Abstract
The ubiquitin-proteasome system (UPS) is the mainstay of protein quality control which regulates cell cycle, differentiation and various signal transduction pathways in eukaryotic cells. The timely and selective degradation of surplus and/or aberrant proteins by the UPS is essential for normal cellular physiology. Any disturbance, delay or exaggeration in the process of selection, sequestration, labeling for degradation and degradation of target proteins by the UPS will compromise cellular and tissue homeostasis. High blood glucose or hyperglycemia caused by diabetes disrupts normal vascular function in several target organs including the retina and kidney resulting in the development of diabetic retinopathy (DR) and diabetic nephropathy (DN). We and others have shown that hyperglycemia and oxidative stress modulate UPS activity in the retina and kidney. The majority of studies have focused on the kidney and provided insights into the contribution of dysregulated UPS to microvascular damage in DN. The eye is a unique organ in which a semi-fluid medium, the vitreous humor, separates the neural retina and its anastomosed blood vessels from the semi-solid lens tissue. The complexity of the cellular and molecular components of the eye may require a normal functioning and well tuned UPS for healthy vision. Altered UPS activity may contribute to the development of retinal microvascular complications of diabetes. A better understanding of the molecular nature of the ocular UPS function under normal and diabetic conditions is essential for development of novel strategies targeting its activity. This review will discuss the association of retinal vascular cell UPS activity with microvascular damage in DR with emphasis on alterations of the PA28 subunits of the UPS.
Keywords: Diabetic Retinopathy, Diabetic Nephropathy, Age-related Macular Degeneration, PA28 Proteasome Regulatory Subunits, Ubiquitin Proteasome System
INTRODUCTION
The ubiquitin-proteasome system (UPS) is the main pathway for regulation of protein turnover and biological function in the eukaryotic cells. It governs a wide variety of regulatory pathways, from cell-cycle control and transcription to development. The UPS comprises various types of components encoded by several hundred genes. These cover proteins involved in monitoring protein structure, detection, labeling and degradation of target proteins, and proteins that regulate the catalytic activity of the proteasome. In general, during conventional ubiquitination, the 8.5 kDa ubiquitin protein is covalently conjugated to any target protein that ultimately determines its stability by UPS. Besides regulating the target protein turnover or clearance by the proteasomes, ubiquitination of some proteins regulates non- proteolytic processes such as endocytosis, protein localization/targeting, complex assembly and regulation of the duration and intensity of signaling by effector molecules.1-2
The grinder UPS machinery comprises a barrel-shaped 20S proteolytic core particle (CP) with 28 subunits complemented with either the 19S regulatory complex comprising 19 individual proteins and/or the 11S regulatory heptamers (Fig. 1). The proteolytic sites of the core particle are housed and concealed within the proteasome barrel, which guarantees inertness of the proteolytic activity. Thus, in the absence of the regulatory subunits, the 20S complex is considered as an inherently repressed enzyme. Accessibility and delivery of target proteins for degradation within the 20S barrel relies on the function of proteasome regulators. Selection of target proteins for ubiquitination and degradation is accomplished by the specificity of the ubiquitin ligases (E2 and E3 enzymes). This selection is balanced by interplays among different chaperons, ubiquitin ligases, ubiquitin ligase adapter proteins and target substrates.
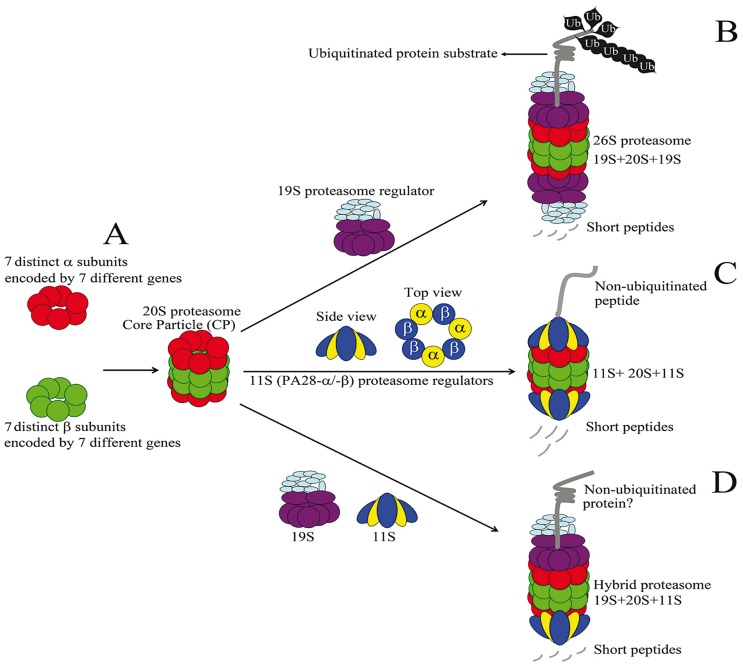
Figure 1.
Organization and assembly of mammalian proteasomes. A) The 20S proteasome or core protein (CP) is formed by the illustrated stacking assembly of a and β subunits making a 28-subunit catalytic barrel. B) Binding of 19S proteasome regulators to both ends of the CP generates the 26S proteasome. 26S proteasome is specialized in ATPdependent degradation of ubiquitinated proteins. C) Binding of heptameric 11S proteasome regulators (PA28, 3a/4β) or PA28-g (not shown) to both ends of the CP results in proteasome formations specialized in non-ATP dependent degradation of non-ubiquitinated short peptides. D) Simultaneous binding of 11S and 19S proteasome regulators to CP results in hybrid proteasome formation. Hybrid proteasomes are poorly studied but are suggested to be involved in ATP-dependent degradation of non-ubiquitinated proteins.68
For protein degradation, ubiquitinated substrates will primarily dock onto the proteasome at its 19S regulatory particle mediated by various ubiquitin receptors. Ubiquitinated substrates will be subsequently transferred into the internal pore of the CP, where they are hydrolyzed. Substrate translocation into the CP requires its ATP-dependent tethering into the CP channel allowing the unfolding of the substrate. The 19S regulatory subunit is comprised of six distinct ATPases assembled in a ring complex, which drives unfolding as well as translocation of the substrate. Entry of the target proteins into the CP requires removal of the ubiquitin tags, which is mediated by activity of the deubiquitinating enzymes (DUBs). The proteolytic function of the CP is endowed by β-type subunits (β1, β2, and β5) of the inner surface of the chamber formed by two abutting β rings. These subunits are members of the N-terminal nucleophile (Ntn) hydrolase family and essentially possess a single residue active site that is located on the N-terminal threonine of each proteolytic subunit. Each active site has specificity for cleavage of a broad range of target peptide sequences. Thus, based on their preference to cleave within specific residues, the β1, β2 and β5 subunits are classified to account for caspase-like, trypsin-like and chymotrypsin- like catalytic activities.3-4
RETINAL UPS AND ITS REGULATION BY OXIDATIVE STRESS
The mammalian visual organ represents a very complex mixture of cells organized in the form of soft tissue (retina), semi-solid tissue (lens), and hard tissue (cornea). Each cellular component of the eye has distinct profiles of gene expression that are specifically linked to their physiology. The regulation of protein quality and proper turnover in these cells is critical for maintenance of healthy visual function, and is mainly mediated by the UPS. Known roles of UPS in the eye include regulation of development and differentiation, signal transduction, physiology, waste protein disposal and response to stress. The normal aging process and several stress related pathologies with subsequent deposition of the cytotoxic obsolete protein aggregates in ocular cells are associated with deficient UPS function in the eye. Aging and oxidative stress result in reduced proteasome peptidase activity in the retina, retinal pigment epithelium (RPE) cells and lens tissue, and are believed to account for the pathogenesis of age-related macular degeneration (AMD) and cataracts. The most widely studied role for UPS is regulating the differentiation of lens fiber cells and maintenance of lens clearance by controlling the quality and amount of the major lens proteins, the crystallins.5
In the retina, due to functional adaptation to light/dark visual cycles, regulation of protein turnover via ubiquitination follows a circadian pattern. Ubiquitin immunoreactivity in human and rodent eyes is detectable throughout all retinal cells with prominent intensity in retinal ganglion and RPE cells. Immunohistological detection of ubiquitin in dark-adapted rats revealed faint staining in the retina. In contrast, the light-exposed retina showed very strong immunoreactivity in cells occupying a region between the outer plexiform layer (OPL) and ganglion cell (GC) layer. The highest immunoreactivity was in undetermined cells within the OPL that resembled blood vessels.6-7 The underlying reason for enhanced ubiquitin immunoreactivity in light exposed retina remains unknown. Enhanced oxidative stress, however, could provide a reasonable explanation.
The retina has the highest level of metabolic activity among different tissues, and contains high concentrations of easily oxidized polyunsaturated fatty acids (PUFA) in an oxygen- rich environment.8 This renders the retina highly prone to damage induced by oxidative stress. Oxidative stress is caused by an increase in the levels of reactive oxygen intermediates (ROI) generated as byproducts of mitochondrial respiration and/or spontaneous oxygenation of PUFA.9-10 Thus, an imbalance between the production of ROI and their elimination by the host’s defense antioxidant system results in oxidative stress. Indeed in photoreceptors, which are transducers of visible light signals, blue light promotes the generation of reactive oxygen radicals arising from mitochondrial electron transport.11 Elevated oxidative stress in the retina is believed to contribute to the pathogenesis of several eye diseases including AMD and DR.12-13
The initial steps in degradation of the cargo phototransduction proteins in rod-type photoreceptor outer segments require UPS activity.14 Shedding of photoreceptor outer segments is essential for normal physiology of photoreceptors and occurs in a circadian fashion. The take-up of retinal outer segments rich in oxidized long chain PUFA by RPE cells is postulated to promote oxidative stress and cell death resulting in the development of AMD.15 Despite the normally high oxidizing potential of retinal tissue, there is limited knowledge on the role of the UPS under normal and pathological conditions of the retina.
A LINK BETWEEN NEURODEGENERATIVE DISEASES AND RETINAL UPS
Several neurodegenerative diseases including Parkinson, Alzheimer’s and Lewy body (LB) are linked to impairment of UPS activity. Interestingly some retinal lesions homologous to their CNS phenotypes also exist in patients with neurodegenerative conditions. However, the role of UPS in mediating the pathogenesis of these diseases requires further investigation.
β-amyloid (Aβ) plaque formation and intraneuronal accumulation of ubiquitin conjugates are the neuropathological hallmarks of Alzheimer’s disease (AD) and the Aβ peptide itself is known to be the 20S proteasome substrate.16-17 It is believed that in AD, proteasomal activity is inhibited by insoluble Aβ aggregates.18 Analysis of AD transgenic mouse model APP(SWE)/PS1(∆E9), which produces a mouse/human amyloid precursor protein, and a mutant human presenilin-1, revealed the deposition of Aβ plaques in the retina earlier than the brain.19 These findings demonstrate functional similarity between UPS activity in the CNS and retina, and also present the retina as a suitable model for studying the UPS in neurodegenerative conditions.
The formation of ubiquitin and SNCA/α- synuclein-containing fibrillar aggregates, the LB, is the characteristic feature of Parkinson disease (PD). LB are heterogeneous aggregates composed of more than 90 proteins, including gene products implicated in PD. In PD, neuronal loss is found in predilection sites for LB, thus, LB formation is considered as a suitable marker for neurodegeneration. In addition to ubiquitin and α-synuclein as a proteasome substrate, parkin protein is also found in LB. Misfolded forms of the α-synuclein, aberrant parkin protein (caused by mutations in the PARK2 gene), and oxidative stress have causative roles in the pathogenesis of PD.20-21 Parkin is a RING E3 ubiquitin ligase and mutations in the PARK2 gene are reported in approximately 50% of patients with autosomal recessive PD, and in 10-15% of sporadic early onset cases of PD.22 PD patients with LB manifest various degrees of visual hallucinations.23 The relation between visual impairment and PD- related genetic mutations are not well-known. However, altered immune localization of both a-synuclein and β-synuclein, as well as the presence of pale inclusions with unknown content and no similarity to LB, was reported in the retina from PD patients.24 Parkin is broadly expressed in the mammalian retina but its functional role, as well as other LB-associated proteins such as a-synuclein in the normal and PD retina, and their association with UPS activity awaits further investigation.25
UPS IN RENAL DISORDERS AND DIBETIC NEPHROPATHY
Proteasome dysfunction has also been implicated in the pathogenesis of several renal diseases. A myriad of genetic disorders with renal involvement including Liddle’s syndrome and von Hippel-Lindau (VHL) disease are tied to UPS dysfunction.26 Other renal pathologies with or without an inherited pattern that are directly or indirectly regulated by UPS include ischemic acute renal failure, tubulointerstitial fibrosis, glomerulopathy in kidney transplants, class A immunoglobulin (IgA) nephropathy, lupus nephritis and DN.27-33 The last four conditions are prominently linked to immune cell response and affect the renal blood filtration units, the glomerulus. Due to the ameliorative effects of systemic proteasome inhibition in these renal pathologies, the ability to modulate UPS activity holds promise in battling the above nephropathies. The lesion sites for the above- mentioned glomerulopathies mainly lie within the intraglomerular capillaries, especially the mesangial components of the glomeruli. Proteasome inhibitors have been demonstrated to improve mesangial appearance and function. We also have found that members of UPS, cullin-1, cullin-3 and the 11S proteasome regulators PA28-β and PA28-g are specifically up-regulated in intraglomerular capillaries of mice with established DN.27
The triggers promoting exclusive proteasome activation in glomerular capillaries and mechanisms by which this overactivation regulates downstream signaling events is yet unclear. The widely studied downstream pathway regulated by the UPS system and linked with the pathogenesis of DN is the Nuclear Factor-Kappa B (NF-kB) family of transcription factors. The NF-kB signaling pathway responds to a broad set of stress signals via activating the downstream NF-kB family of transcription factors. The canonical NF-kB pathway is activated by interaction of inflammatory mediators including TNF-a, IL-1β, and LPS (lipopolysaccharide) with their respective receptors allowing the phosphorylation of the inhibitor of -kB (IkB) kinase (IkK) complexes. Phosphorylation of IkBs by IKKB promotes its ubiquitination followed by proteasome-mediated degradation. The liberated NF-kB dimer then translocates into the nucleus and promotes transcription of target genes.34-35 Reportedly the specific inhibition of the proteasome with MG- 132 in experimental diabetes prevented NF-kB activation and DN. MG132 treatment of diabetic mice was associated with lower amounts of inflammation, proteinuria, basement membrane thickening and glomerular mesangial expansion, which are typical features of DN. 19, 36 These suggest that inhibition of UPS is sufficient to alleviate adverse effects of NF-kB activation on the development of DN.
PROTEASOME ACTIVATOR PA28 COMPOSITION AND FUNCTION
The 11S regulatory subunits of the mammalian proteasome, also called REG, are ring-shaped conical heptameric complexes which associate with the CP of the proteasome. The 11S subunit proteins are 28-kDa PA28-a, -β, and-g (REG a, β, and g). The PA28-a and PA28-β genes are reversely oriented on the mouse chromosome 14 with a gap of 6 kbp suggesting that they are derived from gene duplication of a common ancestral gene. The PA28-a and PA28-β genes are induced by interferon gamma (IFN-g) and their protein products share approximately 50% amino acid identity, while PA28-g is not induced by IFN-g.37-38 Although 11S regulatory subunits stimulate proteasome peptidase activity on short peptides, they do not stimulate degradation of large ubiquitinated or non-ubiquitinated protein substrates.
The PA28-a and PA28-β (PA28-a/-β) are the most extensively studied UPS REG proteins. The deletion of both PA28-a/-β genes in mice results in impaired processing and generation of certain antigenic epitopes by the proteasome.39 PA28-g, also called Ki antigen, shares 35% homology with the PA28-a/-β proteins. PA28-g was discovered as a major autoantigen in patients with lupus erythematosus.40 The precise role of PA28-g in regulating the proteasome activity is not well understood, with PA28-g null mice showing some defects in processing of certain antigens.41 Fibroblasts isolated from PA28-g null mice, however, showed impaired cell cycle progression and spontaneous apoptosis during logarithmic growth.42 The proteasome substrates for PA28-g-mediated degradation, which regulate cell cycle progression and apoptosis, remain unknown.
The heptameric form of PA28-a/-β composed of 3 a and 4 β subunits (PA28-3a/4β), are proposed to be the common form of the 11S REG in eukaryotic cells. Expression of the recombinant PA28-a in Escherichia coli results in heptameric complex formation, whereas expression of the recombinant PA28-β yields only monomers. However, co-expression of PA28-a with PA28-β results in formation of heptamers composed of 3 a and 4 β subunits with an approximate molecular weight of 194 kDa. There are functional differences in stimulating the peptidase activity of the 20S proteasome by PA28 proteins. The expression of PA28-a alone could moderately stimulate peptide-hydrolyzing activity. In contrast, isolated PA28-β subunits do neither associate with 20S proteasome nor stimulate peptide hydrolysis under identical conditions.43
The concurrent expression of both a and β isoforms, however, is concomitant with a marked increase in peptidase activity when compared to the homo-oligomers. Thus, the formation of the PA28-a/-β heptameric complex is required for optimal peptidase activity of the 20S proteasome.43 Biochemical studies indicated that PA28 does not directly activate the proteasome peptidase activity. Recent structural and biochemical analyses have revealed that PA28 accomplishes its task by facilitating substrate entry into the CP. This occurs through its binding to the outer α-rings of the 20S proteasome and inducing a change in the orientation of the N-terminal tail of CP α-subunits.44-46 It has been suggested that the 11S-regulated production of the peptide precursors (8-10 residues long) for antigen presentation is mediated through maintaining an open pore conformation of CP allowing facilitated entry of the substrate and peptide product exit from the proteasome. This results in reduced proteasome processivity allowing the release of longer peptides suitable for antigen presentation.47 As indicated, PA28-a/-β null mice are viable with no obvious anatomical and immunological defects, but lack the capacity to process specific substrates, the identity of which remains elusive. 48
The PA28 proteins are in high abundance in immune tissues. They are involved in the production of a certain subset of antigenic peptides for presentation by class I molecules of the major histocompatibility complex (MHC). Thus, the role of the 11S components of the proteasome was considered to be regulation of the immune response.49 However, it is now demonstrated that almost all cells and tissues, including the retina, express PA28-a/-β and their expression is not restricted to induction by IFN-g. Analysis of the promoter of the PA28-β gene in dendritic cells (DC) revealed a NF-kB binding site, which is strikingly absent from the PA28-a promoter.50 This differential capacity for induction by NF-kB transcription factors in DC suggests a disparate regulatory mechanism for expression or function of PA28-a or PA28-β genes in different cell types.
Non-immune related functions of the 11S proteins are largely unknown and require further clarification. However, TRAF6 (tumor necrosis factor receptor-associated factor 6), which is a RANK (receptor activator of NF-kB) adapter protein, has been suggested to be a non-antigenic protein substrate for PA28-a/-β.51 TRAF6 is an E3 ubiquitin ligase and a member of the TRAF proteins which serve as adapter molecules coupling the tumor necrosis factor receptor (TNFR) super family to intracellular signaling events.52 PA28-mediated degradation of TRAF6 and regulation of the PA28-β protein by NF-kB proteins are suggestive of undisclosed functions for REG proteins in regulating cellular scenarios linked to inflammation.
PA28 IN MICROVASCULAR RESPONSES TO ELEVATED GLUCOSE LEVELS AND OXIDATIVE STRESS
Diabetes is a metabolic diseases caused by high blood glucose levels resulting in adverse complications in certain tissues in the form of DR, DN, neuropathy, non-healing foot ulcers, hearing impairment, and cardiovascular disorders. The majority of the deleterious complications of diabetes occur in organs with microvascular engagement including the retina and kidney. The retinal vasculature and renal intraglomerular capillaries, particularly the perivascular supporting cells including pericytes/mesangial cells, are primarily affected by diabetic hyperglycemia. This is marked with loss of pericytes (PC) in the retinal vasculature and expansion of mesangial cells of the kidney with net outcome of retinal vascular rarefaction and renal failure in DR and DN, respectively.53
The underlying mechanism for the higher susceptibility of PC to high glucose compared to endothelial cells (EC) in retinal vasculature is not known. The in vivo effect of hyperglycemia in retinal vascular cells is recapitulated in vitro by showing that cultured retinal PC treated with high glucose (25mM-40mM) are highly susceptible to oxidative stress and cell death as compared to EC.54-55 The precise link between exposure to high glucose, its influence on UPS, and selective death of PC in the diabetic retina is unclear but the endoplasmic reticulum (ER) and oxidative stress are prime candidates for romoting cell death by apoptosis. High glucose treatment of PC induces ER stress, which in turn has the potential to impair proteasome activity.56-57 Alternatively, oxidative stress can impair UPS activity. Oxidative stress caused by hydrogen peroxide (H2O2) blocked proteasome activity and increased the levels of ubiquitinated proteins, thus linking oxidative stress to UPS modulation.58 We hypothesized that impaired or dysregulated proteasome activity in retinal PC, compared to EC, could account for their higher susceptibility to hyperglycemia mediated cytotoxicity.
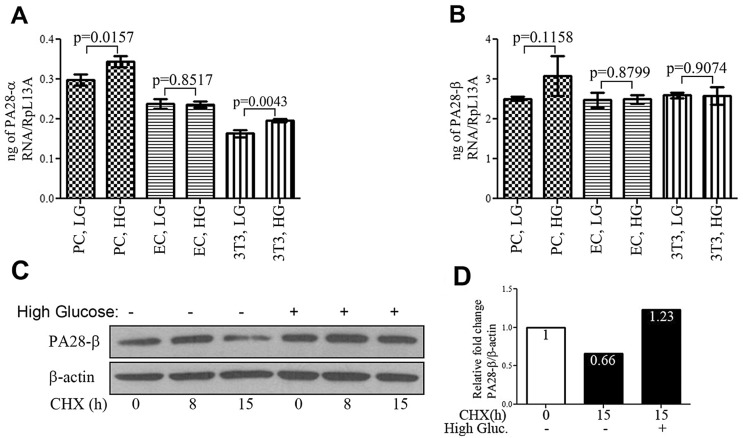
Oxidative stress is closely linked to the regulation of proteasome’s proteolytic activity. The PA28-a/-β genes are involved in adaptation of the cellular response to oxidative stress by enhancing the degradation of oxidized proteins in cultured mouse fibroblasts.59 We recently showed that high glucose treatment resulted in increased levels of PA28-a/-β proteins in cultured retinal PC but not in EC. Real-time PCR and cycloheximide (CHX) chase experiments indicated that the rise in the levels of these proteins in PC was due to increased protein stability rather than increased transcription (Fig. 2). Thus, there are functional differences in the UPS response in retinal EC and PC under high glucose conditions.27
Figure 2.
High glucose increased the stability of the PA28-β protein in cultured retinal pericytes (PC). (A-B) Real-time polymerase chain reaction (PCR) showing that high glucose treatment of retinal PC and endothelial cells (EC) results in modest or no change in transcript levels for either PA28-a or PA28-β. NIH3T3 cells were used as control for treatment with high glucose and PCR analysis. (C) Chase assay using protein synthesis inhibitor, cycloheximide (CHX) showing that treatment with high glucose for 5 days increased PA28-β stability. (D) Quantification of the Western blot for PA28-β by normalizing the ratio of PA28-β signal intensity to β-Actin. The percent change for the signal is represented above each bar.
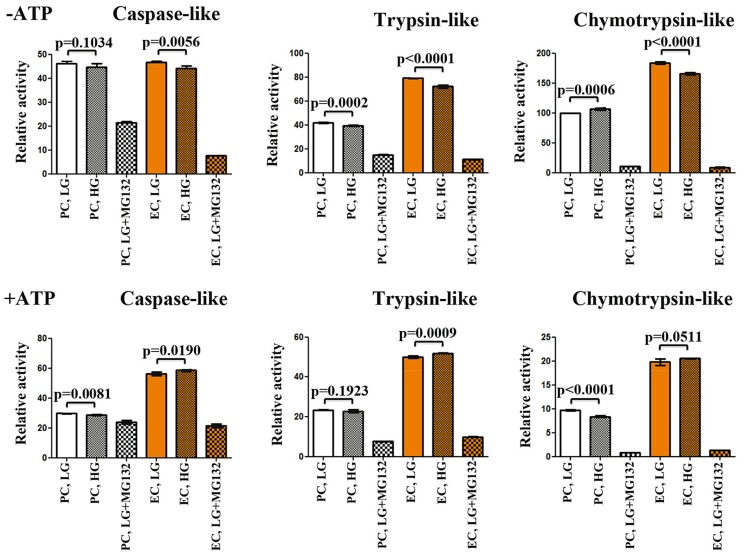
The explanation for the observed discrepancy in response to high glucose between EC and PC, in regard to oxidative stress and the modulation of PA28-a/-β proteins could potentially be due to their intrinsic differences in total UPS peptidase capacity. Indeed, using various fluorogenic peptide substrates to test proteasome’s chymotrypsin-like, trypsin-like and caspase-like peptidase activities in the absence and presence of ATP, we observed significantly higher proteasome peptidase activity in retinal EC as compared to PC (Fig. 3). Intriguingly, while high glucose reduced the chymotrypsin-like peptidase activity of the PC, it increased the peptidase activity for all substrates in EC under high glucose conditions.27 The enhanced peptidase activity of retinal EC and reduced chymotrypsin-like peptidase activity in PC under high glucose conditions together with enhanced PA28-a/β levels only in PC is likely suggestive of less efficiency of PC to cope with high glucose-mediate cytotoxicity.
Figure 3.
Cultured retinal endothelial cells (EC) have higher efficiency than pericytes (PC) to degrade proteasome substrates in vitro. (A) Proteasome peptidase activity in the absence of ATP. There is no significant difference in caspaselike peptidase activity between PC and EC. The proteasome inhibitor, MG132 at 200 μM had lesser efficiency to block the caspase-like activity of PC compared to EC. EC, but not PC, showed significant reduction in caspase-like activity after high glucose. EC had almost two-fold higher trypsin-like peptidase capacity than PC. Both PC and EC showed reduced peptidase activity after high glucose. EC showed higher chymotrypsin-like peptidase activity than PC. High glucose moderately increased the peptidase activity in PC but significantly reduced it in EC. MG132 potently inhibited the chymotrypsin like activity for both PC and EC. (B) Peptidase activity in the presence of 0.5 mM ATP. EC show higher peptidase activity for all tested proteasome substrates compared with PC. PC have reduced chymotrypsin-like peptidase activity after high glucose treatment. MG132 treatment dramatically reduced chymotryptic activity of the proteasome. Sample replicates for all assays (n=6). P values are shown for low and high glucose treatment conditions. Error bars, mean ± standard error.
We have also demonstrated that similar to PC, retinal proteasome activity is subject to hyperglycemia-mediated modulation in vivo by showing enhanced levels of the PA28-a/-β proteins in retinas of mice with established diabetes. PA28-g protein expression was not detectable in normal or diabetic mouse retinas.27 The increase in PA28-a/-β protein levels in both diabetic retinas and PC under high glucose conditions corresponded with elevated oxidative stress.60 PA28-a/-β proteins are regulated by nuclear factor erythroid 2- related factor 2 (Nrf2) transcription factor. Nrf2 is an important component of responses to oxidative stress and regulates H2O2-induced expression of both PA28- a/-β as well as other antioxidant genes.61 A likely explanation for the increased PA28 proteins in the retina and PC after hyperglycemia may be an adaptive response to assist with UPS activity to degrade certain substrates.
DYSREGULATION OF UPS IN DIABETIC NEPHROPATHY
The kidney is also a target of hyperglycemia and oxidative stress in diabetic patients. The prominent renal lesion is microvascular damage within the kidney filtration units or glomerulus resulting in mesangial expansion, hypercellularity and increased thickening of the glomerular basement membrane. Mesangial cells are specialized intraglomerular PC that provide a network of cells and fibers that support capillary loops in the glomerulus. Reduced or inefficient renal filtration capacity in diabetic patients necessitates dialysis or kidney transplantation.62 Due to the similar vulnerability to high glucose between retinal and intraglomerular PC, we investigated whether PA28-a/-β proteins were also elevated in the diabetic mouse kidneys. We found induction of the PA28-β/-g proteins together with Cullin-1 and Cullin-3 ubiquitin ligase proteins exclusively within the glomerular capillaries of diabetic mice with DN. Despite the fact that we could not differentiate the expression intensity of the 11S regulators in EC or PC of the glomerulus, the immunostaining signal for PA28 and Cullin proteins correlated with the severity of DN.27 Thus, hyperglycemia regulates UPS activity in vascular units of the glomerulus. Increased level of PA28 proteins in the glomerulus under DN conditions is also consistent with reported oxidative stress in DN and might play a protective role against oxidative damage.63-64
As PA28 proteasome regulators protect against oxidative stress, we anticipated that the increased levels of these proteins in the kidneys of mice with DN could ameliorate DN. However, this increase in intraglomerular capillaries of diabetic mice did not appear to protect against progression of DN. Thus, initial activation of PA28 proteins may protect against oxidative stress induced by hyperglycemia but their chronic activity could exacerbate the pathogenesis of DN. In agreement with the latter possibility, recent findings introduce proteasome inhibition as a potent target for amelioration of DN in experimental diabetic mouse models.19,65 However, the authors did not investigate whether retinal vascular integrity was maintained in mice with systemic proteasome inhibition. Based on similarity in the pathophysiology of DR and DN with exclusive PC damage, we predict that diabetic mice with systemic proteasome inhibition are protected against DR. This notion is supported by the highly significant correlation between DR and development of preclinical morphological changes of DN in the majority of type I diabetic patients.66 In addition, the incidence of DR is increased dramatically 5 years before the onset of proteinuria in type1 diabetes.67 Thus, presumably there is a shared susceptibility background for the pathobiology of both DR and DN. This overlap in DR and DN susceptibility necessitates evaluating the effects of proteasome inhibition in protection against DR lesions.
CONCLUDING REMARKS AND FUTURE PERSPECTIVE
In summary, proteasome regulatory proteins, the PA28, have distinct yet unknown roles in regulating the response of PC and EC to hyperglycemia. Developing specific inhibitors against these proteins or characterizing their protein targets will be valuable in developing future therapies for DR and DN or other microvascular complications of diabetes. However, additional studies are needed to advance our understanding of cell specific regulation of UPS activity. After showing elevated PA28 protein levels in retinal PC with high glucose, and also in diabetic glomerular capillaries, it will be valuable to determine whether altered PA28 activity in PC or EC units of vasculature is responsible for developing DR or DN. Addressing this question requires analysis of genetically modified mice that allow conditional inactivation or over-expression of either PA28- a/-β and PA28-g genes. These mouse models will allow cell-specific inactivation of targeted genes in EC or PC units of the vasculature after crossing with VE-Cadherin and PDGF-Rβ -Cre transgenic mice, respectively. Transgenic mice designed to temporally over-express the PA28 genes in PC or EC compartments of the vasculature will also allow us to determine the possible role elevated PA28 proteins play in the development and progression of DR or DN. These studies will advance our knowledge regarding the role proteasome plays in maintaining tissues homeostasis and how its alterations contribute to the development and progression of diabetes complications.
Acknowledgments
This work was supported by grants EY016995, EY021357, and P30-EY016665, from the National Institute of Health and an unrestricted departmental award from Research to Prevent Blindness. NS is a recipient of a Research Award from the American Diabetes Association, 1-10- BS-160 and Retina Research Foundation.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Kravtsova-Ivantsiv Y, Ciechanover A. Non- canonical ubiquitin-based signals for proteasomal degradation. J Cell Sci. 2012;125:539–548. doi: 10.1242/jcs.093567. [DOI] [PubMed] [Google Scholar]
- 2.Ravid T, Hochstrasser M. Diversity of degradation signals in the ubiquitin-proteasome system. Nat Rev Mol Cell Biol. 2008;9:679–690. doi: 10.1038/nrm2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciechanover A, Schwartz AL. The ubiquitin- proteasome pathway: the complexity and myriad functions of proteins death. Proc Natl Acad Sci U S A. 1998;95:2727–2730. doi: 10.1073/pnas.95.6.2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lecker SH, Goldberg AL, Mitch WE. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J Am Soc Nephrol. 2006;17:1807–1819. doi: 10.1681/ASN.2006010083. [DOI] [PubMed] [Google Scholar]
- 5.Shang F, Taylor A. Role of the ubiquitin-proteasome in protein quality control and signaling. implication in the pathogenesis of eye diseases. Prog Mol Biol Transl Sci. 2012;109:347–396. doi: 10.1016/B978-0-12-397863-9.00010-9. [DOI] [PubMed] [Google Scholar]
- 6.Loeffler KU, Mangini NJ. Immunolocalization of ubiquitin and related enzymes in human retina and retinal pigment epithelium. Graefes Arch Clin Exp Ophthalmol. 1997;235:248–254. doi: 10.1007/BF00941767. [DOI] [PubMed] [Google Scholar]
- 7.Naash MI, Al-Ubaidi MR, Anderson RE. Light exposure induces ubiquitin conjugation and degradation activities in the rat retina. Invest Ophthalmol Vis Sci. 1997;38:2344–2354. [PubMed] [Google Scholar]
- 8.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fessel JP, Porter NA, Moore KP, Sheller JR, Roberts LJ. Discovery of lipid peroxidation products formed in vivo with a substituted tetrahydrofuran ring (isofurans) that are favored by increased oxygen tension. PNAS. 2002;99:16713–16718. doi: 10.1073/pnas.252649099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang JH, Basinger SF, Gross RL, Wu SM. Blue light-induced generation of reactive oxygen species in photoreceptor ellipsoids requires mitochondrial electron transport. Invest Ophthalmol Vis Sci. 2003;44:1312–1319. doi: 10.1167/iovs.02-0768. [DOI] [PubMed] [Google Scholar]
- 12.Barot M, Gokulgandhi MR, Mitra AK. Mitochondrial dysfunction in retinal diseases. Curr Eye Res. 2011;36:1069–1077. doi: 10.3109/02713683.2011.607536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jarrett SG, Boulton ME. Consequences of oxidative stress in age-related macular degeneration. Mol Aspects Med. 2012;33:399–417. doi: 10.1016/j.mam.2012.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obin MS, Jahngen-Hodge J, Nowell T, Taylor A. Ubiquitinylation and ubiquitin-dependent proteolysis in vertebrate photoreceptors (rod outer segments). Evidence for ubiquitinylation of Gt and rhodopsin. J Biol Chem. 1996;271:14473–14484. doi: 10.1074/jbc.271.24.14473. [DOI] [PubMed] [Google Scholar]
- 15.Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J Lipid Res. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alves-Rodrigues A, Gregori L, Figueiredo-Pereira ME. Ubiquitin, cellular inclusions and their role in neurodegeneration. Trends Neurosci. 1998;21:516–520. doi: 10.1016/s0166-2236(98)01276-4. [DOI] [PubMed] [Google Scholar]
- 17.Zhao X, Yang J. Amyloid-beta peptide is a substrate of the human 20S proteasome. ACS Chem Neurosci. 2010;1:655–660. doi: 10.1021/cn100067e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cecarini V, Bonfili L, Cuccioloni M, Mozzicafreddo M, Rossi G, Buizza L, et al. Crosstalk between the ubiquitin-proteasome system and autophagy in a human cellular model of Alzheimer’s disease. Biochimica Et Biophysica Acta-Molecular Basis of Disease. 2012;1822:1741–1751. doi: 10.1016/j.bbadis.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, Miller CA, Ko MK, Black KL, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage. 2011;54 Suppl 1:S204–217. doi: 10.1016/j.neuroimage.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011;70:532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakabayashi K, Tanji K, Odagiri S, Miki Y, Mori F, Takahashi H. The Lewy Body in Parkinson’s Disease and Related Neurodegenerative Disorders. Mol Neurobiol. 2013;47:495–508. doi: 10.1007/s12035-012-8280-y. [DOI] [PubMed] [Google Scholar]
- 22.Nuytemans K, Theuns J, Cruts M, Van Broeckhoven C. Genetic etiology of Parkinson disease associated with mutations in the SNCA, PARK2, PINK1, PARK7, and LRRK2 genes: a mutation update. Hum Mutat. 2010;31:763–780. doi: 10.1002/humu.21277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120:131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devos D, Tir M, Maurage CA, Waucquier N, Defebvre L, Defoort-Dhellemmes S, et al. ERG and anatomical abnormalities suggesting retinopathy in dementia with Lewy bodies. Neurology. 2005;65:1107–1110. doi: 10.1212/01.wnl.0000178896.44905.33. [DOI] [PubMed] [Google Scholar]
- 25.Esteve-Rudd J, Campello L, Herrero MT, Cuenca N, Martin-Nieto J. Expression in the mammalian retina of parkin and UCH-L1, two components of the ubiquitin-proteasome system. Brain Res. 2010;1352:70–82. doi: 10.1016/j.brainres.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 26.Reinstein E, Ciechanover A. Narrative review: protein degradation and human diseases: the ubiquitin connection. Ann Intern Med. 2006;145:676–684. doi: 10.7326/0003-4819-145-9-200611070-00010. [DOI] [PubMed] [Google Scholar]
- 27.Aghdam SY, Gurel Z, Ghaffarieh A, Sorenson CM, Sheibani N. High glucose and diabetes modulate cellular proteasome function: Implications in the pathogenesis of diabetes complications. Biochem Biophys Res Commun. 2013;432:339–444. doi: 10.1016/j.bbrc.2013.01.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coppo R, Camilla R, Alfarano A, Balegno S, Mancuso D, Peruzzi L, et al. Upregulation of the immunoproteasome in peripheral blood mononuclear cells of patients with IgA nephropathy. Kidney Int. 2009;75:536–541. doi: 10.1038/ki.2008.579. [DOI] [PubMed] [Google Scholar]
- 29.Hainz N, Thomas S, Neubert K, Meister S, Benz K, Rauh M, et al. The proteasome inhibitor bortezomib prevents lupus nephritis in the NZB/W F1 mouse model by preservation of glomerular and tubulointerstitial architecture. Nephron Exp Nephrol. 2012;120:47–58. doi: 10.1159/000334955. [DOI] [PubMed] [Google Scholar]
- 30.Itoh M, Takaoka M, Shibata A, Ohkita M, Matsumura Y. Preventive effect of lactacystin, a selective proteasome inhibitor, on ischemic acute renal failure in rats. J Pharmacol Exp Ther. 2001;298:501–507. [PubMed] [Google Scholar]
- 31.Sureshkumar KK, Hussain SM, Marcus RJ, Ko TY, Khan AS, Tom K, et al. Proteasome inhibition with bortezomib: an effective therapy for severe antibody mediated rejection after renal transplantation. Clin Nephrol. 2012;77:246–253. doi: 10.5414/cn107156. [DOI] [PubMed] [Google Scholar]
- 32.Takaoka M, Itoh M, Hayashi S, Kuro T, Matsumura Y. Proteasome participates in the pathogenesis of ischemic acute renal failure in rats. Eur J Pharmacol. 1999;384:43–46. doi: 10.1016/s0014-2999(99)00664-0. [DOI] [PubMed] [Google Scholar]
- 33.Tashiro K, Tamada S, Kuwabara N, Komiya T, Takekida K, Asai T, et al. Attenuation of renal fibrosis by proteasome inhibition in rat obstructive nephropathy: possible role of nuclear factor kappaB. Int J Mol Med. 2003;12:587–592. [PubMed] [Google Scholar]
- 34.Wada J, Makino H. Inflammation and the pathogenesis of diabetic nephropathy. Clin Sci (Lond) 2013;124:139–152. doi: 10.1042/CS20120198. [DOI] [PubMed] [Google Scholar]
- 35.Wertz IE, Dixit VM. Signaling to NF-kappaB: regulation by ubiquitination. Cold Spring Harb Perspect Biol. 2010;2:a003350. doi: 10.1101/cshperspect.a003350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui W, Li B, Bai Y, Miao X, Chen Q, Sun W, et al. Potential role for Nrf2 activation in the therapeutic effect of MG132 on diabetic nephropathy in OVE26 diabetic mice. Am J Physiol Endocrinol Metab. 2013;304:E87–99. doi: 10.1152/ajpendo.00430.2012. [DOI] [PubMed] [Google Scholar]
- 37.Fabunmi RP, Wigley WC, Thomas PJ, DeMartino GN. Interferon gamma regulates accumulation of the proteasome activator PA28 and immunoproteasomes at nuclear PML bodies. J Cell Sci. 2000;114:29–36. doi: 10.1242/jcs.114.1.29. [DOI] [PubMed] [Google Scholar]
- 38.Strehl B, Seifert U, Kruger E, Heink S, Kuckelkorn U, Kloetzel PM. Interferon-gamma, the functional plasticity of the ubiquitin-proteasome system, and MHC class I antigen processing. Immunol Rev. 2005;207:19–30. doi: 10.1111/j.0105-2896.2005.00308.x. [DOI] [PubMed] [Google Scholar]
- 39.Yamano T, Sugahara H, Mizukami S, Murata S, Chiba T, Tanaka K, et al. Allele-selective effect of PA28 in MHC class I antigen processing. J Immunol. 2008;181:1655–1664. doi: 10.4049/jimmunol.181.3.1655. [DOI] [PubMed] [Google Scholar]
- 40.Francoeur AM, Peebles CL, Gompper PT, Tan EM. Identification of Ki (Ku, p70/p80) autoantigens and analysis of anti-Ki autoantibody reactivity. J Immunol. 1986;136:1648–1653. [PubMed] [Google Scholar]
- 41.Barton LF, Runnels HA, Schell TD, Cho Y, Gibbons R, Tevethia SS, et al. Immune defects in 28-kDa proteasome activator gamma-deficient mice. J Immunol. 2004;172:3948–3954. doi: 10.4049/jimmunol.172.6.3948. [DOI] [PubMed] [Google Scholar]
- 42.Murata S, Kawahara H, Tohma S, Yamamoto K, Kasahara M, Nabeshima Y, et al. Growth retardation in mice lacking the proteasome activator PA28gamma. J Biol Chem. 1999;274:38211–38215. doi: 10.1074/jbc.274.53.38211. [DOI] [PubMed] [Google Scholar]
- 43.Song X, von Kampen J, Slaughter CA, DeMartino GN. Relative functions of the alpha and beta subunits of the proteasome activator, PA28. Journal of Biological Chemistry. 1997;272:27994–28000. doi: 10.1074/jbc.272.44.27994. [DOI] [PubMed] [Google Scholar]
- 44.DeMartino GN, Slaughter CA. The proteasome, a novel protease regulated by multiple mechanisms. J Biol Chem. 1999;274:22123–22126. doi: 10.1074/jbc.274.32.22123. [DOI] [PubMed] [Google Scholar]
- 45.Forster A, Masters EI, Whitby FG, Robinson H, Hill CP. The 1.9 A structure of a proteasome-11S activator complex and implications for proteasome- PAN/PA700 interactions. Mol Cell. 2005;18:589–599. doi: 10.1016/j.molcel.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 46.Ma CP, Willy PJ, Slaughter CA, DeMartino GN. PA28, an activator of the 20 S proteasome, is inactivated by proteolytic modification at its carboxyl terminus. J Biol Chem. 1993;268:22514–22519. [PubMed] [Google Scholar]
- 47.Sijts EJ, Kloetzel PM. The role of the proteasome in the generation of MHC class I ligands and immune responses. Cell Mol Life Sci. 2011;68:1491–1502. doi: 10.1007/s00018-011-0657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Murata S, Udono H, Tanahashi N, Hamada N, Watanabe K, Adachi K, et al. Immunoproteasome assembly and antigen presentation in mice lacking both PA28 alpha and PA28 beta. Embo Journal. 2001;20:5898–5907. doi: 10.1093/emboj/20.21.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun Y, Sijts AJAM, Song MX, Janek K, Nussbaum AK, Kral S, et al. Expression of the proteasome activator PA28 rescues the presentation of a cytotoxic T lymphocyte epitope on melanoma cells. Cancer Research. 2002;62:2875–2882. [PubMed] [Google Scholar]
- 50.Ossendorp F, Fu N, Camps M, Granucci F, Gobin SJP, van den Elsen PJ, et al. Differential expression regulation of the alpha and beta subunits of the PA28 proteasome activator in mature dendritic cells. Journal of Immunology. 2005;174:7815–7822. doi: 10.4049/jimmunol.174.12.7815. [DOI] [PubMed] [Google Scholar]
- 51.Takayanagi H, Ogasawara K, Hida S, Chiba T, Murata S, Sato K, et al. T-cell-mediated regulation of osteoclastogenesis by signalling cross-talk between RANKL and IFN-gamma. Nature. 2000;408:600–605. doi: 10.1038/35046102. [DOI] [PubMed] [Google Scholar]
- 52.Deng L, Wang C, Spencer E, Yang LY, Braun A, You JX, et al. Activation of the I kappa B kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell. 2000;103:351–361. doi: 10.1016/s0092-8674(00)00126-4. [DOI] [PubMed] [Google Scholar]
- 53.Cade WT. Diabetes-related microvascular and macrovascular diseases in the physical therapy setting. Phys Ther. 2008;88:1322–1335. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Huang Q, Sheibani N. High glucose promotes retinal endothelial cell migration through activation of Src, PI3K/Akt1/eNOS, and ERKs. Am J Physiol Cell Physiol. 2008;295:C1647–1657. doi: 10.1152/ajpcell.00322.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Romeo G, Liu WH, Asnaghi V, Kern TS, Lorenzi M. Activation of nuclear factor-kappaB induced by diabetes and high glucose regulates a proapoptotic program in retinal pericytes. Diabetes. 2002;51:2241–2248. doi: 10.2337/diabetes.51.7.2241. [DOI] [PubMed] [Google Scholar]
- 56.Menendez-Benito V, Verhoef LG, Masucci MG, Dantuma NP. Endoplasmic reticulum stress compromises the ubiquitin-proteasome system. Hum Mol Genet. 2005;14:2787–2799. doi: 10.1093/hmg/ddi312. [DOI] [PubMed] [Google Scholar]
- 57.Zhong Y, Wang JJ, Zhang SX. Intermittent but not constant high glucose induces ER stress and inflammation in human retinal pericytes. Adv Exp Med Biol. 2012;723:285–292. doi: 10.1007/978-1-4614-0631-0_37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dong X, Liu J, Zheng H, Glasford JW, Huang W, Chen QH, et al. In situ dynamically monitoring the proteolytic function of the ubiquitin-proteasome system in cultured cardiac myocytes. Am J Physiol Heart Circ Physiol. 2004;287:H1417–1425. doi: 10.1152/ajpheart.01233.2003. [DOI] [PubMed] [Google Scholar]
- 59.Pickering AM, Koop AL, Teoh CY, Ermak G, Grune T, Davies KJ. The immunoproteasome, the 20S proteasome and the PA28alphabeta proteasome regulator are oxidative-stress-adaptive proteolytic complexes. Biochem J. 2010;432:585–594. doi: 10.1042/BJ20100878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mohamed IN, Soliman SA, Alhusban A, Matragoon S, Pillai BA, Elmarkaby AA, et al. Diabetes exacerbates retinal oxidative stress, inflammation, and microvascular degeneration in spontaneously hypertensive rats. Mol Vis. 2012;18:1457–1466. [PMC free article] [PubMed] [Google Scholar]
- 61.Pickering AM, Linder RA, Zhang H, Forman HJ, Davies KJ. Nrf2-dependent induction of proteasome and Pa28alphabeta regulator are required for adaptation to oxidative stress. J Biol Chem. 2012;287:10021–10031. doi: 10.1074/jbc.M111.277145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kanwar YS, Sun L, Xie P, Liu FY, Chen S. A Glimpse of Various Pathogenetic Mechanisms of Diabetic Nephropathy. Annu Rev Pathol. 2011;6:395–423. doi: 10.1146/annurev.pathol.4.110807.092150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Koya D, Hayashi K, Kitada M, Kashiwagi A, Kikkawa R, Haneda M. Effects of antioxidants in diabetes-induced oxidative stress in the glomeruli of diabetic rats. Journal of the American Society of Nephrology. 2003;14:S250–S253. doi: 10.1097/01.asn.0000077412.07578.44. [DOI] [PubMed] [Google Scholar]
- 64.Singh DK, Winocour P, Farrington K. Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol. 2011;7:176–184. doi: 10.1038/nrendo.2010.212. [DOI] [PubMed] [Google Scholar]
- 65.Luo ZF, Qi W, Feng B, Mu J, Zeng W, Guo YH, et al. Prevention of diabetic nephropathy in rats through enhanced renal antioxidative capacity by inhibition of the proteasome. Life Sci. 2011;88:512–520. doi: 10.1016/j.lfs.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 66.Klein R, Zinman B, Gardiner R, Suissa S, Donnelly SM, Sinaiko AR, et al. The relationship of diabetic retinopathy to preclinical diabetic glomerulopathy lesions in type 1 diabetic patients - The Renin- Angiotensin System Study. Diabetes. 2005;54:527–533. doi: 10.2337/diabetes.54.2.527. [DOI] [PubMed] [Google Scholar]
- 67.Kofoed-Enevoldsen A, Jensen T, Borch-Johnsen K, Deckert T. Incidence of retinopathy in type I (insulin-dependent) diabetes: association with clinical nephropathy. J Diabet Complications. 1987;1:96–99. doi: 10.1016/s0891-6632(87)80064-8. [DOI] [PubMed] [Google Scholar]
- 68.Tanahashi N, Murakami Y, Minami Y, Shimbara N, Hendil KB, Tanaka K. Hybrid proteasomes. Induction by interferon-gamma and contribution to ATP-dependent proteolysis. Journal of Biological Chemistry. 2000;275:14336–14345. doi: 10.1074/jbc.275.19.14336. [DOI] [PubMed] [Google Scholar]