Abstract
Purpose
To explore retinal ganglion cell (RGC) dysfunction in glaucoma suspects and patients with early primary open angle glaucoma (POAG) using pattern electroretinography (PERG).
Methods
Twenty glaucoma suspects (glaucomatous optic disc appearance), 15 early POAG (based on abnormal discs and abnormal visual fields) and 16 normal controls were enrolled. Transient PERG was recorded in response to 0.8° and 16° black and white checkerboard stimuli. Amplitude and peak time (latency) of the P50 and N95 components of the PERG response, and the ratio of N95 amplitude in response to 0.8° and 16° checks were measured.
Results
N95 peak time (latency) was significantly increased in both early manifest POAG and glaucoma suspects as compared to normal controls (P<0.001). In early POAG, N95 amplitude in response to small (0.8°) checks and the small/large check ratio were reduced in comparison to normal eyes (P<0.05). However, in glaucoma suspects no significant N95 amplitude reduction was observed. No significant difference was observed among the study groups in terms of P50 amplitude or peak time.
Conclusion
The N95 PERG response demonstrated uncoupled peak time and amplitude alterations in glaucoma. N95 peak time was significantly increased both in glaucoma suspects and early POAG; N95 amplitude reduction was present only in early POAG. PERG may detect RGC dysfunction (increased latency) before cell death (decreased amplitude) occurs.
Keywords: Pattern Electroretinography, Glaucoma Suspect, Primary Open Angle Glaucoma
INTRODUCTION
Glaucoma is the second leading cause of blindness worldwide and the most common cause of irreversible blindness.1 Detection of glaucoma at its earliest stages is a major goal for clinicians and researchers. Standard achromatic perimetry (SAP) is an integral tool for early recognition of visual field loss. Among several limitations of SAP, the subjective nature of the test has led to interest in alternative, objective information provided by electrophysiological responses, especially those evoked by pattern stimulation.2-4
Retinal ganglion cells (RGCs) and their axons are primary targets in the pathophysiology of glaucoma5 and pattern electroretinography (PERG) has been shown to reflect RGC function.6 A number of studies have shown alterations of PERG in glaucoma7-9, ocular hypertension10-11 and experimental models of glaucoma12-14; there is also evidence supporting that PERG is able to predict progression of glaucomatous neuropathy before SAP15 and that it may detect early RGC dysfunction in glaucoma before visual field defects develop.16
PERG is recorded noninvasively from the cornea when a high contrast checkerboard or grating pattern with equal alternating bright and dark elements is viewed on a cathode ray tube (CRT) monitor. Generation of PERG waves is the consequence of non-linear responses produced by different levels of retinal illumination at adjacent points.17 The frequency of checkerboard reversal determines whether the transient response (<6 reversals per second) or the steady-state response (>10 reversals per second) is elicited. When transient PERG is elicited, the waveform consists of a positive (P50) and a negative (N95) component. Dissimilar spatial tuning properties of the positive and negative PERG components reflect their different origins.18-19 Recent research on PERG in an animal model revealed that P50 originates from both spiking and non-spiking activity of ON and OFF pathways whereas the N95 wave originates from the spiking activity of RGCs.6
Although the role of PERG in detecting glaucomatous damage has generally been accepted,20-21 the mechanisms and patterns of PERG alterations in glaucoma suspects have not been well established. The purpose of this study was to evaluate PERG components in early manifest primary open angle glaucoma (EMPOAG) and glaucoma suspects (GSs) in comparison to normal controls.
METHODS
Thirty-one eyes of 16 normal subjects with mean age of 40.42±12.1 (range, 26-59) years, 38 eyes of 20 GSs with mean age of 44.95±13.23 (range, 23-66) years, and 29 eyes of 15 patients with EMPOAG with mean age of 46.97±13.3 (range, 22-63) years were recruited.
Normal subjects had best-corrected visual acuity (BCVA) of at least 20/30, IOP less than 21mmHg, normal optic disc appearance and normal 24-2 SITA-standard SAP. GSs had signs suggestive of glaucomatous optic nerve damage (vertical cup to disc ratio >0.7; vertical cup to disc ratio asymmetry ≥0.2, localized thinning of the neural rim, splinter disc hemorrhage, beta zone peripapillary atrophy) on fundus examination, but normal IOP and also normal SAP. EMPOAG patients had glaucomatous optic nerve damage together with abnormal SAP defined in the presence of at least two of the following three criteria: glaucoma hemifield test (GHT) “outside normal limits”, pattern standard deviation outside the 95% normal limit, and a cluster of three points on the pattern deviation plot with p values less than 5% including one point with p value less than 1%.
Exclusion criteria for all subjects included angle closure glaucoma, high myopia (>5D),22 ocular or systemic pathologies which may cause PERG abnormalities such as diabetes mellitus23 and retinal dystrophies,24 prior intraocular surgery except for uncomplicated cataract extraction, age less than 18 years or more than 65 years, unreliable SAP (>33% fixation loss, > 20% false positive and false negative rates). Eyes with advanced visual field defects (MD<-16 dB) or fixation threat, defined as differential light sensitivity of 10 dB or worse at either or both test points closest to the point of fixation in the upper and lower hemifields were also excluded.25
All participants underwent a complete ophthalmologic examination including dry refraction and determination of BCVA, slit lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, and dilated stereoscopic fundus examination; these were followed (on a separate session) by ultrasonic pachymetry and SAP (Humphrey visual field analyzer 750, Carl-Zeiss Meditec, Dublin, CA, USA) using the SITA standard strategy, program 24-2. Each patient had previous experience with SAP (at least 2 reliable examinations within the previous 6 months).
A commercially available electrophysiology instrument (RETI-port/scan, Roland Consult, Brandenburg, Germany), was used to measure PERG responses. The stimulus was presented on a CRT monitor subtending 29 by 29 degrees of visual angle at a distance of 50 cm. The checkerboard had mean luminance of 120 cd/m2, contrast of 99% and check size of 0.8° or 16°. Temporal frequency was 4 Hz to evoke transient responses. PERG was recorded with corneal Dawson-Trick-Litzcow (DTL) electrodes placed near the lower limbus, and as reference, gold cup electrodes at the outer ipsilateral canthus according to International Society for Clinical Electrophysiology of Vision (ISCEV) protocols.26 All subjects were asked to fixate on a small cross at the center of the screen. We analyzed the amplitudes and latencies of P50 and N95 in response to 0.8° and 16° checks.
Sample size was calculated based on the main outcome measure (N95 amplitude in response to small checks) which required 16 cases in each group to be able to determine a difference of at least 3 μv among the study groups with a 80% power when the standard deviation for the mentioned parameter in each group was assumed to be 3 μv.
To present data we used mean±SD, median and range. To compare the results after checking for normality of data based on the Kolmogorov- Smirnov test and box plots, the Kruskal-Wallis and chi-square tests were used. To consider the correlation of eyes in bilateral cases, the generalized estimating equation (GEE) analysis and for multiple comparisons the Bonferroni method were employed. All statistical analyses were performed using SPSS software version 21.0 (SPSS Co., Chicago, IL, USA). P-values less than 0.05 were considered as statistically significant.
RESULTS
Table 1 provides details on PERG data of the study groups. N95 latency in response to both small and large stimuli was significantly increased in both EMPOAG eyes and GSs as compared to controls (P<0.001 for all comparisons). However, EMPOAG eyes and GSs were comparable in this regard (P=0.35, P=1.00, respectively). P50 latency was comparable among EMPOAG, GS and normal subjects in response to both large (P=0.193) and small checks (P=0.317).
Table 1.
PERG parameters in the study population (N=98)
| Normal (a) | GS (b) | EMPOAG (c) | P†† | MC | |||
|---|---|---|---|---|---|---|---|
| Latency | |||||||
| L P50B-W | |||||||
| Mean±SD | 51± 2 | 52± 2 | 52± 3 | 0.193 | - | ||
|
|
|||||||
| Median (R) | 50 (48 to 57) | 53 (48 to 57) | 51 (48 to 60) | ||||
|
|
|||||||
| S P50B-W | |||||||
| Mean±SD | 56± 3 | 57± 3 | 57± 3 | 0.317 | - | ||
|
|
|||||||
| Median (R) | 56 (51 to 64) | 57 (50 to 63) | 57 (51 to 64) | ||||
|
|
|||||||
| L N95B-W | |||||||
| Mean±SD | 101± 7 | 110± 11 | 108± 9 | <0.001 | (a,b),(a,c) | ||
|
|
|||||||
| Median (R) | 102 (86 to 119) | 108 (93 to 131) | 106 (96 to 131) | ||||
|
|
|||||||
| S N95B-W | |||||||
| Mean±SD | 102± 3 | 110± 7 | 108± 7 | <0.001 | (a,b),(a,c) | ||
|
|
|||||||
| Median (R) | 102 (95 to 109) | 110 (98 to 130) | 105 (98 to 121) | ||||
|
| |||||||
| Amplitude | |||||||
| L P50B-W | |||||||
| Mean±SD | 9.88± 2.44 | 10.57± 3.53 | 8.73± 2.43 | 0.037 | (b,c) | ||
|
|
|||||||
| Median (R) | 9.27 (5.92 to 15.7) | 10.25 (3.76 to 18.1) | 8.84 (3.25 to 13.5) | ||||
|
|
|||||||
| S P50B-W | |||||||
| Mean±SD | 9.63± 3.54 | 9.34± 3.78 | 7.17± 3.17 | 0.012 | (a,c)(b,c) | ||
|
|
|||||||
| Median (R) | 8.93 (5.22 to 19.5) | 8.93 (3.14 to 17.6) | 6.72 (1.98 to 14.7) | ||||
|
|
|||||||
| L N95B-W | |||||||
| Mean±SD | 11.53± 2.72 | 11.95± 3.16 | 9.83± 2.48 | 0.007 | (b,c) | ||
|
|
|||||||
| Median (R) | 11.1 (6.77 to 17.3) | 11.4 (5.6 to 20) | 10.2 (4.49 to 16.8) | ||||
|
|
|||||||
| S N95B-W | |||||||
| Mean±SD | 12.6± 4.02 | 12.09± 3.85 | 8.76± 3.16 | <0.001 | (a,c),(b,c) | ||
|
|
|||||||
| Median (R) | 12.2 (6.81 to 23) | 11.4 (5.32 to 20.6) | 9.03 (1.49 to 14.4) | ||||
|
|
|||||||
| Ratio | |||||||
| Mean±SD | 1.09± 0.21 | 1.01± 0.2 | 0.89± 0.28 | 0.004 | (a,c) | ||
|
|
|||||||
| Median (R) | 1.1 (0.65 to 1.48) | 1 (0.58 to 1.43) | 0.9 (0.25 to 1.39) | ||||
GS, glaucoma suspect; EMPOAG, early manifest glaucoma; SD; standard deviation; R, range; L P50 B-W, P50 wave response to large (16°) black-white stimulus; S P50 B-W, P50 wave response to small (0.8°) black-white stimulus; L N95 B-W, N95 wave response to large (16°) black-white stimulus; S N95 B-W, N95 wave response to small (0.8°) back-white stimulus MC, Multiple comparisons based on Bonferroni method.
Based on GEE analysis
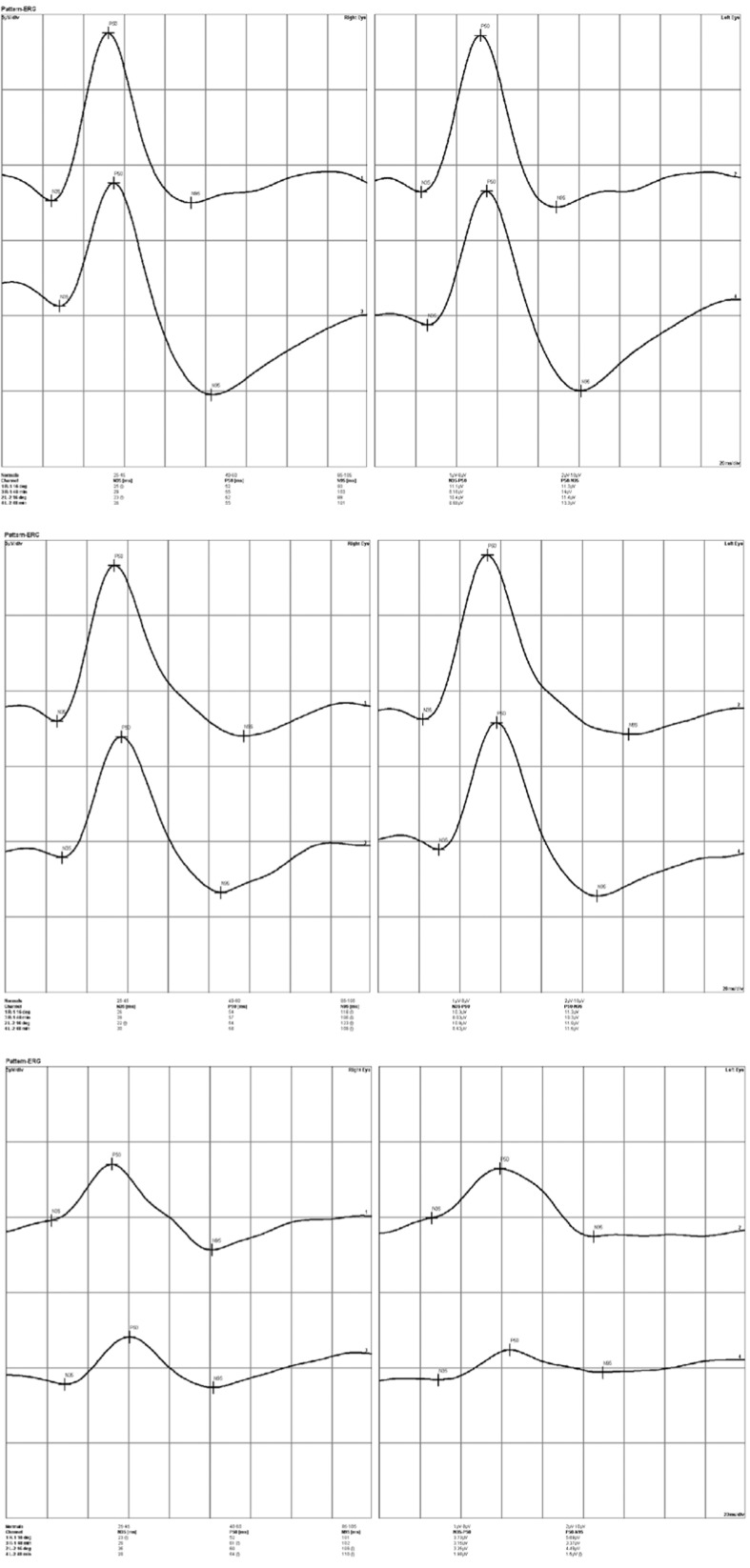
N95 amplitude in response to small checks (0.8°) was significantly lower in EMPOAG eyes as compared to GSs and control eyes (P<0.001 for both comparisons). N95 amplitude in response to large checks (16°) was significantly lower in EMPOAG eyes as compared to GSs (P=0.007) but not controls (P=0.06). P50 amplitude in response to small checks was significantly reduced in EMPOAG eyes as compared to GSs (P=0.03) and controls (P=0.02). Using large checks, these reductions were significant when compared to GSs (P=0.03) but not control eyes (P=0.3). GSs were comparable to normal controls in all amplitude parameters. Figure 1 depicts three representative PERG tracings in a normal control, a GS, and a case of EMPOAG.
Figure 1.
Pattern electroretinographic (PERG) tracings of the right and left eyes (left and right columns, respectively) in three representative cases. Within each printout the upper tracing represents the PERG response to large (16 degree) checks while the lower tracing is the response to small (0.8 degree) checks. Upper image set, PERG in a normal control, note the amplitude and peak time of the P50 and N95 waves; middle image set, PERG tracings in a glaucoma suspect, note the increased latency despite normal amplitudes; lower image set, PERG tracings in a case of early manifest glaucoma, note both the increased latency and reduced amplitude of both waves.
PERG ratio was computed by dividing N95 amplitude in response to 0.8° checks by the amplitude in response to 16° checks. This ratio was 1.09±0.21 in normal controls, 1.01±0.2 in GSs (P=0.5) and 0.89±0.28 in EMPOAG (P=0.004).
DISCUSSION
PERG allows detection of small changes in the electrical activity of RGCs. In the current study, PERG revealed RGC dysfunction in both EMPOAG and GSs, however the pattern of abnormality was different: glaucoma suspects displayed increased latency while eyes with EMPOAG demonstrated both increased latency and reduced amplitudes.
Previous studies have reported significant reduction in PERG amplitudes in early glaucoma.3,7-27 It has been demonstrated that the origin of the N95 wave is the RGC4,19 and that its amplitude may be an indirect measure of RGC population.28 Ventura et al28 stressed that RGC health is necessary for generation of PERG waves and reported a inverse correlation between N95 amplitude and the severity of glaucoma such that amplitude reduction was present in 19% of GSs versus 33% of EMPOAG patients. Later, in a longitudinal study progressive deterioration of RGC function in GS patients was confirmed by PERG parameters.29 According to North et al,27 N95 amplitude is sensitive for detecting diffuse damage in early POAG and able to detect RGC dysfunction before irreversible cell loss occurs; this matter was exemplified by more significant amplitude attenuation in early glaucoma as compared to OHT. Our results are in line with these studies in revealing reduced P50 and N95 amplitude in response to both large and small checks in EMPOAG. Amplitude reduction in manifest glaucoma may reflect both missing and sick RGCs.
Amplitude reduction was not observed in GS patients in the current study. This discrepancy with previous studies may be due to use of different recording techniques. Ventura et al recorded steady state responses in their studies9,28-29 as compared to the standard transient PERG employed in the present study. High reversal (>16 reversals/second) pattern stimuli pose a metabolic challenge to RGCs30 which may be the cause of greater amplitude reduction at higher temporal frequencies in glaucoma.20 Reduced ability to follow high temporal frequency stimuli represents altered temporal properties of the visual system. In transient PERG, this alteration becomes manifest as increased latency rather than decreased amplitude. Another possible reason for this discrepancy is that the number of RGCs varies widely, from 777,000 to 1.7 million in normal eyes,31 which may result in large amplitude variability among normal subjects.32 Consequently, in patients with larger baseline amplitudes a small to modest loss in RGCs may not reduce the amplitudes below normal limits.33 This may explain why amplitude reduction was not observed in GSs in our study.
Application of the PERG ratio (ratio of the N95 wave amplitude in response to 0.8° and 16° checks) may circumvent the issue mentioned above. In normal subjects the amplitudes of responses to large and small checks are highly correlated; this means an individual with large 0.8° amplitudes also has large 16° amplitudes but the ratio remains larger than 1. In early glaucoma, the amplitude of the response to small checks becomes affected out of proportion to that of large checks and thus the ratio falls below 1.20 In the current study PERG ratio was significantly reduced in EMPOAG as compared to normal controls and GSs. Although GS patients also had slightly smaller ratios than normal subjects (which may indicate very early RGC loss) the difference failed to reach statistical significance. Bode et al34 reported that this ratio is able to identify high risk GSs four years before conversion to manifest glaucoma.
As mentioned above, peak time latency indicates altered temporal properties of RGCs. N95 latency was significantly delayed in EMPOAG with both check sizes as compared to normal controls. The relatively spared P50 latency we observed may be due to its distinct origin in the distal retina.6 The same phenomenon was seen in GSs in whom N95 latency was longer than normal controls.
There is disparity in the literature on peak time assessment in glaucoma and no constant pattern has been observed.7,9 Porciatti and Ventura reported that a reduced number of neurons results in lower amplitudes but the phase remains constant.35 Our results are in agreement with two recent studies in which progressive retinal changes were monitored. Bode et al34 reported 0.8° peak time as a good predictor of conversion in a group of glaucoma suspects and Ventura et al29 reported longitudinal reduction in both amplitude and phase (increased latency) in glaucoma suspects. These findings are consistent with large clinical trials illustrating that functional parameters (provided by perimetry) may precede ONH changes in 35% to 86% of patients: VF defects were first seen in 35% of patients in the Ocular Hypertension Treatment Study, the corresponding figure was 60% in the European Glaucoma Prevention Study and 86% in the Early Manifest Glaucoma Trial.36
PERG amplitude and peak time represent distinct aspects of RGC dysfunction; amplitude reduction may be a sign of lost RGCs, dysfunctional RGCs, or a combination of both,21,35 whereas delayed PERG peak time may indicate that viable but stressed RGCs are responding in a slow manner. New evidence has suggested that retrograde axonal transport becomes dysfunctional before the axons and RGCs are actually lost.37 Our results in GS eyes may indicate that although viable RGCs may not display anatomical changes or become reduced in number, their dysfunction may become manifest as reduced temporal response to visual stimuli.21 Reduced activity of RGCs (delayed peak time) without reduced amplitude may arise from synaptic and transport delay which are known to be the first changes prior to RGC death.37-38 With further damage, both latency and amplitude become affected as observed in our EMPOAG patients.
These findings may have implications in clinical practice; research on the mechanisms of RGC degeneration has revealed that early events in glaucomatous neurodegeneration are loss of synaptic connectivity at the dendritic level together with both anterograde and retrograde axonal transport deficits.5,39 It is believed that at this stage, dysfunctional axonal transport is not associated with significant degeneration of RGC soma and axons, and that these deficits are reversible. This reversibility was confirmed in studies showing a significant increase in amplitude and decrease in peak time of PERG waves after surgical40 or medical IOP reduction in ocular hypertensive and glaucoma patients.41 Furthermore, it has been demonstrated that neuronal activity can eliminate apoptosis,39 therefore early detection of functional deficits prior to RGC death may raise the prospect of enhancing synaptic function in sick RGCs and preventing vision loss in glaucoma.42
Our results are in agreement with previous studies and suggest that P50 and N95 amplitudes in response to small checks may have the greatest discriminatory power especially in patients with unreliable visual fields and GSs; these functional parameters may also help determine the appropriate time for treatment. To the best of our knowledge, this is the first study on PERG parameters in an Iranian population; as mentioned by Ventura et al9 some discrepancies between our results and previous reports may stem from race specific differences in RGC electrical activity. Another possible confounder is our relatively small sample size; our subjects were selected based on very restricted criteria which may explain some minor differences in certain parameters. Glaucoma suspects exemplify shortcomings of current technologies to identify early glaucomatous damage. Our results suggest that the clinical suspicion of damage may actually be associated with visual dysfunction demonstrated by electrophysiological changes. Abnormal PERG parameters may therefore serve as an additional risk factor together with well- established risk factors of glaucoma9 and help identify patients at higher risk of progression.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol. 1996;80:389–393. doi: 10.1136/bjo.80.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bach M, Speidel-Fiaux A. Pattern electroretinogram in glaucoma and ocular hypertension. Doc Ophthalmol. 1989;73:173–181. doi: 10.1007/BF00155035. [DOI] [PubMed] [Google Scholar]
- 3.Graham SL, Drance SM, Chauhan BC, Swindale NV, Hnik P, Mikelberg FS, et al. Comparison of psychophysical and electrophysiological testing in early glaucoma. Invest Ophthalmol Vis Sci. 1996;37:2651–2662. [PubMed] [Google Scholar]
- 4.Holder GE. Pattern electroretinography (PERG) and an integrated approach to visual pathway diagnosis. Prog Retin Eye Res. 2001;20:531–561. doi: 10.1016/s1350-9462(00)00030-6. [DOI] [PubMed] [Google Scholar]
- 5.Calkins DJ. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog Retin Eye Res. 2012;31:702–719. doi: 10.1016/j.preteyeres.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luo X, Frishman LJ. Retinal pathway origins of the pattern electroretinogram (PERG). Invest Ophthalmol Vis Sci. 2011;52:8571–8584. doi: 10.1167/iovs.11-8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parisi V, Manni G, Centofanti M, Gandolfi SA, Olzi D, Bucci MG. Correlation between optical coherence tomography pattern electroretinogram and visual evoked potentials in open-angle glaucoma patients. Ophthalmology. 2001;108:905–912. doi: 10.1016/s0161-6420(00)00644-8. [DOI] [PubMed] [Google Scholar]
- 8.Bayer AU, Maag KP, Erb C. Detection of optic neuropathy in glaucomatous eyes with normal standard visual fields using a test battery of short- wavelength automated perimetry and pattern electroretinography. Ophthalmology. 2002;109:1350–1361. doi: 10.1016/s0161-6420(02)01100-4. [DOI] [PubMed] [Google Scholar]
- 9.Ventura LM, Porciatti V, Ishida K, Feuer WJ, Parrish RK. Pattern electroretinogram abnormality and glaucoma. Ophthalmology. 2005;112:10–19. doi: 10.1016/j.ophtha.2004.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bach M, Unsoeld AS, Philippin H, Staubach F, Maier P, Walter HS, et al. Pattern ERG as an early glaucoma indicator in ocular hypertension: A long- term, prospective study. Invest Ophthalmol Vis Sci. 2006;47:4881–4887. doi: 10.1167/iovs.05-0875. [DOI] [PubMed] [Google Scholar]
- 11.Parisi V, Miglior S, Manni G, Centofanti M, Bucci MG. Clinical ability of pattern electroretinograms and visual evoked potentials in detecting visual dysfunction in ocular hypertension and glaucoma. Ophthalmology. 2006;113:216–228. doi: 10.1016/j.ophtha.2005.10.044. [DOI] [PubMed] [Google Scholar]
- 12.Porciatti V, Saleh M, Nagaraju M. The pattern electroretinogram as a tool to monitor progressive retinal ganglion cell dysfunction in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2007;48:745–751. doi: 10.1167/iovs.06-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marx MS, Podos SM, Bodis-Wollner I, Howard-Williams JR, Siegel MJ, Teirelbaum CS, et al. Flash and pattern electroretinograms in normal and laser-induced glaucomatous primate eyes. Invest Ophthalmol Vis Sci. 1986;27:378–386. [PubMed] [Google Scholar]
- 14.Marx MS, Podos SM, Bodis-Wollner I, Lee PY, Wang RF, Severin C. Signs of early damage in glaucomatous monkey eyes: low spatial frequency losses in the pattern ERG and VEP. Exp Eye Res. 1988;46:173–184. doi: 10.1016/s0014-4835(88)80075-7. [DOI] [PubMed] [Google Scholar]
- 15.Bode SF, Jehle T, Bach M. Pattern electroretinogram in glaucoma suspects: new findings from a longitudinal study. Invest Ophthalmol Vis Sci. 2011;52:4300–4306. doi: 10.1167/iovs.10-6381. [DOI] [PubMed] [Google Scholar]
- 16.Bach M, Sulimma F, Gerling J. Little correlation of the pattern electroretinogram (PERG) and visual field measures in early glaucoma. Doc Ophthalmol 1997- 1998;94:253–263. doi: 10.1007/BF02582983. [DOI] [PubMed] [Google Scholar]
- 17.Thompson DA, Drasdo N. Computation of the luminance and pattern components of the bar pattern electroretinogram. Doc Ophthalmol. 1987;66:233–244. doi: 10.1007/BF00145237. [DOI] [PubMed] [Google Scholar]
- 18.Bach M, Holder GE. Check size tuning of the pattern electroretingoram:a reappraisal. Doc Ophthalmol 1996- 1997;92:193–202. doi: 10.1007/BF02583290. [DOI] [PubMed] [Google Scholar]
- 19.Zrenner E. The physiological basis of the pattern electroretinogram. Prog Retin Eye Res. 1990;9:427–464. [Google Scholar]
- 20.Bach M, Hoffmann MB. Update on the pattern electroretinogram in glaucoma. Optom Vis Sci. 2008;85:386–395. doi: 10.1097/OPX.0b013e318177ebf3. [DOI] [PubMed] [Google Scholar]
- 21.Ventura LM, Porciatti V. Pattern electroretinogram in glaucoma. Curr Opin Ophthalmol. 2006;17:196–202. doi: 10.1097/01.icu.0000193082.44938.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oner A, Gumus K, Arda H, Karakucuk S, Mirza E. Pattern electroretinographic recordings in eyes with myopia. Eye Contact Lens. 2009;35:238–241. doi: 10.1097/ICL.0b013e3181b343d9. [DOI] [PubMed] [Google Scholar]
- 23.Arden GB, Hamilton AM, Wilson-Holt J, Ryan S, Yudkin JS, Kurtz A. Pattern electroretinograms become abnormal when background diabetic retinopathy deteriorates to a preproliferative stage: possible use as a screening test. Br J Ophthalmol. 1986;70:330–335. doi: 10.1136/bjo.70.5.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robson AG, Michaelides M, Saihan Z, Bird AC, Webster AR, Moore AT, et al. Functional characteristics of patients with retinal dystrophy that manifest abnormal parafoveal annuli of high density fundus autofluorescence; a review and update. Doc Ophthalmol. 2008;116:79–89. doi: 10.1007/s10633-007-9087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heijl A, Leske M, Bengtsson B, Hyman L, Bengtsson B, Hussein M. Reduction of intraocular pressure and glaucoma progression: results from the early manifest glaucoma trial. Arch Ophthalmol. 2002;120:1268–1279. doi: 10.1001/archopht.120.10.1268. [DOI] [PubMed] [Google Scholar]
- 26.Holder GE, Brigell MG, Hawlina M, Meigen T, Bach M. ISCEV standard for clinical pattern electroretinography. Doc Ophthalmol. 2007;114:111–116. doi: 10.1007/s10633-007-9053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.North RV, Jones AL, Drasdo N, Wild JM, Morgan JE. Electrophysiological evidence of early functional damage in glaucoma and ocular hypertension. Invest Ophthalmol Vis Sci. 2010;51:1216–1222. doi: 10.1167/iovs.09-3409. [DOI] [PubMed] [Google Scholar]
- 28.Ventura LM, Sorokac N, Santos RDL, Feuer WJ, Porciatti V. The relationship between retinal ganglion cell function and retinal nerve fiber thickness in early glaucoma. Invest Ophthalmol Vis Sci. 2006;47:3904–3911. doi: 10.1167/iovs.06-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ventura LM, Golubev I, Feuer WJ, Porciatti V. Pattern electroretinogram progression in glaucoma suspects. J Glaucoma. 2013;22:219–225. doi: 10.1097/IJG.0b013e318237c89f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porciatti V, Sorokac N, Buchser W. Habituation of retinal ganglion cell activity in response to steady state pattern visual stimuli in normal subjects. Invest Ophthalmol Vis Sci. 2005;46:1296–1302. doi: 10.1167/iovs.04-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jonas JB, Schmidt AM, Muller-Bergh JA, Schldrzer-Schlotzer UM, Naumann GO. Human optic nerve fiber count and optic disc size. Invest Ophthalmol Vis Sci. 1992;33:2012–2018. [PubMed] [Google Scholar]
- 32.Bach M. Electrophysiological approaches for early detection of glaucoma. Eur J Ophthalmol. 2001;11(Suppl 2):S41–S49. doi: 10.1177/112067210101102s05. [DOI] [PubMed] [Google Scholar]
- 33.Hood DC, Xu L, Thienprasiddhi P, Greenstein VC, Odel JG, Grippo TM, et al. The pattern electroretinogram in glaucoma patients with confirmed visual field deficits. Invest Ophthalmol Vis Sci. 2005;46:2411–2418. doi: 10.1167/iovs.05-0238. [DOI] [PubMed] [Google Scholar]
- 34.Bode SF, Jehle T, Bach M. Pattern electroretinogram in glaucoma suspects: new findings from a longitudinal study. Invest Ophthalmol Vis Sci. 2011;52:4300–4306. doi: 10.1167/iovs.10-6381. [DOI] [PubMed] [Google Scholar]
- 35.Porciatti V, Ventura LM. Physiologic significance of steady-state pattern electroretinogram losses in glaucoma: clues from simulation of abnormalities in normal subjects. J Glaucoma. 2009;18:535–542. doi: 10.1097/IJG.0b013e318193c2e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malik R, Swanson WH, Garway-Heath DF. ‘Structure-function relationship’ in glaucoma: past thinking and current concepts. Clin Exp optom. 2012;40:369–380. doi: 10.1111/j.1442-9071.2012.02770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buckingham BP, Inman DM, Lambert W, Oglesby E, Calkins DJ, Steele MR, et al. Progressive ganglion cell degeneration precedes neuronal loss in a mouse model of glaucoma. J Neurosci. 2008;28:2735–2744. doi: 10.1523/JNEUROSCI.4443-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber AJ, Harman CD. Structure-function relations of parasol cells in the normal and glaucomatous primate retina. Invest Ophthalmol Vis Sci. 2005;46:3197–3207. doi: 10.1167/iovs.04-0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan JE. Retina ganglion cell degeneration in glaucoma: an opportunity missed? A review. Clin Experiment Ophthalmol. 2012;40:364–368. doi: 10.1111/j.1442-9071.2012.02789.x. [DOI] [PubMed] [Google Scholar]
- 40.Sehi M, Grewal DS, Goodkin ML, Greenfield DS. Reversal of retinal ganglion cell dysfunction after surgical reduction of intraocular pressure. Ophthalmology. 2010;117:2329–2336. doi: 10.1016/j.ophtha.2010.08.049. [DOI] [PubMed] [Google Scholar]
- 41.Ventura LM, Porciatti V. Restoration of retinal ganglion cell function in early glaucoma after intraocular pressure reduction: a pilot study. Ophthalmology. 2005;112:20–27. doi: 10.1016/j.ophtha.2004.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morquette JB, Polo AD. Dendritic and synaptic protection: is it enough to save the retinal ganglion cell body and axon? J Neuroophthalmol. 2008;28:144–154. doi: 10.1097/WNO.0b013e318177edf0. [DOI] [PubMed] [Google Scholar]