Abstract
AIMS:
1. To study the distribution of various Rotterdam classified phenotypes of polycystic ovarian syndrome (PCOS) women, in our population. 2. To compare the four phenotypes with respect to anthropometric, clinical, and metabolic parameters. 3. To report the prevalence of insulin resistance (IR) and metabolic syndrome in these women.
SETTINGS AND DESIGN:
Private practice, Prospective cross-sectional comparative study.
MATERIALS AND METHODS:
Women attending gynecology outpatient with the primary complains of irregular menses and/or infertility were evaluated. Each of them underwent detailed clinical examination, transvaginal sonography, and biochemical and hormonal assays. Four hundred and ten women with a clinical diagnosis of PCOS based on Rotterdam criteria were included in the study. The four phenotypes were 1) PCO complete, that is oligo/anovulation (O) + polycystic ovaries (P) + hyperandrogenism (H) 2) P + O, 3) P + H, and 4) O + H. All women were also evaluated for metabolic syndrome (American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI), modified Adult Treatment Panel (ATP) III 2005 guidelines) and IR (homeostatic model assessment-IR (HOMA-IR)).
STATISTICAL ANALYSIS:
Statistical Package for Social Sciences (SPSS) version 18.
RESULTS:
Largest group was PCOS complete (65.6%) followed by P + O (22.2%); H + O (11.2%); and P + H (0.9%). Overall prevalence of metabolic syndrome was 35.07%. Hyperandrogenic phenotyptes; H + O (50%) and P + H + O (37.04%), had significantly higher prevalence of metabolic syndrome than normoandrogenic P + O phenotype (10%) (P ≤ 0.001). Body mass index (BMI) ≥ 25 (P = 0.0004; odds ratio (OR) = 3.07 (1.6574–5.7108, 95% CI)), waist circumference (WC) ≥ 80 cm (P = 0.001; OR = 3.68 (1.6807–8.0737, 95% CI)) and family history of diabetes (P = 0.019; OR 1.82 (1.1008–3.0194, 95% CI)), were strongly associated with the presence of metabolic syndrome. The overall prevalence of IR in PCOS women was 30.44% (HOMA-IR cutoff ≥ 3.8) and 34.94% (HOMA-IR cutoff ≥ 3.5).
CONCLUSIONS:
The prevalence of metabolic syndrome and IR was 35.07 and 30.44%, respectively. The hyperandrogenic phenotypes have significantly higher metabolic morbidity compared to normoandrgenic phenotype. BMI > 25, WC ≥ 80 cm, and family history of diabetes carry the highest risk for developing metabolic syndrome.
KEY WORDS: Insulin resistance, metabolic syndrome, polycystic ovarian syndrome, phenotypes
INTRODUCTION
Polycystic ovarian syndrome (PCOS) was first described by Valisnere in 1721[1] as, “Young, married peasant women, moderately obese, and infertile with two larger than normal ovaries, bumpy, shiny and whitish, just like pigeon eggs”. In 1935, Stein and Leventhal published a case series of seven women with amenorrhea, hirsuitism, obesity, and ovaries with a grossly polycystic appearance.[2] The syndrome is one of the commonest endocrinopathies in women of reproductive age causing significant menstrual and fertility issues and over the past few decades its metabolic, cardiovascular, and reproductive risks have become apparent.[3]
In spite of tremendous scientific interest and research, controversy continues regarding almost all aspects of PCOS. Diagnosis, prevalence, etiology, pathophysiology, management, long-term risks, etc., Also, clinical and biochemical features of these women may vary according to race, ethnicity, and criteria used for diagnosis.[4] Diagnosis of PCOS continues to be controversial primarily because of the heterogenous nature of the condition which may change during the lifetime of the woman. Currently, the commonest criteria used for diagnosis of PCOS is the “Rotherdam criteria” which includes any two of the following three features:
1) Oligo/anovulation (O), 2) clinical and/or biochemical hyperandrogenemia (H), 3) polycystic ovaries on ultrasound (P), with exclusion of other known disorders of hyperandrogenemia. This generates four different phenotypes: 1) P + H + O (PCOS complete), 2) P + O, 3) H + O, and 4) P + H (5). Diagnosis of PCOS is important, because it is associated with increased risks of insulin resistance (IR), noninsulin dependent diabetes mellitus and metabolic syndrome. All of which have long-term consequences.[3,4,5] PCOS women have a 11-fold higher risk of having metabolic syndrome, which is a cluster of endocrine disturbances like IR, dyslipidemia, obesity, hypertension, atherosclerosis, and endothelial dysfunction,[6,7,8,9,10] compared to age matched controls.[11] The original National Cholesterol Education Program-Adult Treatment Panel III (NCEP-ATP III) criteria in 2001 defines metabolic syndrome as the co-occurrence of three or more of the following risk factors:1) Waist circumference (WC) ≥ 88 cm in women, 2) blood pressure ≥130/85 mmHg, 3) fasting serum glucose ≥110 mg/dl, 4) fasting serum triglyceride ≥150 mg/dl, and 5) fasting high density lipoprotein <50 mg/dl.[12] Currently, modified American Heart Association/National Heart, Lung, and Blood Institute (AHA/NHLBI) definition (ATP III 2005) includes the following changes: 1) Defining the ethnic specific difference in central obesity by using the World Health Organization (WHO) recommendation for WC as ≥80 cm in Asian women and 2) reduced threshold for impaired fasting glucose to 100 mg/dl in accordance with American Diabetes Association revised definition.[13]
Hyperinsulinemia and IR are thought to be key pathological factors for PCOS and metabolic syndrome.[14,15] IR is a metabolic disorder characterized by impairment of insulin function. Diagnosis of IR is not standardized.[16] Many techniques are followed, for example: Fasting plasma glucose, 2 h 75 g oral glucose tolerance test (OGTT), glucose/insulin ratio, homeostasis model assessment for IR (HOMA-IR), quantitative insulin sensitivity check index (Quiki) and euglycemic hyperinsulinemic clamp which is considered the gold standard.[17,18] Superiority of one over the other is not established. In the present study, HOMA-IR was used to diagnose IR. Prevalence of acanthosis nigricans was also reported. Acanthosis nigricans is a brown to black poorly defined; velvety hyperpigmentation of the skin; usually present in the posterior and lateral folds of neck, axilla, and groin. This clinical finding correlates well with IR.[19]
The prevalence of metabolic syndrome and IR in PCOS has been studied in very few different populations and ethnic groups. Also, there are limited data on differences between various phenotypes with respect to long-term metabolic risk. Especially the newer phenotypes generated by the Rotterdam criteria are inadequately studied and reported. This study was undertaken to characterize the various phenotypes of PCOS. Their distribution, clinical, anthropometric, and biochemical features and to report the prevalence and risk factors for metabolic syndrome and IR in the different phenotypes on our population.
MATERIALS AND METHODS
This prospective cross-sectional comparative study was conducted from January 2009 to December 2012, in a private hospital with a large gynecologic practice. All women in the reproductive age group (18-45) with the primary complains of menstrual irregularities with or without infertility were evaluated in detail for PCOS. All those with a final diagnosis of PCOS were included in the study. PCOS was diagnosed according to the 2003 Rotterdam criteria (European Society of Human Reproduction and Embryology/American Society for Reproductive Medicine (ESHRE/ASRM) PCOS consensus workshop group) with at least two of the following three features; (i) oligo or anovulation (ii) clinical and/or biochemical hyperandrogenism, and (iii) ultrasound appearance of polycystic ovaries. Four hundred and ten women with PCOS qualified for the study.
All participants gave written informed consent and this study was approved by our ethics committee.
All anthropometric measurements were done following standard protocol and calibrated instruments by a single technician. All clinical findings like F-G score, acanthosis nigricans, galactorrhea, etc., were evaluated by single gynecologist. All sonographies were performed by single gynecologist (author) (voluson E8). All biochemical and hormonal tests were carried out in the laboratory of the private hospital where this study was conducted (miniVidas, bioMerieux), except serum sex hormone binding globulin (SHBG) and free testosterone, which were outsourced to a standard national laboratory (Thyrocare Technologies Limited). Evaluation of reports and final diagnosis of PCOS was made by single gynecologist (author).
Oligo and anovulation was defined as: Menstrual cycles <21 days or >35 days, clinical hyperandrogenism was defined as modified Ferriman Gallway score ≥8. Biochemical hyperandrogenism as a free androgen index (FAI) of >4.5 (total testosterone, nmol/l/SHBG, nmol/l × 100). Polycystic ovary was defined as ≥12 follicles per ovary and/or ovarian volume ≥10 cm3. All participants gave fasting (>12 h) blood samples for plasma glucose, insulin, lipid profile, follicle stimulating hormone (FSH), luteinizing hormone (LH), prolactin, thyroid stimulating hormone (TSH), total testosterone, and SHBG. Sonography was performed in early follicular phase. Metabolic syndrome was defined according to the modified AHA/NHLBI ATP III (2005) definition. It was diagnosed if at least three of the following five features were present: 1) WC of ≥ 80 cm, 2) blood pressure of ≥130/85 mmHg, 3) fasting blood sugar of ≥100 mg/dl, 4) triglycerides of ≥150 mg/dl, and 5) high density lipoprotein (HDL) of ≤50 mg/dl.
IR was estimated using the HOMA-IR (fasting insulin μU/ml × fasting glucose (mg/dl)/405). IR was present with a HOMA-IR value of ≥3.8.
Statistical analysis
Quantitative variables were expressed as mean ± standard deviation (SD) and were compared between groups using one way analysis of variance (ANOVA), ANOVA, and “Tamhane” post hoc statistical tests. Quantitative variables were expressed as frequencies in percentages and were analyzed by χ2 tests. Multivariate logistic regression analysis was used to examine independent predictors of metabolic syndrome. Statistical analysis was done using SPSS version 18. A P value of less than 0.05 was considered statistically significant.
RESULTS
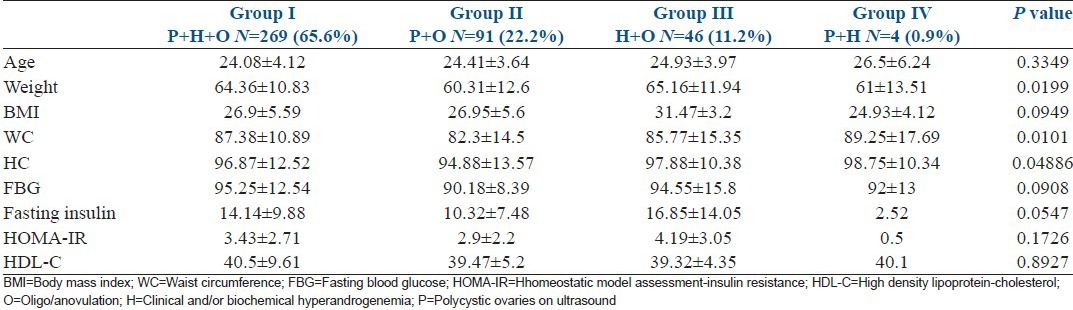
The largest phenotypic group was women with all three features, that is, PCO complete P + O + H (65.6%). Followed by P + O (22.2%), the normoandrogenic phenotype; and then H + O (11.2%), the non-PCO phenotype. The ovulatory phenotype, H + P was the least common (0.9%). The mean and SD of clinical and demographical characteristics of these four phenotypes are presented in Table 1. The hyperandrogenic phenotype, that is, P + O + H, H + O, as defined by National Institute of Child Health and Human Development (NICHD, 1990) comprised 76.8%. The newer phenotypes which were added after the Rotterdam criteria (2003), that is, P + O and P + H comprised 23.1%.
Table 1.
Anthropometric and clinical data of different polycystic ovary syndrome phenotypes
The overall prevalence of metabolic syndrome was 35.07%. In the hyperandrogenic anovulatory phenotype H + O metabolic syndrome was present in 50% of women. In PCO complete P + H + O 37.4% and in P + O phenotype the prevalence of metabolic syndrome was low, that is, 10%.
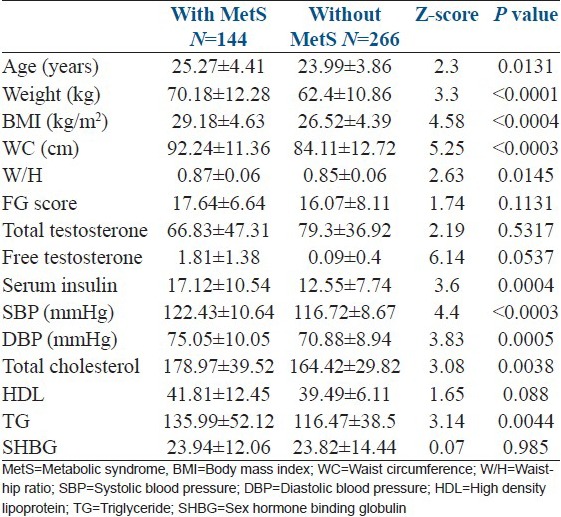
Clinical and biochemical parameters of PCOS women with and without metabolic syndrome was compared [Table 2]. There was statistically significant difference between the two groups in age (P = 0.013), BMI (P < 0.0004), WC (P < 0.0003), waist-hip ratio (W/H) (P = 0.014), serum fasting insulin (P = 0.0004), total cholesterol (P = 0.004), triglycerides (P = 0.03), and systolic and diastolic blood pressure (P < 0.00003 and 0.0005, respectively).
Table 2.
Clinical and biochemical parameters of polycystic ovary syndrome women with and without metabolic syndrome
The prevalence of metabolic syndrome increased with age [Figure 1] and with increasing BMI [Figure 2]. The age adjusted prevalence of metabolic syndrome in different phenotypes is shown in Figure 3.
Figure 1.
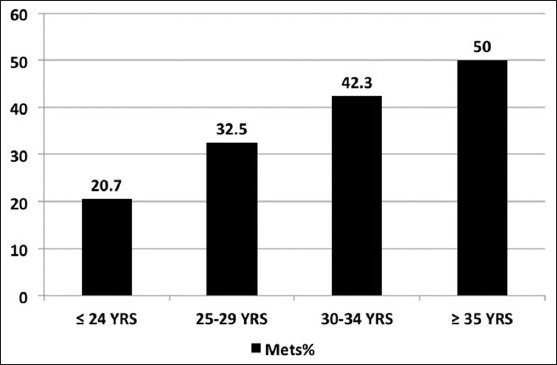
Distribution of metabolic syndrome by age
Figure 2.
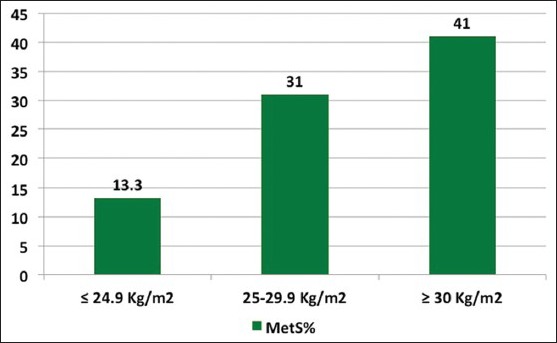
Distribution of metabolic syndrome by body mass index
Figure 3.
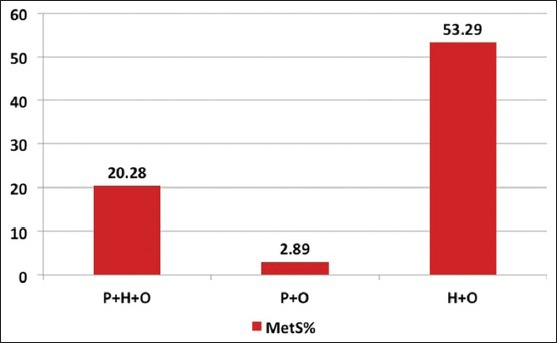
Age adjusted prevalence of metabolic syndrome in different polycystic ovary syndrome phenotypes
In obese women (BMI ≥ 30), prevalence of metabolic syndrome was 41%, against 13.3% in lean women (BMI ≤ 24.9). Overall 62.5% of PCOS women were overweight, 73.9% had WC above the cutoff of ≥80 cm. Acanthosis nigricans was detected in 37.5% of all PCOS women. In women with metabolic syndrome and IR, approximately 65% women showed this classic sign.
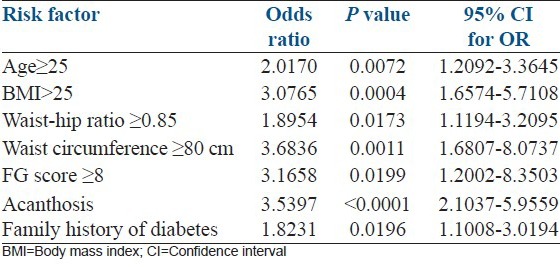
Logistic regression analysis showed that BMI (OR = 3.07 (1.65-5.71 95% CI); P = 0.0004), WC ≥ 80 cm (OR = 3.68 (1.68-8.07 95% CI); P = 0.001), acanthosis nigricans (OR 3.53 (2.10-5.92 95% CI); P < 0.0001) and family history of diabetes (OR = 1.82 (1.10-3.01 95% CI); P = 0.02), were associated with the risk of having metabolic syndrome [Table 3].
Table 3.
Logistic regression analysis showing the predictive association of clinical variables and presence of metabolic syndrome
IR (HOMA-IR ≥ 3.8) was present in 30.44% of all PCOS women. Since there is no consensus on cutoff levels of HOMA-IR to define presence of IR, multiple cutoffs of HOMA-IR were used. With a HOMA-IR cutoff of ≥3.5 and ≥2.5, IR was present in 34.95 and 50.52% of PCOS women, respectively. Prevalence of IR varied in different phenotypes. It was highest in hyperandrogenic anovulatory phenotype H + O (35.13%) followed by P + H + O (31.58%) and P + O (21.05%).
DISCUSSION
The phenotypic distribution in our study in order of decreasing prevalence was: P + O + H; P + O; O + H; and P + H (65.6, 22.2, 11.2, and 0.9%, respectively). In few similar studies the phenotypic distribution was quite similar. Turkish population 44.09, 14.17, 18.9, and 14.1%; Bulgarian 53.6, 11, 12.8, and 22.6%; United States 58, 13, 14, and 14%; and Iranian 32.1, 46.8, 14.8, and 6.3%, respectively.[20,21,22,23] PCO complete (P + O + H) was the largest group in most reports. Normoandrogenic phenotype (P + O) comprised a significant proportion of PCOS women. Ovulatory phenotype P + H, was the least common group, possibly because these woman are mostly asymptomatic and unlikely to present in gynecology outpatient. Also, women with hyperandrogenic symptoms like acne and hirsutism are more likely to consult dermatology.
The overall prevalence of metabolic syndrome in our population of PCOS women was 35.07%. Similar studies from different populations have reported prevalence of metabolic syndrome. American 43-46%,[11] Italian 8.2%,[24] Iranian 24.9%,[23] German 33.8%,[25] and Indian 46.2 and 37.5%.[26,27]
The differences in the above studies may be as a result of different body weights, dietary habits, lifestyle, and genetic factors in different countries and ethnic groups. Also different criteria for diagnosis of metabolic syndrome like NCEP ATP III and International Diabetic Federation (IDF) criteria. A recent consensus definition incorporating IDF and AHA/NHLB risk factors have been introduced.[28] Our study has used this definition for the definition of metabolic syndrome. Thus, our findings are comparable with the other Indian study[27] reporting 37.5% prevalence of metabolic syndrome in PCOS women with infertility.
Our study also aimed to characterize and compare the prevalence of metabolic complications of the four PCOS phenotypes. The hyperandrogenic phenotypes had a four- to five-fold higher (37-50%) prevalence of metabolic syndrome compared to the non-hyperandrogenic phenotype (10%). Suggesting that P + O created by the Rotterdam criteria, one of the new phenotypes, have a mild metabolic profile. Many studies have reported similarly.[22,23,29,30]
IR, hyperinsulinemia, and hyperandrogenemia are likely pathogenic factors in PCOS.[31,32,33] Polycystic morphology on ultrasound, its mechanism and importance is not understood. Studies however report lower incidence of metabolic syndrome, IR, lower cholesterol, and low density lipoproteins in women with PCO ovary.[22,23,29,30,34]
In our study obese women (BMI ≥ 30) had three- to four-fold higher prevalence of metabolic syndrome compared to lean women (BMI ≤ 24.9), 41 vs 13.3%, which is similar to other studies.[23,25,27] Surprisingly, Shroff et al.,[22] reported not a single case of metabolic syndrome PCOS women with BMI < 30. In our lean PCOS women with metabolic syndrome the major components were; abnormal WC and low HDL cholesterol. Thus, significant number of lean women had abnormal WC and W/H ratio.
IR was reported based on HOMA-IR values, which could be one of the limitations of this study. Diagnosis of IR is not standardized.[35] The gold standard for establishing IR is euglycemic hyperinsulinemic clamp. However, this elaborate procedure is not suitable as a screening method. HOMA-IR calculation correlates very well with euglycemic hyperinsulinemic clamp and is often used as a surrogate marker. However, the cutoff or threshold values for HOMA-IR are not established. Using a cutoff of 3.8 may under estimate the true prevalence of IR.[16,17,18] Approximately, 50-70% of all women with PCOS have some degree of IR.[36] True prevalence is difficult to determine with no universally accepted parameter to measure IR. As reported by us using different cutoff for HOMA-IR, the prevalence may range from 30 to 50%.
Currently there is lack of consensus on how best to evaluate insulin sensitivity. Homeostatis measurements (fasting glucose/insulin ratio or HOMA-IR values) and minimal model tests (OGTT) represent the easiest screening methods.[36] Possibly OGTT is the best, simple test to provide information about both IR and glucose intolerance. Diagnosis of glucose intolerance gives greater scope for treatment options and prognosis. So, from clinical prognosis and management point of view doing 75 g 2 h OGTT is the most informative. HOMA-IR is not of much practical importance.
Multivariate logistic regression analysis showed that age ≥25 years, WC ≥ 80 cm, and BMI and family history of diabetes were strong risk factors for metabolic syndrome. These correlate well with other studies which concluded that WC is closely related with obesity related risk factors as compared with BMI.[27,37]
The major limitations of our study is the absence of control group which should be age and BMI matched to the PCOS group. Nonavailability of educated and informed volunteers to participate in such studies is the main hurdle.
CONCLUSION
An appropriate diagnosis of PCOS and accurate identification of phenotype is very important as it has long-term health implications for women. These women need to be informed and counseled about their present and long-term risks. Our study is the first to characterize phenotypes of PCOS in a large cohort of women and to show the data of various metabolic complications of these phenotypes. Our findings highlights that normoandrogenic phenotype has least metabolic risks. Also, significant proportion of lean PCOS women have abnormal WC, W/H ratio, and metabolic abnormalities. Therefore, we recommend screening all PCOS women for metabolic abnormalities.
ACKNOWLEDGEMENT
I would like to acknowledge that this work has been possible only with the support of the entire staff of Obstetrics and Gynecology Department of KCHPL. Special thanks to Dr. Luna Samanta, Dr. Tanaya Jena and Sunita Panigrahi for the statistical calculations. Dr. Shovana Senapati and Dr. Sheetal Agarwal for clinical support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Insler V, Lunenfeld B. Polycystic ovarian disease: A challenge and controversy. Gynecol Endocrinol. 1990;4:51–70. doi: 10.3109/09513599009030691. [DOI] [PubMed] [Google Scholar]
- 2.Stein KF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181–91. [Google Scholar]
- 3.Ehrmann DA. Polycystic ovary syndrome. N Engl J Med. 2005;352:1223–36. doi: 10.1056/NEJMra041536. [DOI] [PubMed] [Google Scholar]
- 4.Kauffman RP, Baker VM, Dimarino P, Gimpel T, Castracane VD. Polycystic ovarian syndrome and insulin resistance in white and Mexican American women: A comparison of two distinct populations. Am J Obstet Gynecol. 2002;187:1362–9. doi: 10.1067/mob.2002.126650. [DOI] [PubMed] [Google Scholar]
- 5.Rotterdam ESHRE/ASRM sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–7. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 6.Maruyama C, Imamura K, Teramoto T. Assessment of LDL particle size by triglyceride/HDL-Cholesterol ratio in nondiabetic, healthy subjects without prominent hyperlipidemia. J Atheroscler Thromb. 2003;10:186–91. doi: 10.5551/jat.10.186. [DOI] [PubMed] [Google Scholar]
- 7.Wild RA, Painter PC, Coulson PB, Carruth KB, Ranney GB. Lipoprotein Lipid concentrations and cardiovascular risk in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1985;61:946–51. doi: 10.1210/jcem-61-5-946. [DOI] [PubMed] [Google Scholar]
- 8.Guzick DS, Talbott EO, Sutton-Tyrell K, Herzog HC, Kuller LH, Wolfson SK., Jr Carotid atherosclerosis in women with polycystic ovary syndrome: Initial results from a case-control study. Am J Obstet Gynecol. 1996;174:1224–9. doi: 10.1016/s0002-9378(96)70665-8. [DOI] [PubMed] [Google Scholar]
- 9.Birdsall MA, Farquhar CM, White HD. Association between Polycystic ovaries and extent of coronary artery disease in women having cardiac catheterization. Ann Intern Med. 1997;126:32–5. doi: 10.7326/0003-4819-126-1-199701010-00005. [DOI] [PubMed] [Google Scholar]
- 10.Paradisi G, Steinberg HO, Hempfling A, Cronin J, Hook G, Shepard MK, et al. Polycystic ovary syndrome is associated with endothelial dysfunction. Circulation. 2001;103:1410–5. doi: 10.1161/01.cir.103.10.1410. [DOI] [PubMed] [Google Scholar]
- 11.Dokras A, Bochner M, Hollinrake E, Markham S, Vanvoorhis B, Jagasia DH. Screening women with polycystic ovary syndrome for metabolic syndrome. Obstet Gynecol. 2005;106:131–7. doi: 10.1097/01.AOG.0000167408.30893.6b. [DOI] [PubMed] [Google Scholar]
- 12.Lepor NE, Vogel RE. National Cholesterol Education Program Adult Treatment Panel III. Summary of the third report of the National Cholesterol Education Program Adult Treatment Panel III. Rev Cardiovas Med. 2001;2:160–5. [PubMed] [Google Scholar]
- 13.Grundy SM, Cleeman JL, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. American Heart Association, National Heart, Lung, and Blood Institute. Diagnosis and management of the metabolic syndrome: An American Heart Association/National Herat, Lung and Blood Institute Scientific Statement. Circulation. 2005;112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 14.Dunaif A, Segal KR, Shelley DR, Green G, Dobrjansky A, Licholai T. Evidence for distinctive and Intrinsic defects in insulin action in polycystic ovary syndrome. Diabetes. 1992;41:1257–66. doi: 10.2337/diab.41.10.1257. [DOI] [PubMed] [Google Scholar]
- 15.Haffner SM, D’Agostino R, Jr, Festa A, Bergman RN, Mykkanen L, Karter A, et al. Low insulin sensitivity (S (i)=0) in diabetic and nondiabetic subjects in the insulin resistance atherosclerosis study: Is it associated with components of the metabolic syndrome and non-traditional risk factors? Diabetes Care. 2003;26:2796–803. doi: 10.2337/diacare.26.10.2796. [DOI] [PubMed] [Google Scholar]
- 16.Lebovitz HE. Insulin resistance: Definition and consequences. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S135–48. doi: 10.1055/s-2001-18576. [DOI] [PubMed] [Google Scholar]
- 17.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: Studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. doi: 10.2337/diacare.23.1.57. [DOI] [PubMed] [Google Scholar]
- 18.Mather K. Surrogate measures of insulin resistance: Of rats, mice and men. Am J Physiol Endocrinol Metab. 2009;296:E398–9. doi: 10.1152/ajpendo.90889.2008. [DOI] [PubMed] [Google Scholar]
- 19.Flier JS, Eastman RC, Minaker KL, Matteson D, Rowe JW. Acanthosis nigricans in obese women with hyperandrogenism. Characterization of an insulin-resistant state distinct from the type A and B syndromes. Diabetes. 1985;34:101–7. doi: 10.2337/diab.34.2.101. [DOI] [PubMed] [Google Scholar]
- 20.Yilmaz M, Isaoglu U, Delibas IB, Kadanali S. Anthropometirc, clinical and laboratory comparison for our phenotypes of polycystic ovary syndrome based on Rotterdam criteria. J Obstet Gynaecol Res. 2011;37:1020–6. doi: 10.1111/j.1447-0756.2010.01478.x. [DOI] [PubMed] [Google Scholar]
- 21.Kavardzhikova S, Pechivanov B. Clinical, hormonal and metabolic characteristics of different phenotyps of polycystic ovary syndrome, in Bulgarian population. Akush Ginekol (Sofia) 2010;49:32–7. [PubMed] [Google Scholar]
- 22.Shroff R, Syrop CH, Davis W, Van Voorhis BJ, Dokras A. RRisk of metabolic complications in the new PCOS phenotypes based on the Rotterdam criteria. Fertil Steril. 2007;88:1389–95. doi: 10.1016/j.fertnstert.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 23.Mehrabian F, Khani B, Kelishadi R, Kermani N. The prevalence of metabolic syndrome and insulin resistance according to the phenotypic subgroups of polycystic ovary syndrome in a representative sample of Iranian females. J Res Med Sci. 2011;16:763–9. [PMC free article] [PubMed] [Google Scholar]
- 24.Carmina E, Napoli N, Longo RA, Rini GB, Lobo RA. Metabolic syndrome in polycystic ovary syndrome (PCOS): Lower prevalence in southern Italy than in the USA and the influence of criteria for the diagnosis of PCOS. Eur J Endocrinol. 2006;154:141–5. doi: 10.1530/eje.1.02058. [DOI] [PubMed] [Google Scholar]
- 25.Hahn S, Tan S, Sack S, Kimmig R, Quadbeck B, Mann K, et al. Prevalence of the metabolic syndrome in German women with polycystic ovary syndrome. Exp Clin Endocrinol Diabetes. 2007;115:130–5. doi: 10.1055/s-2007-967093. [DOI] [PubMed] [Google Scholar]
- 26.Bhattacharya SM. Metabolic Syndrome in females with polycystic ovary syndrome and International Diabetes Federation criteria. J Obstet Gynaecol Res. 2008;34:62–6. doi: 10.1111/j.1447-0756.2007.00685.x. [DOI] [PubMed] [Google Scholar]
- 27.Mandrelle K, Kamath MS, Bondu DJ, Chandy A, Aleyamma TK, George K. Prevalence of metabolic syndrome in women with polycystic ovary syndrome attending an infertility clinic in a tertiary care hospital in south India. J Hum Reprod Sci. 2012;5:26–31. doi: 10.4103/0974-1208.97791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidermiology and Prevention; National Heart, Lung and Blood Institute; American Heart Associaation; World Heart Federation; International Atherosclerosis Society and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 29.Apridonidze T, Essah PA, Iuorno MJ, Nestler JE. Prevalence and characteristics of the metabolic syndrome in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2005;90:1929–35. doi: 10.1210/jc.2004-1045. [DOI] [PubMed] [Google Scholar]
- 30.Glueck CJ, Papanna R, Wang P, Goldenberg N, Sieve-Smith L. Incidence and treatment of metabolic syndrome in newly referred women with confirmed polycystic ovarian syndrome. Metabolism. 2003;52:908–15. doi: 10.1016/s0026-0495(03)00104-5. [DOI] [PubMed] [Google Scholar]
- 31.Ciaraldi TP, el-Roeiy A, Madar Z, Reichart D, Olefsky JM, Yen SS. Cellular mechanisms of insulin resistance in Polycystic ovarian syndrome. J Clin Endocrinol Metab. 1992;75:577–83. doi: 10.1210/jcem.75.2.1322430. [DOI] [PubMed] [Google Scholar]
- 32.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocrinol Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 33.Cara JF, Rosenfield RL. Insulin-like growth factor I and Insulin potentiate luteinizing hormone-induced androgen synthesis by rat ovarian thecal-interstitial cells. Endocrinology. 1988;123:733–9. doi: 10.1210/endo-123-2-733. [DOI] [PubMed] [Google Scholar]
- 34.Kauffman RP, Baker TE, Baker VM, Diamarino P, Castracane VD. Endocrine and metabolic difference among phenotypic expressions of polycystic ovary syndrome according to the 2003 Rotterdam consensus criteria. Am J Obstet Gynecol. 2008;198:670.e1–7. doi: 10.1016/j.ajog.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 35.Lunger F, Wildt L, Seeber B. Accurate screening for insulin resistance in PCOS women using fasting insulin concentrations. Gynecol Endocrinol. 2013;29:541–4. doi: 10.3109/09513590.2013.774362. [DOI] [PubMed] [Google Scholar]
- 36.Legro RS, Castracane VD, Kauffman RP. Detecting insulin resistance in polycystic ovary syndrome: Purposes and pitfalls. Obstet Gynecol Surv. 2004;59:141–54. doi: 10.1097/01.OGX.0000109523.25076.E2. [DOI] [PubMed] [Google Scholar]
- 37.Janssen I, Katzmarzyk PT, Ross P. Waist circumference and not body mass index explains obesity related health risks. Am J Clin Nutr. 2004;79:379–84. doi: 10.1093/ajcn/79.3.379. [DOI] [PubMed] [Google Scholar]


