Abstract
Purpose
To report two cases of retinitis pigmentosa (RP) associated with vasoproliferative tumors (VPTs) and Coats-like fundus.
Case Reports
Two patients with RP presented with recent loss of vision due to combined VPTs and Coats-like retinal vascular alterations. One patient had two VPTs with adjacent capillary nonperfusion, telangiectasia and aneurysmal vascular changes in one eye. The other patient had prominent VPT with Coats-like retinal vascular alterations in both eyes. These lesions received treatment resulting in improved vision in both patients.
Conclusion
Although rare, VPTs and Coats-like retinal vascular alterations including retinal exudation associated with telangiectatic vessels, aneurysmal changes and capillary nonperfusion may occur in patients with RP.
Keywords: Capillary Nonperfusion, Coats Disease, Retinitis Pigmentosa, Telangiectasia, Vasoproliferative Tumors
INTRODUCTION
Retinal vasoproliferative tumors (VPTs) are uncommon pinkish-yellow dome-shaped masses in the peripheral retina with vascular and glial components.1,2 They are thought to be reactive proliferations rather than true tumors.2 Approximately 74% of VPTs are idiopathic while 26% are secondary to inflammatory, vascular, traumatic, dystrophic or degenerative ocular disorders. The most common reported causes are chronic untreated pars planitis (28%) and retinitis pigmentosa (21%).1
Retinitis pigmentosa (RP) is a rare dystrophic disease with progressive loss of rod and cone photoreceptors.1,3An association seems to exist between Coats disease and RP.4,5 Although RP has been a frequent (21%) underlying disease in secondary VPTs,1 only a small number of patients with RP develop VPTs.6
Coats disease is a rare idiopathic ocular vascular occlusive disease.7,8 In a large case series of 158 eyes with classic Coats disease VPTs occurred in only 6% of patients. 9
Herein, we report two patients who presented with a rare combination of RP, VPTs and Coats like fundus appearance.
CASE REPORTS
Case 1
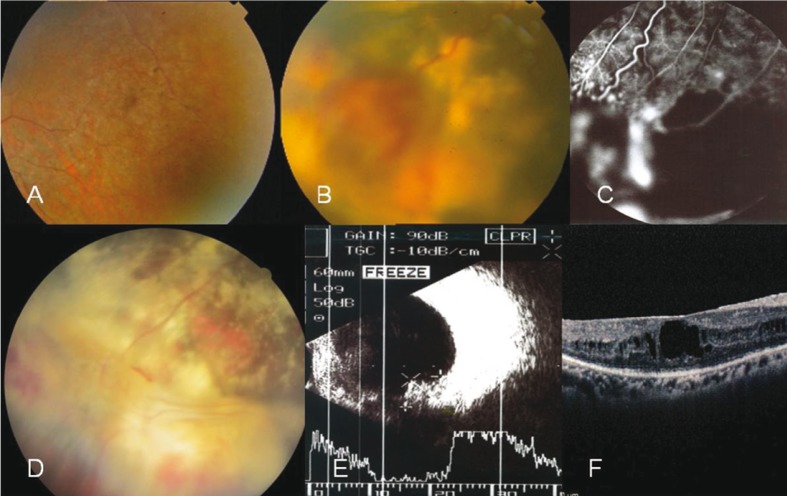
A 30-year-old female and a known case of RP was referred with complaints of recently decreased vision in her right eye. On examination, best corrected visual acuity (BCVA) was 20/200 and slit lamp examination was unremarkable except for trace cells in the anterior vitreous of both eyes. Fundus examination showed typical bilateral bone-spicule pigmentation and macular edema which was later confirmed by optical coherence tomography (OCT) (Fig. 1A). Two VPTs were present in the inferotemporal quadrant of the right eye measuring 8×6×5.5 mm and 4×3.5×2mm with extensive subretinal hard exudates. Fluorescein angiography (FA) showed some areas of capillary nonperfusion in the peripheral retina with telangiectatic and aneurysmal vascular changes around a leaking VPT lesion (Fig. 1). These changes resembled FA changes seen in eyes with Coats disease.
Figure 1.
RP associated with vasoproliferative tumor (VPT) and Coats like fundus appearance. A, Fundus appearance of the right eye in case 1 shows typical RP changes. B, VPT with hard exudates, and preretinal and vitreous hemorrhage around the lesion is present in the same eye. C, Fluorescein angiography reveals multiple points of leakages on the VPT lesion in addition to light bulbs, telangiectatic vessels, localized aneurysmal changes and capillary nonperfused areas around and far from the main lesion. D, Fundus appearance, two months after cryotherapy; note the regressed VPT and coats like fundus appearance, with consequent gliosis and residual hard exudates. E, Ultrasonic appearance of the lesion with high internal reflectivity before treatment. F, OCT findings include full thickness cystic edema involving the macular area and microcystic changes in the inner nuclear layer with deranged macular contour (before treatment).
Laser photocoagulation was applied on the VPT lesions in order to decrease their thickness and improve adjacent areas of nonperfusion. After 2 weeks, complementary cryotherapy of the VPTs was performed and the patient received an intravitreal injection of bevacizumab 2 weeks later. After 2 months, complete regression of the VPTs was noted together with persistent but stable subretinal exudates (Fig. 1D). The macular edema had resolved and visual acuity was improved to 20/60 at the last visit. There was no recurrence of the condition after 2 years of follow up.
Case 2
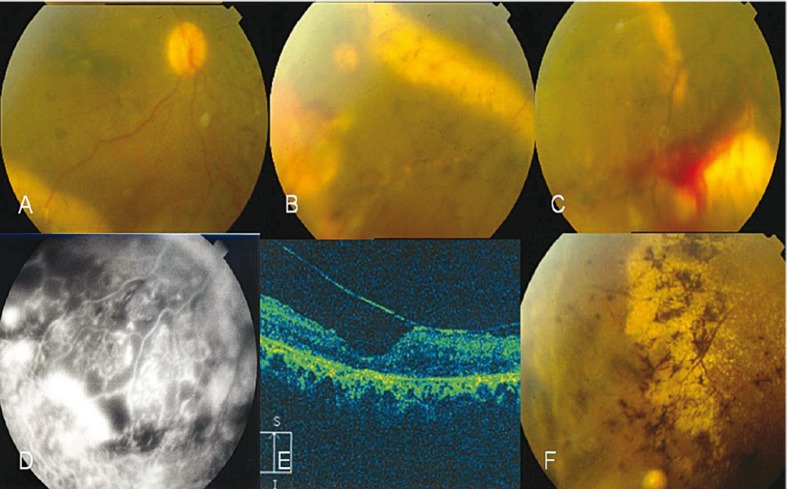
A 31-year-old female previously diagnosed with RP presented to our clinic complaining of decreased vision in her right eye over the past 4 days. BCVA was counting fingers at 1 meter in her right eye and counting fingers at 0.5 meter in the left one. Positive ocular findings included mild posterior subcapsular lens opacities, trace cells in the anterior chambers in both eyes, and trace blood and pigment in the anterior vitreous of the right eye. Fundus examination (Figure 2A-C) revealed typical RP changes in both eyes, and macular distortion and wrinkling in the right eye. An inferotemporal exudative retinal detachment with a VPT lesion measuring 5×5×4 mm was present in the right eye and an inferotemporal VPT measuring 5×4×3mm with surrounding hard exudates was also present in the left eye. We performed FA, ultrasonography and OCT (Figure 2C-G). FA revealed capillary nonperfusion with leaking aneurysmal and telangiectatic vessels not related to RP and the VPT.
Figure 2.
Retinitis pigmentosa (RP) associated with vasoproliferative tumor (VPT) and Coats like fundus in both eyes of case 2. A, Hazy fundus photograph of the right eye shows a dilated vein and far peripheral hard exudates inferotemporally. B, Peripheral view of the fundus in the right eye shows hard exudate precipitations around the VPT along the stuffed venules by hard exudates and telangiectatic vessels on VPT with bony spicular changes. C, VPT with telangiectatic vessels and hard exudates, note preretinal hemorrhages on and around the lesion in the left eye. D, Peripheral lesion with fluorescence surrounding the capillary nonperfusion; note light bulbs and aneurysmal telangiectatic vessels in the right eye. E, Epiretinal membrane with antero-posterior traction in OCT (OD). F, Regressed inferotemporal lesion with consequent gliosis and epiretinal membranous changes (OD).
Laser photocoagulation was performed over the lesions and nonperfused areas in both eyes and after 2 weeks, cryotherapy was performed. A dense vitreous hemorrhage developed in the right eye over the following days which lasted for 3 months. As the condition was disabling, deep vitrectomy with membranectomy and ILM-peeling was performed. After 3 months, the patient showed improvement in retinal appearance (Figure 2G-I). Lesion size and the associated exudates were notably decreased and vision was improved to 20/200 in the right eye but remained unchanged in the left one. There was no recurrence of the condition after 2 years of follow up.
DISCUSSION
VPTs are usually located in the peripheral retina in the inferotemporal quadrant.1 While primary VPTs are single unilateral lesions, secondary tumors are usually bilateral and/ or multiple as observed in our second patient. Given the peripheral location of most lesions, FA is often technically difficult or impossible. FA shows rapid filling of the tumor in the early phases and late increasing hyperfluorescence, compatible with diffuse leakage.1 Telangiectatic dilatations of retinal vessels, retinal exudations, and exudative retinal detachment are common features of VPT and Coats disease. Capillary nonperfusion is a known feature of Coats disease but not a constant finding in VPT lesions.
Based on our findings, we hypothesize that Coats-like vascular changes and VPTs seen in eyes with RP may have a common underlying etiology or may represent different stages of the same disease process.
The term “Coats-type RP” is an atypical form of RP and various studies have suggested that 1%-4% of RP cases will show such a response in later stages of the disease.4,5,10 In the adult type of Coats disease no gender preference is notable.10 The cause of the inferior predilection for VPT and telangiectatic vessels remains to be determined.
In conclusion, although such an association is rare, both Coats-like retinal vascular alterations and VPTs may develop in patients with RP.
Footnotes
Conflicts of Interest
None.
REFERENCES
- 1.Shields CL, Shields JA, Barrett J, De Potter P. Vasoproliferative tumors of the ocular fundus. Classification and clinical manifestations in 103 patients. Arch Ophthalmol. 1995;113:615–623. doi: 10.1001/archopht.1995.01100050083035. [DOI] [PubMed] [Google Scholar]
- 2.Hiscott P, Mudhar H. Is vasoproliferative tumour (reactive retinal glioangiosis) part of the spectrum of proliferative vitreoreinopathy? Eye (Lond) 2009;23:1851–1858. doi: 10.1038/eye.2008.351. [DOI] [PubMed] [Google Scholar]
- 3.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 4.Morgan WE, Crawford JB. Retinitis pigmentosa and Coats’ disease. Arch Ophthalmol. 1968;79:146–149. doi: 10.1001/archopht.1968.03850040148006. [DOI] [PubMed] [Google Scholar]
- 5.Khan JA, Ide CH, Strickland MP. Coats’-type retinitis pigmentosa. Surv Ophthalmol. 1988;32:317–332. doi: 10.1016/0039-6257(88)90094-x. [DOI] [PubMed] [Google Scholar]
- 6.Medlock RD, Shields JA, Shields CL, Yarian DL, Beyrer CR. Retinal hemangioma-like lesions in eyes with retinitis pigmentosa. Retina. 1990;10:274–277. doi: 10.1097/00006982-199010000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Morris B, Foot B, Mulvihill A. A population-based study of Coats disease in the United Kingdom I: epidemiology and clinical features at diagnosis. Eye (Lond) 2010;24:1797–1801. doi: 10.1038/eye.2010.126. [DOI] [PubMed] [Google Scholar]
- 8.London NJ, Shields CL, Haller JA. Coats Disease. In: Ryan SJ, editor. Retina. 4th ed. Philadelphia: Mosby; 2013. pp. 1058–1070. [Google Scholar]
- 9.Shields JA, Shields CL, Honavar SG, Demirci H. Clinical variations and complications of Coats disease in 150 cases: the 2000 Sanford Gifford Memorial Lecture. Am J Ophthalmol. 2001;131:561–571. doi: 10.1016/s0002-9394(00)00883-7. [DOI] [PubMed] [Google Scholar]
- 10.Kan E, Yilmaz T, Aydemir O, Güler M, Kurt J. Coats-like retinitis pigmentosa: Reports of three cases. Clin Ophthalmol. 2007;1:193–198. [PMC free article] [PubMed] [Google Scholar]