured by dissolving known quantity of both the formulations in phosphate buffer at pH 7.4. Absorbance was measured after suitable dissolutions at 254 nm in UV-visible spectrophotometer (Shimadzu, UV-1700) and % drug content was calculated from the obtained absorbance values.[20]
In vitro diffusion studies
Diffusion studies were performed in a vertical Franz diffusion cell using egg membrane as a barrier layer. The formulation was applied on the membrane placed between donor and acceptor compartment. Phosphate buffer pH 7.4 was used as dissolution media. Constant stirring and temperature control was maintained by keeping the whole set up on a magnetic stirrer with temperature control. The temperature was maintained at 37±1°C. The samples were withdrawn at 0, 30 60, 90, 120, and 150 min. The receptor compartment was replaced with phosphate buffer pH 7.4 at each time interval to maintain sink conditions. The results were compared with the CPG.[21]
Determination of flux
The flux (J) was determined as the angular coefficient of a curve obtained by plotting the cumulative amount of the permeated drug versus time. The permeability coefficient (Kp) was calculated using the following equation:

Where, C is the initial concentration of drug in the vehicle applied to the donor phase.
Pharmacodynamic evaluation
Experimental animals
Male albino rats of Wistar strain weighing 180-230 g were used for the study. They were housed in polypropylene cages and maintained under standard laboratory conditions with a 12-12 h light-dark cycle as well as free access to standard rat pellet diet (Lipton, India Ltd.) and drinking water. They were acclimatized to laboratory conditions for 10 days before starting the experiment. The experimental protocol was approved by the institutional animal ethical committee (Ref. no: 930/a/06/CPCSEA).
Left hind paw method
Anti-inflammatory activity was evaluated by carrageenan induced hind paw edema in animals. Group I, control group received carrageenan (1%). Group II received Aloe vera gel and carrageenan. Group III served as standard group and received CPG (Pyrex® Gel) and followed by carrageenan. Group IV received PAG followed by carrageenan. Edema was induced on the left hind paw of the rats by injecting 0.1 ml of 1% (w/v) of carrageenan. Aloe vera gel, PAG, and CPG were applied 30 min before carrageenan administration. In order to measure paw volume, animals were marked with a permanent marker at left hind paw ankle. The paw volume was measured at intervals of 0 min, 1 h, 2 h, 3 h, and 4 h by mercury displacement method using plethysmometer.[22] The percent inhibition of paw edema in drug treated group was compared with carrageenan control group and calculated using the formula:

Where Vc is the inflammatory increase in paw volume in control group and Vt is the inflammatory increase in paw volume in standard or test group.
Statistical analysis
The data was expressed as mean±standard deviation (SD). Statistical analysis was performed by one-way analysis of variance (ANOVA) test for multiple comparisons formed by Tukey-Krammer test. Statistical significance was set at p < 0.05.
RESULTS AND DISCUSSION
Physical appearance
The prepared gel was a yellowish white viscous creamy preparation with a smooth homogenous texture and glossy appearance.
Weight of the gel
The collection of Aloe vera leaflets was carried out and leaflets were thoroughly washed. The mucilage isolated showed a weight of 174.8 g out of which 104.7 ml of juice was obtained after serial filtrations.
Percent practical yield
Transgel was prepared by dispersing 1% of carbopol, 0.5% of piroxicam, and 0.5% of methyl paraben in weighed quantity (50 g) of aloe juice. The prepared gel was weighed. Practical yield was obtained as 48.63 g and theoretical yield was 50.5 g, thereby showing percentage yield as 96.3%.
Measurement of pH
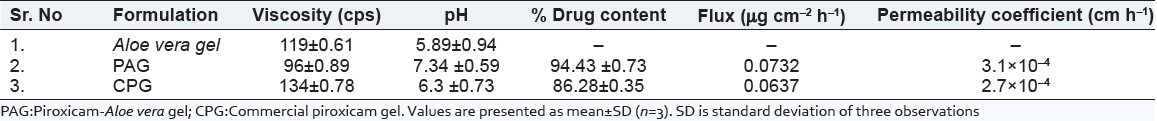
Surface pH of the gel was found to be 5.89 and 6.3 for Aloe gel and CPG, respectively. As Aloe vera is highly acidic, it may induce irritation on application to the skin. Hence, it was treated with alkali. As piroxicam is basic moiety, it also caused the elevation in pH of final gel formulation and it was measured as 7.34 [Table 1].
Table 1.
Physicochemical parameters of gel formulations
Viscosity studies
Viscosity of the Aloe vera gel, PAG, and CPG was observed as 119, 96, and 134 cps, respectively. Viscosity of PAG indicated that the sol-gel behavior was due to incorporation of piroxicam as base moiety in the gel formulation. The viscosity was higher for the Aloe vera gel [Table 1].
Drug content
Drug content determines the loading capacity of Aloe gel formulation. From the results it was observed that the percentage drug content was highest for the PAG compared to CPG implying more loading capacity of Aloe gel [Table 1].
Diffusion studies
Study was conducted for 3 h and the samples were analyzed at 252 nm. The results are shown in the Figure 1. These implies that the amount of drug permeating through the membrane was more than 68% for PAG and nearly 63% for CPG implying that the drug release was effective from the test formulation compared to CPG due to presence of Aloe which provides an aqueous base for hydrating tissues of the skin.
Figure 1.
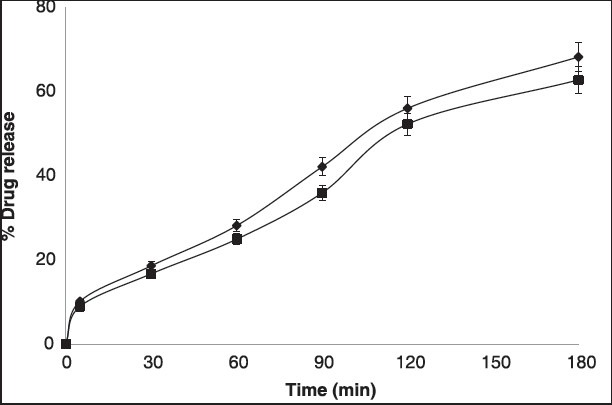
% Drug release of piroxicam from both prepared and commercial transgels. Values are presented as mean (n = 6)±standard deviation (SD)
Determination of flux
The flux (J) and permeability coefficients (Kp) of the formulated gel and marketed gel are shown in Table 1 and Figure 2. A plot of the cumulative amounts of piroxicam versus time was plotted to calculate flux [Figure 2]. The flux and permeability coefficient values of PAG were found be higher than that of CPG, which may due to the effect of acemannan present in Aloe vera and has been reported as an effective penetration enhancer.
Figure 2.
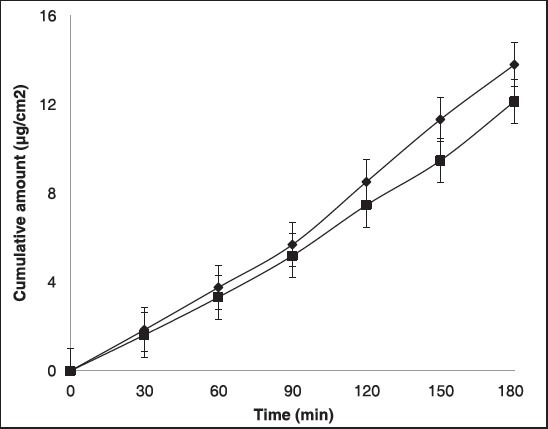
Cumulative amount of drug versus time plot, each value represents mean (n = 6)±SD
Pharmacodynamic evaluation
Piroxicam, an NSAID which is a nonselective COX inhibitor is used in the treatment of osteoarthritis and rheumatoid arthritis. It is an oxicam derivative in which 4-hydroxy-1,2-benzothiazine-1,1 dioxide moiety is responsible for its COX inhibition. Inhibition of COX alone does not account for its anti-inflammatory action. It also acts on oxidative stress which is dependent on the reduction of nitric oxide (NO) levels in osteoarthritis. The anti-inflammatory activity of all formulated gels were assessed by carrageenan induced left hind paw method. The results of in vivo anti-inflammatory activity of PAG on carrageenan induced paw edema in rats were measured as mean paw edema volumes in Table 2. Anti-inflammatory effect of PAG was evaluated after application of gel and subplantar injection of carrageenan in rats. The presence of piroxicam in deeper skin was highest in PAG compared to CPG. In this study, the percent inhibition was observed as highest for the PAG with p < 0.001 at all time intervals compared to CPG as shown in Figure 3. The percentage inhibition of edema was assessed to measure the therapeutic efficiency of PAG. The inhibition of edema was observed to be less for plain aloe gel compared to commercial piroxicam gel (considered as reference) and PAG. Test formulation had shown better inhibition of edema due to enhanced permeability of the piroxicam. Better inhibition shown by PAG is due the synergistic effect of Aloe vera on piroxicam, which has mild anti-inflammatory activity. These results suggest the potentials of Aloe vera as an aqueous gel base for transdermal application of piroxicam, a hydrophobic moiety.
Table 2.
Effect of transgel formulations on carrageenan-induced paw edema in rats
Figure 3.
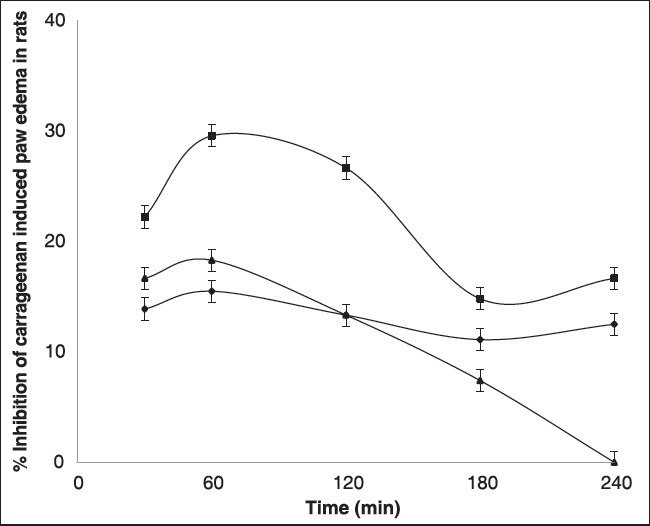
Percent inhibition plot of carrageenan induced rat paw edema in all the groups. Values are expressed as mean (n = 6)±SD
CONCLUSION
In the coming years, topical drug delivery would be used extensively to ensure better patient compliance. Since gels are helpful in enhancing spreadability, adhesion, viscosity, and extrusion; this novel drug delivery has become popular. Moreover, these gels are suitable for loading hydrophobic drugs in water soluble gel bases for better drug diffusion profiles. The PAG showed promising results using carbopol as gelling agent. PAG was formulated in Aloe vera gel base and subjected to physicochemical studies, that is, rheological studies, pH measurement, in vitro release studies, and in vivo release studies through rat skin. In vivo release of the test formulations was performed to determine drug release rate from the gel. From the in vitro studies, PAG showed significantly better release than the CPG. In vivo study was also performed by carrageenan induced paw edema model to reveal the anti-inflammatory potentials of PAG. Aloe vera has a synergistic anti-inflammatory effect in the preparation. It is concluded that Aloe vera gel is an effective gel base to prepare PAG with an enhanced anti-inflammatory profile compared to transdermal gels.
ACKNOWLEDGMENT
The authors are thankful to the management of Sree Vidyanikethan College of Pharmacy, A. Rangampet, Tirupati, Andhra Pradesh, India for providing the necessary facilities to carry out the research work.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Chaudhary H, Rohilla A, Rathee P, Kumar V. Optimization and formulation design of carbopol loaded Piroxicam gel using novel penetration enhancers. Int J Bio Macromol. 2013;55:246–53. doi: 10.1016/j.ijbiomac.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Goosen C, du Plessis J, Muller DG, Janse van Rensburg LF. Correlation between physicochemical characteristics, pharmacokinetic properties and transdermal absorption of NSAID's. Int J Pharm. 1998;163:203–9. doi: 10.1016/s0378-5173(99)00340-3. [DOI] [PubMed] [Google Scholar]
- 3.Boudreau MD, Beland FA. An evaluation of the biological and toxicological properties of Aloe Barbadensis (Miller), Aloe vera. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2006;24:103–54. doi: 10.1080/10590500600614303. [DOI] [PubMed] [Google Scholar]
- 4.Penzes T, Blazso G, Aihner Z, Falkay G, Eros I. Topical absorption of piroxicam from organogels - in vitro and in vivo correlations. Int J Pharm. 2005;298:47–54. doi: 10.1016/j.ijpharm.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 5.Kumar R, Philip A. Modified transdermal technologies: Breaking the barriers of drug permeation via the skin. Trop J Pharm Res. 2007;6:633–44. [Google Scholar]
- 6.Kumar P, Sankar C, Mishra B. Delivery of macromolecules through skin. Ind Pharmacist. 2004;5:7–17. [Google Scholar]
- 7.Allen LV, Popovich NG, Ansel HC. 8th ed. New Delhi: Wolter Kluwer Publishers; 2005. Ansel's Pharmaceutical dosage forms and drug delivery systems; pp. 298–299. [Google Scholar]
- 8.Mutimer MN, Riffikin C, Hill JA, Glickman ME, Cyr GN. Modern ointment base technology. II. Comparative evaluation of bases. J Am Pharm Assoc Am Pharm Assoc (Baltim) 1956;45:212–8. doi: 10.1002/jps.3030450406. [DOI] [PubMed] [Google Scholar]
- 9.Grindlay D, Reynolds T. The Aloe vera phenomenon: A review of the properties and modern uses of the leaf parenchyma gel. J Ethnopharmacol. 1986;16:117–51. doi: 10.1016/0378-8741(86)90085-1. [DOI] [PubMed] [Google Scholar]
- 10.Jani GK, Shah DP, Jain VC, Patel MJ, Vithalan DA. Evaluating mucilage from Aloe Barbadensis Miller as a pharmaceutical excipient for sustained-release matrix tablets. Pharm Technol. 2007;31:90–8. [Google Scholar]
- 11.Beetge E, du Plessis J, Muller DG, Goose C, van Rensburg FJ. The influence of the physiochemical characteristcs and pharmacokinetics properties of selected NSAID's on their transdermal absorption. Int J Pharm. 2000;193:261–4. doi: 10.1016/s0378-5173(99)00340-3. [DOI] [PubMed] [Google Scholar]
- 12.Zapataa PJ, Navarroa D, Guillena F, Castillo S, Martínez Romero D, Valero D, et al. Characterisation of gels from different Aloe spp. as antifungal treatment: Potential crops for industrial applications. Ind Crop Prod. 2012;42:223–30. [Google Scholar]
- 13.Jia Y, Zhao G, Jia J. Preliminary evaluation: The effects of Aloe ferox Miller and Aloe arborescens Miller on wound healing. J Ethnopharmacol. 2008;120:181–9. doi: 10.1016/j.jep.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 14.Kasliwal N, Derle D, Negi J, Gohil J. Effect of permeation enhancers on the release and permeationkinetics of meloxicam gel formulations through rat skin. Asian J Pharm Sci. 2008;3:193–9. [Google Scholar]
- 15.Doliwa A, Santoyo S, Campanero MA, Ygartua P. Sensitive LC determination of piroxicam after in vitro transdermal permeation studies. J Pharm Biomed Anal. 2001;26:531–7. doi: 10.1016/s0731-7085(01)00455-1. [DOI] [PubMed] [Google Scholar]
- 16.Gay CL, Green PG, Richard H, Francoeur ML. Iontophoretic delivery of piroxicam across the skin in vitro. J Contrl Rel. 1992;22:57–67. [Google Scholar]
- 17.Shokri J, Azarmi Sh, Fasihi Z, Hallaj-Nezhadi S, Nokhodchi A, Javadzadeh Y. Effects of various penetration enhancers on percutaneous absorption of piroxicam from emulgels. Res Pharm Sci. 2012;7:225–34. [PMC free article] [PubMed] [Google Scholar]
- 18.Santoyo S, Arellano A, Ygartua P, Martin C. Penetration enhancer effects on the in vitro percutaneous absorption of piroxicam through rat skin. Int J Pharm. 1995;117:219–24. [Google Scholar]
- 19.Shin S, Cho C, Oh I. Enhanced efficacy by percutaneous absorption of piroxicam from the poloxamer gel in rats. Int J Pharm. 2000;193:213–8. doi: 10.1016/s0378-5173(99)00339-7. [DOI] [PubMed] [Google Scholar]
- 20.Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci. 2001;14:101–14. doi: 10.1016/s0928-0987(01)00167-1. [DOI] [PubMed] [Google Scholar]
- 21.Kakkar AP, Gupta A. Gelatin based transdermal therapeutic system. Indian Drugs. 1992;29:308–12. [Google Scholar]
- 22.Crunkhorn P, Mencock SC. Mediators of inflammation induced in rat paw by carregeenan. Br J Pharmacol. 1971;42:392–402. doi: 10.1111/j.1476-5381.1971.tb07124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


