Abstract
Patients with permanent pacemaker or automatic implantable cardioverter-defibrillator (AICD) leads have an increased prevalence of tricuspid regurgitation. However, the roles of cardiac rhythm and lead-placement duration in the development of severe tricuspid regurgitation are unclear.
We reviewed echocardiographic data on 26 consecutive patients who had severe tricuspid regurgitation after permanent pacemaker or AICD placement; before treatment, they had no organic tricuspid valve disease, pulmonary hypertension, left ventricular dysfunction, or severe tricuspid regurgitation. We compared the results to those of 26 control subjects who had these same devices but no more than mild tricuspid regurgitation.
The patients and control subjects were similar in age (mean, 81 ±6 vs 81 ±8 yr; P = 0.83), sex (male, 42% vs 46%; P = 0.78), and left ventricular ejection fraction (0.60 ±0.06 vs 0.58 ± 0.05; P = 0.4). The patients had a higher prevalence of atrial fibrillation (92% vs 65%; P=0.01) and longer median duration of pacemaker or AICD lead placement (49.5 vs 5 mo; P < 0.001). After adjusting for age, sex, and right ventricular systolic pressure by multivariate logistic regression analysis, we found that atrial fibrillation (odds ratio=6.4; P = 0.03) and duration of lead placement (odds ratio=1.5/yr; P = 0.001) were independently associated with severe tricuspid regurgitation.
Out study shows that atrial fibrillation and longer durations of lead placement might increase the risk of severe tricuspid regurgitation in patients with permanent pacemakers or AICDs.
Key words: Atrial fibrillation/complications; cardiac pacing, artificial/adverse effects; defibrillators, implantable/adverse effects; disease progression; echocardiography; electrodes, implanted/adverse effects; pacemaker, artificial/adverse effects; risk factors; tricuspid valve insufficiency/diagnosis/etiology; ventricular dysfunction, right/diagnosis/etiology
Patients with permanent pacemaker (PPM) or automatic implantable cardioverter-defibrillator (AICD) leads have an increased prevalence of significant tricuspid regurgitation (TR). Tricuspid regurgitation can be caused by various mechanisms, including pulmonary hypertension, direct lead interference with valve closure, tricuspid valve leaflet trauma from laceration and perforation, infective endocarditis, and fibrous adherence of pacemaker leads to the tricuspid valve apparatus.1 Patients with long-standing atrial fibrillation (AF) can also develop severe TR even when their tricuspid valves are structurally normal.2 In addition, patients with PPM leads have an increased prevalence of TR.3 Investigators in several large studies concluded that having a PPM or AICD increases the degree of TR in some patients.1,3–5 However, it is unclear whether the chronic presence of device leads results in increased TR. We therefore sought to determine the predictors of severe TR in patients who have PPM or AICD leads. We hypothesized that a longer duration of PPM or AICD lead placement and the presence of AF were the chief risk factors.
Patients and Methods
We obtained our study population by conducting a retrospective search of our echocardiographic laboratory's database to identify consecutive patients who had undergone transthoracic echocardiographic examination at our clinic from May 2002 through October 2009. We identified 589 patients who had been diagnosed with severe TR in accordance with the guidelines of the American Society of Echocardiography (ASE).6 Of these patients, we excluded 563 because they had common organic or secondary causes of TR. The exclusionary criteria were congenital heart disease, more-than-moderate left-sided valvular heart disease, heart transplantation, right ventricular (RV) infarction, prior valvular surgery, severe pulmonary hypertension (RV systolic pressure, >50 mmHg), carcinoid or rheumatic heart disease, the absence of a PPM or AICD, more-than-moderate TR before PPM or AICD implantation, and severe TR that was chiefly due to left ventricular dysfunction. The remaining 26 patients with severe TR and a PPM or AICD constituted the group of patients in our study. Using similar exclusionary criteria, we identified 26 control subjects from our pacemaker clinic who had a PPM or AICD but no more than mild TR as defined in the ASE guidelines. The 2 groups were compared. All echocardiographic measurements were retrospective and had been recorded at the time of the relevant echocardiogram; however, right atrial volumes and tricuspid annular diameters were measured by other means. When multiple echocardiograms were available for review, data were abstracted from the first one that showed severe TR. This retrospective study was approved by the Mayo Clinic Institutional Review Board.
Clinical Data and Echocardiographic Measurements
The demographic characteristics and the clinical and echocardiographic data obtained from the medical records included age, sex, race, and the duration of PPM or AICD implantation; and diagnoses or known histories of AF, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, cerebrovascular accident or transient ischemic attack, coronary artery disease, and hypercholesterolemia (Table I). Standard 2-dimensional and color-flow Doppler echocardiograms, obtained with use of the ACUSON Sequoia™ C512 ultrasound system (Siemens Medical Solutions USA, Inc.; Mountain View, Calif) or the Vivid 7® cardiac ultrasonography system (GE Healthcare; Wausheka, Wis), were interpreted by experienced staff echocardiologists. In accordance with ASE guidelines, TR severity was determined by the size of the TR jet on color-flow images, the relative size of the regurgitant flow in comparison with right atrial area, and hepatic vein systolic flow reversal.7 Left atrial volume was calculated by means of the modified Simpson's rule.8 Right atrial pressure was estimated in accordance with inferior vena caval diameter and the effect of respiration on the diameter.9 Right ventricular size and function were evaluated qualitatively, and RV systolic pressure was estimated through use of the modified Bernoulli equation. Left ventricular ejection fraction was obtained by means of the modified Simpson's rule, from apical 4-and 2-chamber views.8 ProSolv® CardioVascular version 3.0 (ProSolv CardioVascular Inc.; Indianapolis, Ind), an offline data-management system, was used to collect right atrial volume and tricuspid annular diameter data, because these had not been part of the routine echocardiographic studies. Right atrial volume was determined from the apical view by means of the modified Simpson's rule. Tricuspid annular diameter was measured in the apical 4-chamber view during diastole from the point of insertion of the septal tricuspid leaflet to the insertion of the anterior tricuspid leaflet.10
TABLE I. Baseline Clinical Characteristics
Twenty-five patients (96%) had one RV lead, and one patient had 2 RV leads. All 26 control subjects had one RV lead. Pacemaker leads were present in 22 patients (85%) and in 24 control subjects (92%). Automatic implantable cardioverter-defibrillator leads were present in 4 patients (15%) and 2 control subjects (8%). Data on RV apical pacing were collected.
Statistical Analysis
Continuous variables are expressed as mean ± SD, and categorical variables as number and percentage. Differences between groups were determined by means of the 2-sample t test or Wilcoxon rank sum test (for continuous data) and the χ2 test (for categorical data). Multivariate logistic regression analysis was used to identify factors associated with the development of severe TR in the patients. Odds ratios (ORs) and 95% confidence intervals (CIs) were reported for continuous and categorical data. A P value <0.05 was considered to be statistically significant. All analyses were performed with the use of JMP® Pro statistical software version 8.0.2 (SAS Institute Inc.; Cary, NC).
Results
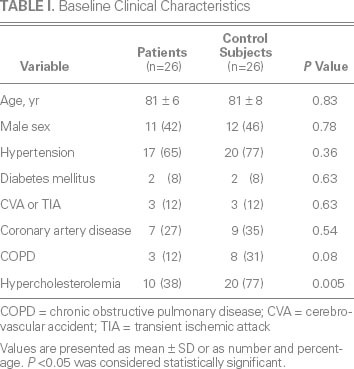
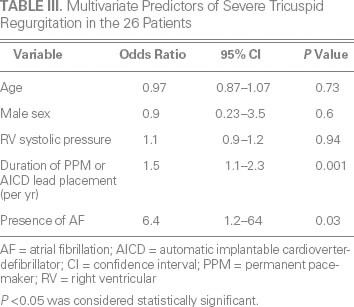
Age, sex, and all comorbid conditions except for hypercholesterolemia were similar between the patients and control subjects (Table I). Left ventricular ejection fraction was similar between the groups (Table II). Median left atrial volume (48 vs 36 mL/m2; P = 0.01), right atrial volume (76 vs 36 mL; P < 0.001) (Fig. 1A), and tricuspid annular diameter (3.7 vs 2.8 cm; P < 0.001) (Fig. 1B) were larger in the patients than in the control subjects. In addition, median RV systolic pressure was slightly higher in the patients (39 vs 31 mmHg; P = 0.04). Neither the number nor the type of leads (AICD vs PPM) differed between the patients and control subjects (P = 0.23 and P = 0.38, respectively). The patients had a significantly higher prevalence of RV apical pacing than did the control subjects (74.9% ± 33% vs 39.7% ± 42%; P = 0.003). The patients also had a higher prevalence of AF (92% vs 65%; P = 0.01) (Fig. 2) and a longer median duration of lead placement (49.5 vs 5 mo; P < 0.001) (Fig. 3). After adjustment for age, sex, and RV systolic pressure in a multivariate logistic regression model (Table III), only the duration of lead placement (OR=1.5/year; 95% CI, 1.1–2.3; P = 0.001) and the presence of AF (OR=6.4; 95% CI, 1.2–64; P = 0.03) were independently associated with severe TR in patients with a PPM or AICD.
TABLE II. Comparison of Echocardiographic Characteristics between Patients and Control Subjects
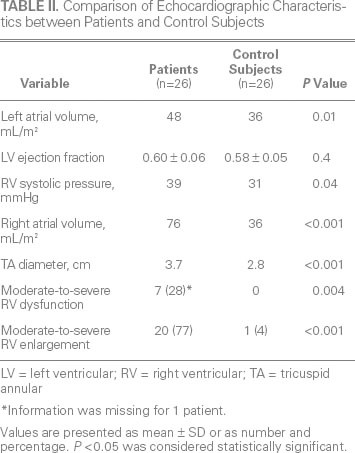
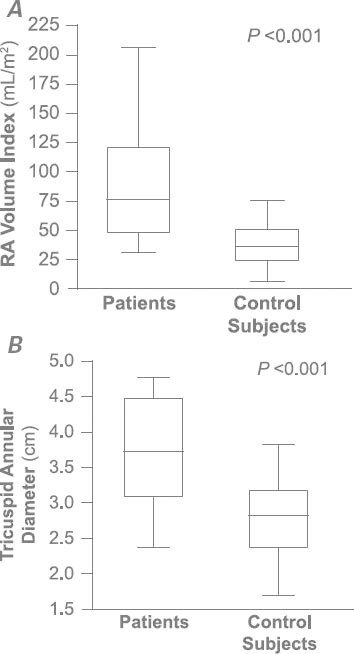
Fig. 1 Patients with permanent pacemaker or automatic implantable cardioverter-defibrillator leads had larger A) right atrial (RA) volumes and B) tricuspid annular diameters than did the control subjects (both P < 0.001).
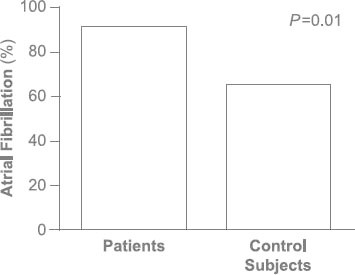
Fig. 2 The prevalence of atrial fibrillation in patients with permanent pacemaker or automatic implantable cardioverter-defibrillator leads was 92%, compared with 65% in the control subjects (P = 0.01).
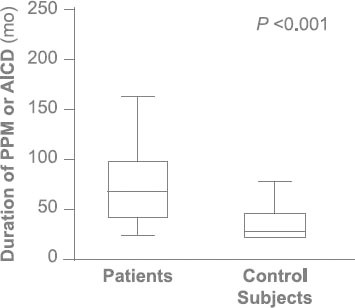
Fig. 3 The median duration of device placement in patients with permanent pacemaker (PPM) or automatic implantable cardioverter-defibrillator (AICD) leads was 49.5 months, compared with 5 months in the control subjects (P < 0.001).
TABLE III. Multivariate Predictors of Severe Tricuspid Regurgitation in the 26 Patients
Discussion
Our study shows that severe TR can occur in patients with a PPM or AICD long after the implantation of device leads, particularly in the presence of AF. After adjustment for age, sex, and RV systolic pressure, the risk of a patient's developing severe TR had an OR of 1.5 for each year of having had the PPM or AICD leads in place, and an OR of 6.4 in the presence of AF.
Data in other studies conflicted in regard to whether the presence of PPM or AICD leads by itself resulted in TR.3,11 Results of one study11 suggested that the degree of TR did not change after PPM or AICD implantation. However, this study had major limitations in that the pertinent echocardiograms were obtained within a short time after device implantation (mean, 1.2 d). Of more importance, there was no statistical comparison of the grades of TR before and after device placement. In contrast, the results of several other larger studies1,3–5 clearly showed that the presence of a PPM or AICD lead increased the degree of TR in some patients.
Few investigators have evaluated risk factors for the development of severe TR in patients who have PPM or AICD leads. In one observational study,5 AICD leads increased TR severity more than PPM leads did. In another study,12 3 or more leads contributed to a higher prevalence of TR, suggesting that either the caliber or the number of leads going through the tricuspid valve leaflets affected the severity of TR.
Additional factors might contribute to an increased degree of TR in patients who have PPM or AICD leads. Klutstein and colleagues4 retrospectively compared 75 patients whose TR increased more than 2 grades with 335 patients whose TR increased less than 2 grades after the implantation of a PPM or AICD. The investigators found that older patients, patients with abnormal left ventricular relaxation, and patients in whom pulmonary hypertension subsequently developed had more severe TR after device leads were implanted. In a cross-sectional study of more than 2,000 patients,7 pulmonary hypertension was shown to be a strong determinant of the severity of TR.
In our case-control study, after we excluded patients with severe pulmonary hypertension and evaluated the influence of heart rhythm and the duration of device-lead placement on the development of severe TR, we found that AF and the duration of lead placement were independent determinants of the development of severe TR. Similarly, Mutlak and colleagues13 suggested previously that age, AF, and annular dilation are the likely mechanisms of severe TR. However, their conclusions were limited because their study lacked a control group. In addition, their study did not consider the effect of implantation duration on the development of severe TR.
Why does AF contribute so extensively to increased degrees of TR in patients with PPM or AICD leads (more than a 6-fold risk)? Atrial fibrillation has been associated with severe functional TR, even in the absence of device leads, because of annular dilation.6 A similar mechanism was implicated when Shiran and Sagie14 showed that increased TR is observed more often in patients who do not undergo a maze procedure at the time of mitral valve surgery. As we have shown,2 chronic AF is a risk factor for the development of severe TR—even in the absence of device leads—in patients who have anatomically normal tricuspid valves. In our current study, the same mechanism probably played a clinically significant role in our patients who had device leads, perhaps because of underdevelopment of the fibrous skeleton in the tricuspid annulus.6
Because RV apical pacing increases mitral regurgitation, we were curious whether it also increased TR. Our 26 patients indeed had a significantly higher prevalence of RV apical pacing than did our control subjects (74.9% ± 33% vs 39.7% ± 42%; P = 0.003). Therefore, in addition to AF and the extended presence of device leads, it is likely that RV apical pacing contributed to the development of TR. Further studies are needed in this regard.
Although it was not possible in our current study to evaluate other possibly influential factors (such as leaflet perforation, lead impingement, or lead entanglement), these complications do worsen over time and could in turn contribute to increased degrees of TR in patients who have a PPM or AICD.1,15
One consequence of the severe TR in our 26 patients was that more of them had moderate-or-greater RV dysfunction than did our control subjects (7 vs 0). Although RV systolic pressure was univariately associated with severe TR in patients with device leads, it was not statistically significant in a multivariate model. The design of our study excluded patients with pulmonary hypertension; accordingly, we could not evaluate the contribution of pulmonary pressures to the development of severe TR.
We did not evaluate the outcomes of our patients; however, patients with severe TR have a worse prognosis than do patients with lower grades of TR.16 This suggests that PPM or AICD implantation in patients with chronic AF could worsen their prognosis because of the development of severe TR.
To our knowledge, this study is the first to evaluate the impact of AF and the effect of the duration of PPM or AICD lead placement on the development of severe TR. We used strict inclusionary criteria to evaluate only patients with isolated TR and to exclude patients with most of the common causes of organic and secondary TR. Our study has the usual limitations of a retrospective study, along with a small sample size. In addition, the case-control design of our study is subject to the limitations of any case-control study.
We conclude that the duration of PPM or AICD lead placement and the presence of AF could be risk factors for the development of severe TR in patients who have PPM or AICD leads. Large prospective studies are needed to further examine the mechanism of severe TR in similar patients.
Footnotes
Address for reprints: Hari P. Chaliki, MD, Division of Cardiovascular Diseases, Mayo Clinic, 13400 E. Shea Blvd., Scottsdale, AZ 85259.
E-mail: chaliki.hari@mayo.edu
References
- 1.Lin G, Nishimura RA, Connolly HM, Dearani JA, Sundt TM 3rd, Hayes DL. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J Am Coll Cardiol 2005;45(10):1672–5. [DOI] [PubMed]
- 2.Najib MQ, Vinales KL, Vittala SS, Challa S, Lee HR, Chaliki HP. Predictors for the development of severe tricuspid regurgitation with anatomically normal valve in patients with atrial fibrillation. Echocardiography 2012;29(2):140–6. [DOI] [PubMed]
- 3.Paniagua D, Aldrich HR, Lieberman EH, Lamas GA, Agatston AS. Increased prevalence of significant tricuspid regurgitation in patients with transvenous pacemaker leads. Am J Cardiol 1998;82(9):1130–2, A9. [DOI] [PubMed]
- 4.Klutstein M, Balkin J, Butnaru A, Ilan M, Lahad A, Rosenmann D. Tricuspid incompetence following permanent pacemaker implantation. Pacing Clin Electrophysiol 2009;32 Suppl 1:S135–7. [DOI] [PubMed]
- 5.Kim JB, Spevack DM, Tunick PA, Bullinga JR, Kronzon I, Chinitz LA, Reynolds HR. The effect of transvenous pacemaker and implantable cardioverter defibrillator lead placement on tricuspid valve function: an observational study. J Am Soc Echocardiogr 2008;21(3):284–7. [DOI] [PubMed]
- 6.Zhou X, Otsuji Y, Yoshifuku S, Yuasa T, Zhang H, Takasaki K, et al. Impact of atrial fibrillation on tricuspid and mitral annular dilatation and valvular regurgitation. Circ J 2002;66 (10):913–6. [DOI] [PubMed]
- 7.Mutlak D, Aronson D, Lessick J, Reisner SA, Dabbah S, Agmon Y. Functional tricuspid regurgitation in patients with pulmonary hypertension: is pulmonary artery pressure the only determinant of regurgitation severity? Chest 2009;135 (1):115–21. [DOI] [PubMed]
- 8.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18(12):1440–63. [DOI] [PubMed]
- 9.Rudski LG, Lai WW, Afilalo J, Hua L, Handschumacher MD, Chandrasekaran K, et al. Guidelines for the echocardiographic assessment of the right heart in adults: a report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J Am Soc Echocardiogr 2010;23(7):685–713. [DOI] [PubMed]
- 10.Hannoush H, Fawzy ME, Stefadouros M, Moursi M, Chaudhary MA, Dunn B. Regression of significant tricuspid regurgitation after mitral balloon valvotomy for severe mitral stenosis. Am Heart J 2004;148(5):865–70. [DOI] [PubMed]
- 11.Leibowitz DW, Rosenheck S, Pollak A, Geist M, Gilon D. Transvenous pacemaker leads do not worsen tricuspid regurgitation: a prospective echocardiographic study. Cardiology 2000;93(1–2):74–7. [DOI] [PubMed]
- 12.de Cock CC, Vinkers M, Van Campe LC, Verhorst PM, Visser CA. Long-term outcome of patients with multiple (> or = 3) noninfected transvenous leads: a clinical and echocardiographic study. Pacing Clin Electrophysiol 2000;23(4 Pt 1):423–6. [DOI] [PubMed]
- 13.Mutlak D, Lessick J, Reisner SA, Aronson D, Dabbah S, Agmon Y. Echocardiography-based spectrum of severe tricuspid regurgitation: the frequency of apparently idiopathic tricuspid regurgitation. J Am Soc Echocardiogr 2007;20(4):405–8. [DOI] [PubMed]
- 14.Shiran A, Sagie A. Tricuspid regurgitation in mitral valve disease: incidence, prognostic implications, mechanism, and management. J Am Coll Cardiol 2009;53(5):401–8. [DOI] [PubMed]
- 15.Sakai M, Ohkawa S, Ueda K, Kin H, Watanabe C, Matsushita S, et al. Tricuspid regurgitation induced by transvenous right ventricular pacing: echocardiographic and pathological observations [in Japanese]. J Cardiol 1987;17(2):311–20. [PubMed]
- 16.Nath J, Foster E, Heidenreich PA. Impact of tricuspid regurgitation on long-term survival. J Am Coll Cardiol 2004;43(3):405–9. [DOI] [PubMed]


