Abstract
Residual muscular ventricular septal defects are surgical challenges, especially after the repair of complex congenital heart defects. We investigated perventricular device closure as a salvage technique in pediatric patients who had postoperative residual muscular ventricular septal defects.
From February 2009 through June 2011, 14 pediatric patients at our hospital had residual muscular ventricular septal defects after undergoing surgical repair of complex congenital heart defects. Ten patients met our criteria for perventricular device closure of the residual defects: significant left-to-right shunting (Qp/Qs >1.5) or substantial hemodynamic instability (a defect ≥2 mm in size). The patients' mean age was 20.4 ± 13.5 months, and their mean body weight was 10 ± 3.1 kg. The median diameter of the residual defects was 4.2 mm (range, 2.5–5.1 mm).
We deployed a total of 11 SQFDQ-II Muscular VSD Occluders (Shanghai Shape Memory Alloy Co., Ltd.; Shanghai, China) in the 10 patients, in accord with conventional techniques of perventricular device closure. The mean procedural duration was 31.1 ±9.1 min. We recorded the closure and complication rates perioperatively and during a 12-month follow-up period. Complete closure was achieved in 8 patients; 2 patients had persistent trivial residual shunts. No deaths, conduction block, device embolism, or other complications occurred throughout the study period.
We conclude that perventricular device closure is a safe, effective salvage treatment for postoperative residual muscular ventricular septal defects in pediatric patients. Long-term studies with larger cohorts might further confirm this method's feasibility.
Key words: Cardiac surgical procedures/instrumentation/methods; equipment design; heart defects, congenital/adverse effects; heart septal defects, ventricular/complications/pathology/therapy; hemodynamics; postoperative complications; prostheses and implants; surgical procedures, minimally invasive/methods; treatment outcome
Muscular ventricular septal defects (VSDs) constitute approximately 20% of all VSDs1 and are possibly associated with other complex congenital heart conditions, such as tetralogy of Fallot and double-outlet right ventricle.2 Surgical closure is the mainstay treatment for most VSDs; however, muscular VSDs cannot be approached adequately without a ventricular incision, especially when associated with a large left-to-right shunt, pulmonary hypertension, or interventricular septal hypertrophy.3–5 After complex congenital heart defects with severe left-to-right shunting are repaired, residual muscular VSDs can significantly increase the patient's risk of death.4,5
Particularly in pediatric patients, postoperative residual muscular VSD poses a surgical challenge. Open surgical repair is associated with repeat cardiopulmonary bypass (CPB), the risk of ventriculotomy because of an inadequate operative field of vision, and postoperative ventricular dysfunction.5,6 Fluoroscopically guided transcatheter device closure is another treatment option; however, restricted vascular access and the technical difficulty of this procedure are well documented in pediatric patients. Radiation exposure and hemodynamic instability during device positioning can also compromise the prognosis.2,7
Perventricular device closure of VSDs with transesophageal echocardiographic (TEE) guidance was first reported by Amin and colleagues.8 This method enables direct access, which is especially suitable in closing apical muscular VSDs.9 We investigated the feasibility of perventricular device closure as a salvage procedure in pediatric patients who had residual muscular VSDs after having undergone repair of complex congenital heart defects.
Patients and Methods
From February 2009 through June 2011, 14 pediatric patients at our hospital were echocardiographically diagnosed to have residual muscular VSD after having undergone repair of complex congenital heart defects. We enrolled the 10 patients who met our criteria for perventricular device closure: substantial hemodynamic instability (a muscular VSD ≥2 mm in size) or significant left-to-right shunting (Qp/Qs >1.5). The mean age of the 10 patients was 20.4 ± 13.5 months, and their mean body weight was 10 ± 3.1 kg (Table I). Residual muscular VSD had been confirmed in 6 patients during the original corrective procedure before chest closure, and 4 patients had been thus diagnosed while recovering in the intensive care unit (ICU).
TABLE I. Preprocedural Data on the 10 Patients
Informed consent was obtained from the patients' parents. This study was approved by the Ethics Committee of our hospital.
Devices Used
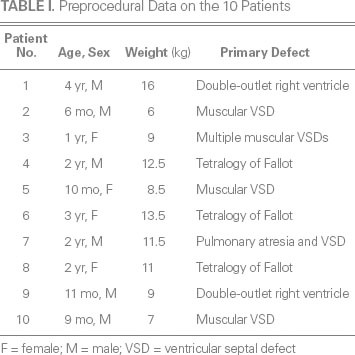
In this study, we used the SQFDQ-II Muscular VSD Occluder and its delivery system (Shanghai Shape Memory Alloy Co., Ltd.; Shanghai, People's Republic of China) (Fig. 1). The self-expandable occluder is made of 0.004-in nitinol wires woven to form 2 symmetric discs with a short connecting waist. Dacron is sewn inside each disc to prevent left-to-right shunting.
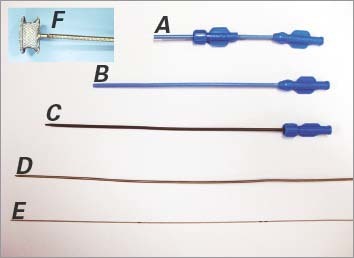
Fig. 1 Photographs show the A) device loading sheath, B) delivery sheath (outer layer), C) delivery sheath (inner layer), D) device cable, and E) guidewire for the F) muscular ventricular septal defect occluder (Shanghai Shape Memory Alloy Co., Ltd.; Shanghai, People's Republic of China).
Echocardiographic Guidance
Using an iE33 xMATRIX Echocardiography System with pediatric probe S7-2t (Koninklijke Philips N.V.; Best, The Netherlands), we performed TEE to evaluate the location and diameter of each patient's muscular VSD. The moderator band was used as a landmark to divide the apical region of the muscular ventricular septum into 2 zones: the right ventricular (RV) infundibular region and the RV inflow region (Fig. 2).10 For muscular VSDs in the RV infundibular region, the midesophageal 4- or 5-chamber view was optimal for evaluation and real-time guidance. For defects in the RV inflow region, the transgastric short-axis view was optimal.
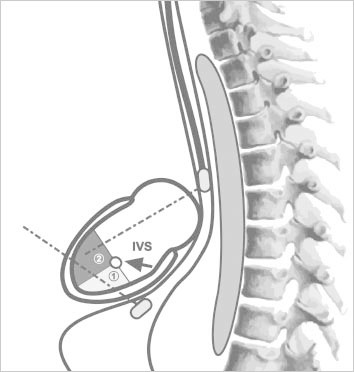
Fig. 2 Diagram shows the right ventricular surface of the interventricular septum (IVS). A mid-muscular ventricular septal defect is in the muscular septal region above the moderator band. With use of the moderator band as a landmark (arrow), the apical portion of the IVS is distinguishable as the right ventricular inflow region (1) and the infundibular region (2).
Closure Technique
Each patient was placed under general anesthesia. Median sternotomy was performed in the 4 patients whose chests were not already open, and all 10 patients were given 1 mg/kg of heparin for systemic anticoagulation. In accordance with each defect's location and diameter, we selected an occluder about 0.5 to 1.5 mm larger than the defect. The technique of perventricular device closure has been described.9,10 The surgeon indented the free wall of the RV with his finger until a position for the RV puncture site was found, perpendicular to the plane of the VSD and clear of the papillary muscles. A pledgeted 4-0 Prolene purse-string suture was placed around the chosen site, and a 20G needle was inserted into the RV cavity in the center of this area. Under TEE guidance, a flexible 0.035-in guidewire was introduced into the RV through the needle and was maneuvered through the VSD into the left ventricle (LV). The needle was removed, and a double-layered delivery sheath was advanced over the wire into the LV. The guidewire and inner layer of the sheath were retrieved together; the outer sheath was left in position, to guide further delivery. During the procedure, the purse-string suture was tightened to prevent excessive blood loss. The previously prepared loader sheath, which contained the occluder device, was connected to the free end of the delivery sheath. The occluder was advanced into the LV, and the left and right discs of the occluder were deployed in stepwise fashion (Figs. 3, 4 and 5). Residual shunting and device-induced valvular regurgitation were checked by means of TEE; if no complications were found, the loader sheath and carrier cable were withdrawn simultaneously. In the patient who had 2 muscular VSDs, we used only one puncture site, to avoid RV dysfunction. The purse-string suture on the RV free wall was tied. The chest was closed, and the patient was taken to the ICU. We recorded the procedural time in each case, from the start of device closure through successful deployment and retrieval of the delivery system. In addition, we determined each patient's left ventricular ejection fraction (LVEF) immediately after the procedure.
Fig. 3 Patient 1. Transesophageal echocardiograms in color-flow Doppler mode show A) an infundibular apical muscular ventricular septal defect after repair of double-outlet right ventricle; B) the guidewire, passed through the defect into the left ventricle; C) the delivery sheath; and D) device closure of the defect.
LA = left atrium; LV = left ventricle; RA = right atrium; RV = right ventricle
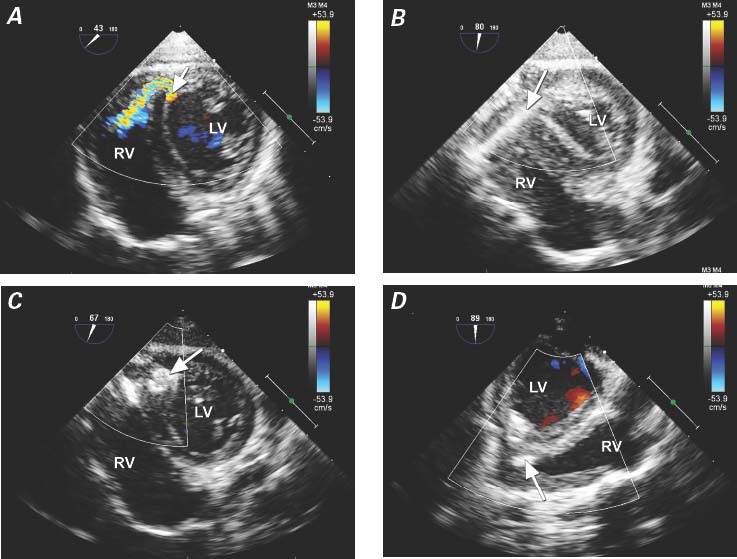
Fig. 4 Patient 6. Transesophageal echocardiograms show a V-shaped apical muscular ventricular septal defect in the right ventricular (RV) inflow region after repair of tetralogy of Fallot. A) Color-flow Doppler mode reveals the defect (arrow) in the posterior portion of the interventricular septum. B) The guidewire (arrow) is passed through the interventricular septum into the left ventricle (LV) by means of a perventricular approach. C) Transgastric short-axis view shows the occluder (arrow), with its left disc not fully opened in the LV side after deployment. D) Midesophageal long-axis view shows the occluder after deployment (arrow).
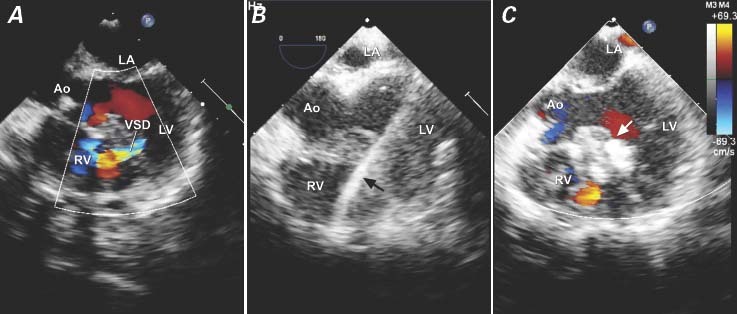
Fig. 5 Patient 8. Transesophageal echocardiograms show the perventricular closure of a residual mid-muscular ventricular septal defect (VSD) after repair of tetralogy of Fallot. A) Color-flow Doppler mode reveals the VSD. B) The guidewire (arrow) is passed through the VSD. C) A 6-mm occluder is deployed (arrow).
Ao = aorta; LA = left atrium; LV = left ventricle; RV = right ventricle
Data Collection
We recorded deaths, perioperative general and procedure-related complications, and lengths of ICU and hospital stays. We defined general complications as those related to the patient's primary congenital disease. Procedure-related complications (those directly due to device closure) included hemodynamic abnormalities, arrhythmias, excessive bleeding during device deployment, residual shunting after the procedure, device embolism or malpositioning that necessitated surgical retrieval, and ventricular dysfunction caused by the procedure.
Follow-Up Protocol. Standard 12-lead electrocardiography (ECG), chest radiography, and transthoracic echocardiography were routinely performed on postoperative day 2 or 3, before each patient's discharge from the hospital, and at 3, 6, and 12 months thereafter.
Statistical Analysis
Data are expressed as median and range for non-normal data, and as mean ± SD for continuous variables. We used SPSS 16.0 for Windows (IBM Corporation; Armonk, NY) for statistical computations.
Results
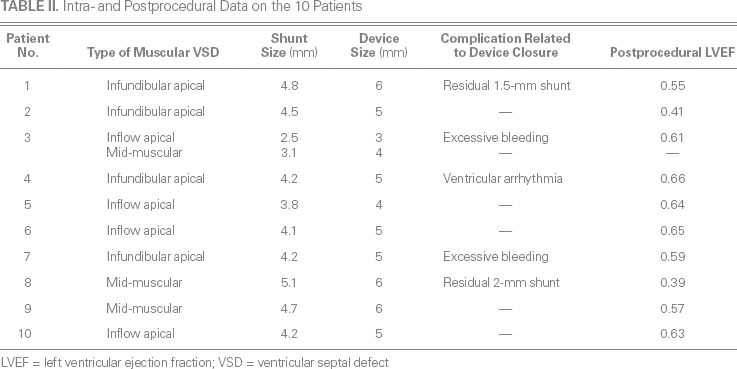
Table II shows intra- and postprocedural data on the patients. Mid-muscular VSD was confirmed in 3 patients (patients 3, 8, and 9), RV infundibular apical muscular VSD in 4 (patients 1, 2, 4, and 7), and RV inflow apical muscular VSD in 4 (patients 3, 5, 6, and 10). The median VSD diameter was 4.2 mm (range, 2.5–5.1 mm). Each defect was closed. The median size of the occluder device was 5 mm (range, 3–6 mm).
TABLE II. Intra- and Postprocedural Data on the 10 Patients
Complications and Outcomes
General Complications. Postprocedural low cardiac output syndrome in patients 2 and 8 resolved after prolonged inotropic support (one patient had LV dysfunction and the other biventricular dysfunction—conditions known before perventricular closure). Acute renal failure in patient 8 resolved after peritoneal dialysis.
Procedure-Related Complications. No patients died. Patient 4 experienced transient ventricular tachycardia and subsequent hemodynamic abnormalities during device closure, but these resolved spontaneously after the delivery system was retrieved. Patients 3 and 7 needed transfusions to overcome excessive blood loss of about 100 mL each during prolonged procedural times. In patients 1 and 8, TEE showed that persistent residual shunts developed (1.5 and 2 mm, respectively) after device implantation. No conduction block, valvular regurgitation, or device embolism was detected in any patient. Postprocedural LVEFs were satisfactory in most patients, indicating that perventricular device closure could avoid the need for secondary CPB and prevent myocardial dysfunction.
Procedural Duration. The mean procedural duration was 31.1 ± 9.1 min (range, 20–45 min). Patients 3 and 7 had the longest procedural times (40 and 45 min, respectively).
Lengths of Stay. The median length of stay in the ICU was 4 days (range, 3–11 d). Patient 2 had a prolonged ICU stay (>10 d) because of a pulmonary infection. The median time of hospitalization was 8 days (range, 7–14 d). Except for patient 2, length of stay was not unusually long for any patient.
Follow-Up Evaluation. All 10 patients survived and completed the 12-month follow-up evaluation. The trivial residual shunts persisted in patients 1 and 8. No other postprocedural complications were noted.
Discussion
All 10 of our pediatric patients with residual muscular VSD successfully underwent perventricular device closure and had good outcomes. Residual muscular VSD, a relatively rare condition,11,12 complicates the postoperative management of cardiac patients and is associated with increased mortality rates.4,5,12 Because of inadequate exposure during the original corrective procedure, this type of VSD can be overlooked. It can be completely obscured when associated with other large left-to-right shunts, pulmonary hypertension, or hypertrophy of the ventricular septum during preoperative screening.3–5 After the correction of primary defects such as tetralogy of Fallot, double-outlet RV, or multiple VSDs, residual shunts can present or even enlarge as a result of increased pressure gradients across the ventricle. Immediate treatment has been recommended in cases of a Qp/Qs >1.5 or significant hemodynamic instability.
Surgical closure of residual muscular VSD in infants and children is a challenge,2,3,5,6,12–14 because the locations of the defects—especially those in the apical or anterior region of the ventricular septum—necessitate repair with the use of CPB. The extensive trabeculation of the RV cavity makes direct-suture or patch closure difficult and can result in further residual shunting.5,6 Right ventriculotomy can impair long-term RV function and increase the risk of arrhythmia.5,6
Transcatheter devices enable the minimally invasive closure of muscular VSDs. Some investigators have used transcatheter closure to good effect in adult patients with postoperative residual muscular VSDs12,14; however, there is less such experience in the pediatric population. Transcatheter closure is not always feasible in small patients because of the disparity between vascular access and the sizes of the necessary sheaths and devices.
In contrast with the foregoing methods, the direct access afforded by perventricular device closure enables the closure of muscular VSDs in different regions of the interventricular septum. The use of this method is not constrained by restricted vascular access in pediatric patients.15–17 Muscular VSDs in the apical inflow, infundibular apical, and mid-muscular regions can be closed successfully.10,12–14 As an off-pump technique, perventricular closure avoids the risk of postoperative ventricular dysfunction or death from repeat CPB so soon after major corrective surgery, and there is no radiation exposure as in the transcatheter approach.18 Patients can undergo single-station hybrid treatment in an ordinary cardiac operating room, without the necessity of visiting the catheterization laboratory. Hemodynamic instability during perventricular closure can be safely managed with the use of parallel CPB.
Technical Considerations
Choosing an appropriate RV puncture site is crucial in perventricular closure. The surgeon can find the site (which should be perpendicular to the muscular VSD) by indenting the RV free wall with his finger, under TEE guidance. The choice of an occluder 1 to 2 mm larger than a single muscular VSD is straightforward. In our previous study,19 we discussed how we have dealt with 2 nearby muscular VSDs.
Adverse Events
Perventricular closure is not bloodless. Excessive bleeding of 100 mL or more can occur, and an intraoperative autologous blood transfusion should be prepared. Intraoperative arrhythmia can necessitate cardioversion to restore a patient's hemodynamic stability. A multidisciplinary team and close intraoperative communication between the surgeon and anesthesiologist are crucial to a successful outcome in this type of hybrid operation with real-time TEE guidance.
Another safety concern is closure-induced ventricular dysfunction. Two of our 10 patients developed low cardiac output syndrome; however, we think that this was due to the primary condition and the corrective surgical procedure, because the patients' ventricular function had already deteriorated before perventricular closure was undertaken. Further study of closure-induced ventricular dysfunction is warranted.
Limitations of the Study
Our study has some limitations. Because of the rarity of postoperative residual muscular VSD, we enrolled only 10 patients. All 10 underwent routine 12-lead ECG during the follow-up period; however, no further 24-hour ambulatory ECG was performed, because every patient was stable in sinus rhythm. Finally, no patient underwent cardiac catheterization, because the anatomy of all heart defects and the Qp/Qs ratios were defined by means of preoperative echocardiography.
On the basis of our experience, we conclude that perventricular device closure is a safe, effective salvage option for treating postoperative residual muscular VSDs in pediatric patients. Long-term studies with larger cohorts might yield the further feasibility of this method.
Footnotes
Address for reprints: Ke Lin, MD, Department of Cardiovascular Surgery, West China Hospital of Sichuan University, No. 37 Guoxue Alley, Chengdu 610041, Sichuan, PRC.
E-mail: edwarddian@gmail.com
References
- 1.Soto B, Becker AE, Moulaert AJ, Lie JT, Anderson RH. Classification of ventricular septal defects. Br Heart J 1980;43(3):332–43. [DOI] [PMC free article] [PubMed]
- 2.Knauth AL, Lock JE, Perry SB, McElhinney DB, Gauvreau K, Landzberg MJ, et al. Transcatheter device closure of congenital and postoperative residual ventricular septal defects. Circulation 2004;110(5):501–7. [DOI] [PubMed]
- 3.Kitagawa T, Durham LA 3rd, Mosca RS, Bove EL. Techniques and results in the management of multiple ventricular septal defects. J Thorac Cardiovasc Surg 1998;115(4):848–56. [DOI] [PubMed]
- 4.Serraf A, Lacour-Gayet F, Bruniaux J, Ouaknine R, Losay J, Petit J, et al. Surgical management of isolated multiple ventricular septal defects. Logical approach in 130 cases. J Thorac Cardiovasc Surg 1992;103(3):437–43. [PubMed]
- 5.Alsoufi B, Karamlou T, Osaki M, Badiwala MV, Ching CC, Dipchand A, et al. Surgical repair of multiple muscular ventricular septal defects: the role of re-endocardialization strategy. J Thorac Cardiovasc Surg 2006;132(5):1072–80. [DOI] [PubMed]
- 6.Myhre U, Duncan BW, Mee RB, Joshi R, Seshadri SG, Herrera-Verdugo O, Rosenthal GL. Apical right ventriculotomy for closure of apical ventricular septal defects. Ann Thorac Surg 2004;78(1):204–8. [DOI] [PubMed]
- 7.Laussen PC, Hansen DD, Perry SB, Fox ML, Javorski JJ, Burrows FA, et al. Transcatheter closure of ventricular septal defects: hemodynamic instability and anesthetic management. Anesth Analg 1995;80(6):1076–82. [DOI] [PubMed]
- 8.Amin Z, Berry JM, Foker JE, Rocchini AP, Bass JL. Intraoperative closure of muscular ventricular septal defect in a canine model and application of the technique in a baby. J Thorac Cardiovasc Surg 1998;115(6):1374–6. [DOI] [PubMed]
- 9.Gan C, Lin K, An Q, Tang H, Song H, Lui RC, et al. Perventricular device closure of muscular ventricular septal defects on beating hearts: initial experience in eight children. J Thorac Cardiovasc Surg 2009;137(4):929–33. [DOI] [PubMed]
- 10.Kumar K, Lock JE, Geva T. Apical muscular ventricular septal defects between the left ventricle and the right ventricular infundibulum. Diagnostic and interventional considerations. Circulation 1997;95(5):1207–13. [DOI] [PubMed]
- 11.Bol-Raap G, Weerheim J, Kappetein AP, Witsenburg M, Bogers AJ. Follow-up after surgical closure of congenital ventricular septal defect. Eur J Cardiothorac Surg 2003;24(4):511–5. [DOI] [PubMed]
- 12.Dua JS, Carminati M, Lucente M, Piazza L, Chessa M, Negura D, et al. Transcatheter closure of postsurgical residual ventricular septal defects: early and mid-term results. Catheter Cardiovasc Interv 2010;75(2):246–55. [DOI] [PubMed]
- 13.Kirklin JK, Castaneda AR, Keane JF, Fellows KE, Norwood WI. Surgical management of multiple ventricular septal defects. J Thorac Cardiovasc Surg 1980;80(4):485–93. [PubMed]
- 14.Lock JE, Block PC, McKay RG, Baim DS, Keane JF. Transcatheter closure of ventricular septal defects. Circulation 1988;78(2):361–8. [DOI] [PubMed]
- 15.Bacha EA, Cao QL, Starr JP, Waight D, Ebeid MR, Hijazi ZM. Perventricular device closure of muscular ventricular septal defects on the beating heart: technique and results. J Thorac Cardiovasc Surg 2003;126(6):1718–23. [DOI] [PubMed]
- 16.Bacha EA, Cao QL, Galantowicz ME, Cheatham JP, Fleishman CE, Weinstein SW, et al. Multicenter experience with perventricular device closure of muscular ventricular septal defects. Pediatr Cardiol 2005;26(2):169–75. [DOI] [PubMed]
- 17.Crossland DS, Wilkinson JL, Cochrane AD, d'Udekem Y, Brizard CP, Lane GK. Initial results of primary device closure of large muscular ventricular septal defects in early infancy using perventricular access. Catheter Cardiovasc Interv 2008;72(3):386–91. [DOI] [PubMed]
- 18.Wagner LK, Eifel PJ, Geise RA. Potential biological effects following high X-ray dose interventional procedures. J Vasc Interv Radiol 1994;5(1):71–84. [DOI] [PubMed]
- 19.Gan C, An Q, Tao K, Tang H, Lui RC, Song H, et al. How to choose an occluder for two nearby muscular ventricular septal defects? Ann Thorac Surg 2009;87(4):1307–8. [DOI] [PubMed]


