Abstract
Nanotechnology (nano: One billionth) is a novel arena with promising applications in the field of medicine, especially pharmaceuticals for safe and targeted drug delivery. The skin is a phenomenal tool for investigation of nanocarriers for drug delivery for topical and dermatological application. The physicochemical characteristics of the nanoparticles, such as rigidity, hydrophobicity, size and charge are crucial to the skin permeation mechanism. Many nanocarriers such as polymeric, inorganic and lipid nanoparticles and nanoemulsions have been developed and some like carbon nanotubes and fullerenes still need further exploration for future use in skin care and dermatological treatments. Risks of nanopollution and cytotoxicity also need to be kept in mind while exploring various nanoparticles for medical use.
Keywords: Nanocarriers, nanodermatology, nanomedicine, nanopollution nanotechnology
INTRODUCTION
The origins of nanotechnology may date back to the 1950's when physicist Richard Feynman proposed developing machines that made smaller copies of themselves. It was eventually observed that matter had unique properties at nanoscale. It was not only the size but also the purposeful engineering that contributed to the uniqueness that nanoparticles penetrate the skin more readily than their bulk counterparts. It is anticipated that nanotechnology will be the fastest growing area for the maintenance of skin health, as well as for the diagnosis and management of cutaneous disease.
Nanotechnology is the latest and fast emerging technology wherein dimension of particle of material is reduced nearly to that of individual molecule or their aggregates.[1] It encompasses the study of particles smaller than 100 nm in size [Figure 1]. It is a promising field with tremendous potential for society and medicine.
Figure 1.

Nanoscopic dimensions
Nanomedicine is defined as the monitoring, repair, construction, and control of human biological systems at the molecular level using engineered nanodevices and nanostructures.[1] It is the medical application of nanotechnology and related research. It covers areas such as nanoparticle drug delivery and possible future applications of molecular nanotechnology and nanovaccinology.
Technologies for the production of nanoparticles have spawned a burgeoning industry that needs still further exploration and refinement. It requires near atom smashing technology; the equivalent of taking large metal marbles of zinc, titanium or iron, and colliding them at near supersonic speeds to make small metal particles of desired size, uniformity, and dispersibility.[2]
These nanoparticles are anticipated to provide more efficacious, specific, and flexible treatment options. Devices and drugs incorporating nanotechnology are touted to be more customized, versatile, and cost effective.
NANOTECHNOLOGY AND DERMATOLOGY
The skin is the largest organ of the human body, presenting a total area of approximately 2 m2. Nanotechnology promises to transform the diagnosis and treatment of dermatological conditions because of its interaction at the sub-atomic level with the skin tissue. The skin represents a marvelous vehicle through which these nanomaterials can be investigated for drug delivery, both with respect to active ingredient delivery and efficacy.
Being the most exposed part to the external environment, it is more prone to the ill-effects of radiation and ultraviolet rays.[3] Any pathology involving the skin is a matter of cosmetic concern. Since the systemic treatment for dermatological problems comes with its potential adverse effects, topical application is the preferred mode due to higher patient compliance and satisfaction.
The skin forms a barrier to the external environment and is impermeable to the drugs due to epidermal cell cohesion and stratum corneum lipids [Figure 1b]. There is a requirement for efficient drug delivery systems past this barrier. Nanotechnology can be used to modify the drug permeation/penetration by controlling the release of active substances and increasing the period of permanence on the skin,[3] besides ensuring a direct contact with the stratum corneum and skin appendages[4] and protecting the drug against chemical or physical instability. Further, the delivery of therapeutic agents without the need for chemical enhancers is desirable to maintain the normal skin barrier function. Treatment with chemical enhancers, such as surfactants and organic solvents, can cause not only a reduction in the barrier function of the skin, but also irritation and damage to the skin.[5]
Figure 1b.
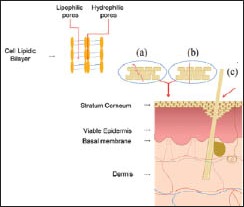
Mechanisms of transport across the skin. (a) intercellular, (b) transcellular and (c) hair-follicles
NANOCARRIERS
Nanostructured carriers are an upcoming option for drug delivery because of their advantages over the conventional formulations. These colloidal particulate systems with size ranging from 10 nm to 1000 nm offer targeted drug delivery, sustained release, protection of labile groups from degradation, low toxicity and drug adhesivity to the skin. Drug-release nanocarriers, such as liposomes, micelles, polymeric and solid lipid nanoparticles as well as inorganic nanoparticles and sub-micrometric emulsions are now available. For example, zinc oxide particles, normally opaque and greasy, vanish and have an elegant feel when broken down into nanoparticles.[2] Emulsions fragmented to nanometer size are less oily, have a better texture, and penetrate skin and hair more deeply when incorporated into emollients and hair conditioners. The physicochemical characteristics of the nanocarriers, such as rigidity, hydrophobicity, size and charge, are crucial to the skin permeation mechanism.[6] The use of nanoscaled carriers in drug delivery is expected to increase specificity of drugs and thus reduce side effects decreasing the dose of administered drugs.[7]
Polymer-based nanoparticles (e.g., nanospheres and nanocapsules) are of interest for skin administration because of controlled release of encapsulated active ingredients, which need to diffuse through the polymeric matrix to permeate the skin. They are structurally stable due to their rigid matrix and are able to maintain their structure for long periods of time when topically applied.[8] For example, Hydrogel (Carbopol® Ultrez 10 National Formulatory) containing dexamethasone as the active ingredient has shown potential use in controlled drug delivery for the treatment of psoriasis.[9] Polymeric nanoparticles encapsulating small inhibitor ribonucleic acids (siRNAs) can selectively inactivate gene expression. Nanoencapsulated siRNAs have been used for the management of pachyonychia congenital and for successful targeted delivery and inhibition of a test gene expressed in melanoma in human trials.[10]
Conceptually, polymeric nanocapsules [Figure 1c] are vesicular particles smaller than 1 μm composed of an oily core surrounded by an ultrathin polymeric wall stabilized by surfactants and/or steric agents.[11] The diffusion of the active ingredient from the oily core depends on the characteristics of the polymeric wall.[12] Thermo-sensitive polymers encapsulate drugs below a critical temperature and dissolve to release the drug above the critical temperature. These are being used for drug delivery at the sites of inflammation or wherever external heat is applied. This is the basis of treatment of localized psoriasis (especially nails and scalp) using methotrexate encapsulated in a thermosensitive polymer.[10]
Figure 1c.
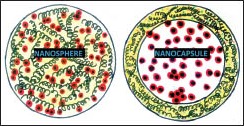
Nanosphere/nanocapsule
Compounds that have been encapsulated in polymeric nanoparticles vary from cosmetics and drugs to peptides and proteins. Pharmaceutical and cosmetic applications of peptides and proteins have been highlighted in recent years and include cancer, infectious disease, autoimmune disease, acquired immunodeficiency syndrome, and anti-aging treatments. Most polymeric systems are retained in the stratum corneum and may improve drug release through the skin, which is dependent on the skin absorption characteristics of the drug as well as the drug release properties. Encapsulated nanobotox is under early clinical trial. Topical paralytic agents, such as α-aminobutyric acid, are being used to transcutaneously relax muscles of facial expression.[10] Volatile anti-microbial gases, such as nitric oxide, have been trapped in nanoparticulate chitosan and has been effectively used in treatment of skin infections and in penetrating abscesses.[10]
Recent advances have shifted our focus to inorganic nanoparticles for specific targeting and control of their cellular actions. Being inorganic, they remain stable for long periods. Inorganic nanoparticles generally possess versatile properties suitable for cellular delivery, including wide availability, rich functionality, good biocompatibility, potential capability of targeted delivery (e.g., selectively destroying cancer cells but sparing normal tissues) and controlled release of carried drugs.[13] These show advantages not only in the cosmetics area, such as in anti-aging and anti-acne treatments, and hydration and skin care products, but also in the treatment of skin diseases such as skin cancer and vitiligo, and for transdermal delivery of substances. Nanosized particles of ZnO and titanium dioxide (TiO2) used in sunscreens are prime examples of inorganic nanoparticles. They are not only transparent but cosmetically desirable. TiO2 is more effective in UVB and ZnO in the UVA range, the combination of these particles assures a broad-band UV protection. However, to solve the cosmetic drawback of these opaque sunscreens, microsized TiO2 and ZnO have been increasingly replaced by TiO2 and ZnO nanoparticles (NPs) (<100 nm). With the use of TiO2 and ZnO NPs, the undesired opaqueness disappears but the required balance between UVA and UVB protection can be altered. Utilization of mixtures of micro- and nanosized ZnO dispersions and nanosized TiO2 particles may improve this situation.[14] Metallic nanoparticles such as silver are being used because for their antiseptic effect. Silver is effective against Methicillin Resistant Staphylococcous aureus,[2] onchomycosis,[15] trichophtyon[16] and dermal leishmaniasis,[17] to name a few.
Gold nanoparticles in particular are an excellent intracellular targeting vector[13] because:
They can be easily tailored to a desirable size from 0.8 nm to 200 nm
Their surface can be modified to impart various functionalities and good biocompatibility
They possess visible light extinction behavior, which makes it possible to track nanoparticle trajectories in the cells.
These gold nanoparticles allow long-wavelength light directed tumor photothermolysis and may allow deeper targeting of cutaneous tumors.[2]
Carbon nanotubes are stable carbon nanoparticles with potential anti-oxidant ability and cytoprotective effect. Carbon nanotubes [Figure 1d]: They have extremely small mean diameters (<100 nm). Their large inner volume allows the loading of small biomolecules while their outer surface can be chemically modified to render themselves various novel features that can be used to load proteins and genes for effective drug delivery.[13] The conductivity of carbon nanotubes can be used to make highly sensitive sub-organellar biomarker sensor, thus, making the diagnosis of chronic skin infections and malignancies possible at an early stage.[10]
Figure 1d.

Carbon nanotubes
Fullerenes [Figure 1e] are 1-nm scale carbon spheres of 60 carbon atoms. Although fullerenes are hydrophobic, they can be organically functionalized by attaching hydrophilic moiety and become water-soluble and capable of carrying genes, proteins and other biomolecules for delivery purposes.[13] Their small size, spherical shape, and hollow interior all provide therapeutic opportunities. Even though fullerenes and their derivatives have been studied as agents for topical application, a multitude of opportunities for their application in the future might be expected.
Figure 1e.
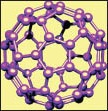
Fullerenes
These have been proposed as rejuvenating cosmetic products like sunscreens, moisturizers, long lasting makeup, etc., Slow release kinetics are important in perfumes, which can yield all day fragrance.[10] Regardless of the desired application (transdermal or topical), the transport characteristics of the nanocarriers are related to its dimensions (hydrodynamic diameter and shape-spherical, elliptical, nail-shaped) and the pathway (via the intercellular route or the hair follicle).
Nanoparticles based on lipid systems are the most common type of nanoparticles studied for topical application.[18] Solid lipid nanoparticles, nanoemulsions, and nanostructured lipid carriers are the main types of matrix nanoparticles while liposomes are the main type of vesicular particles evaluated in permeation studies. Other types cited in the literature include niosomes, cubosomes, bicellar systems, vesicles, and nanodispersions. They have been investigated as delivery systems with respect to production, characterization and application because of their advantageous features. E.g., occlusive properties, increase in skin hydration, modified release profile, increase of skin penetration associated with a targeting effect and avoidance of systemic uptake. Elastic liposomes represent a novel concept, which grants to conventional liposomes the capability to deform and flow through capillaries such as the narrow pores of the skin. It has been found that these deformable fluid vesicles enhance skin permeation and carry the active compound into deeper layers of the skin. Liposomal preparations for skin delivery of dipotassium glycyrrhizinate, an anti-inflammatory agent has been employed in treating acute and chronic dermatitis.[19]
In contrast to the polymeric nanoparticles, they are less stable when applied on the skin surface. After topical application, solid lipid nanoparticles were observed to lose their shape and melt after a period of 2 h due to the interaction between the particle components and skin lipids.[20] This occurrence can reduce the skin barrier function and occlude the skin surface,[21] favoring the skin penetration process. Thus, an increase in the permeation to the receptor medium[22] or in the penetration into the skin layers of the active substance is commonly observed when it is encapsulated in different lipid-based nanoparticles, either matrical or vesicular. The lipid components[20] or the surfactant components of nanoparticles can interact with the skin.[21] Moreover, the addition of other components such as ethanol[22] and magnetic nanoparticles to lipid nanoparticles can even enhance the permeation. Topically delivered nanosized hyaluronic acid, which can easily penetrate the skin is under development. Solid lipid nanoparticles and nanostructured lipid carriers can be synthesized with an active ingredient in the center to delay release.[10]
Due to their unique size and composition-dependent properties, lipid nanoparticles pose the ability to penetrate through several anatomical barriers providing release of their contents and encouraging applications on the field of controlled drug delivery.[23] They are submicron colloidal carriers and their size typically ranges from 40 nm to 1000 nm. They differ from nanoemulsions in the fact that the lipid core is in the solid state [Figure 1e]. They form an occlusive film over the epidermis and enhance skin hydration by minimizing water loss. They also act as a physical UV blocker[17] and hence can be combined with organic sunscreens.
On the contrary, nanoemulsions have a lipophilic interior,[2] they are efficient at transporting hydrophobic substances in aqueous environments. In comparison to nanocapsules, the nanoemulsions present a higher capacity to penetrate and/or permeate the skin, probably due to their flexibility[24] and lack of polymer, which has an affinity with the stratum corneum. They can allow for deeper penetration of water immiscible active ingredients such as antioxidants, retinol, or lipid and increase their effective concentration in target tissues. They are being exploited as cutaneous delivery vehicles for anti-aging products.[2] Nanoemulsions are being created as drug delivery vehicles for topical use, intranasal and intratracheal use, as well as for ingestion and parenteral use. For example, topical nanoemulsions containing gamma-amino-butyric acid, an inhibitory neurotransmitter with muscle relaxing properties, are being studied for wrinkle reduction.[2] They form an ideal vehicle to be used in treatment of acne vulgaris as they are stable for the transport of lipophilic compounds into the skin. Nanoemulsions also do not have the problem of creaming, flocculation, coalescence and sedimentation, which are commonly associated with macro emulsions, thus ensuring better stability of the formulation.
A wide variety of nanomaterials have already entered into modern society and new ones are being developed at a prodigious rate e.g., quantum dots can be coupled to tags such as antibodies, and have proved useful in animal models for visualization of skin tumors without the use of radioactivity.[10] Nanopunch (consisting of copper, nickel, chromium, and silicon) is a latest biopsy tool for collecting samples from challenging sites like nail matrix or fascia.[10] However, it is still a vast field which needs further exploration to come up with efficient drug delivery nanocarriers keeping in mind the size, route, and rheological properties.
NANODERMATOLOGY: THE FUTURE HAZARDS
New technologies are often wondrous and even magical, yet they can be burdened with unintended consequences. These nanoscaled drug delivery vehicles may lead to the emergence of whole new classes of irritants, allergens, haptens, cross-reactants, and unanticipated particle-particle interactions, which may lead to diseases. Theoretically speaking, nanoparticles are harmful because of their extremely small size that leads to an exponential increase in the surface-volume ratio. They can not only get absorbed in the body and cause tissue and cell destruction but may even stay lodged in the environment and lead to nanopollution.[25] Skin exposure to NP-containing sunscreens leads to incorporation of TiO2 and ZnO NPs in the stratum corneum, which can alter specific NP attenuation properties due to particle–particle, particle–skin, and skin–particle–light physicochemical interactions. Both sunscreen NPs induce (photo) cyto- and genotoxicity and have been sporadically observed in viable skin layers especially in case of long-term exposures and ZnO. Studies have shown carbon nanotubes to be cytotoxic and to induce granuloma in lungs of laboratory animals.[26]
Very little is known about the safety aspects of the nano-engineered materials that are being released in our environment, in consumer products, and health care products. Hence, it is critical for us as a society intimately involved with the health of the people to be aware of this new technology, to educate our own members about it, and to play an active role in evaluating this technology and setting policies and guidelines for its safe and fruitful use. Despite all the hype surrounding nanotechnology, only the time will tell it will be a boon or bane.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Venugopal J, Prabhakaran MP, Low S, Choon AT, Zhang YZ, Deepika G, et al. Nanotechnology for nanomedicine and delivery of drugs. Curr Pharm Des. 2008;14:2184–200. doi: 10.2174/138161208785740180. [DOI] [PubMed] [Google Scholar]
- 2.Nasir A. Nanotechnology and dermatology: Part II-risks of nanotechnology. Clinics in Dermatology. Sep-Oct. 2010;28:581–8. doi: 10.1016/j.clindermatol.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 3.Hadgraft J. Skin, the final frontier. Int J Pharm. 2001;224:1–18. doi: 10.1016/s0378-5173(01)00731-1. [DOI] [PubMed] [Google Scholar]
- 4.Guterres SS, Alves MP, Pohlmann AR. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights. 2007;2:147–57. [PMC free article] [PubMed] [Google Scholar]
- 5.Lademann J, Richter H, Teichmann A, Otberg N, Blume-Peytavi U, Luengo J, et al. Nanoparticles: An efficient carrier for drug delivery into the hair follicles. Eur J Pharm Biopharm. 2007;66:159–64. doi: 10.1016/j.ejpb.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 6.Crosera M, Bovenzi M, Maina G, Adami G, Zanette C, Florio C, et al. Nanoparticle dermal absorption and toxicity: A review of the literature. Int Arch Occup Environ Health. 2009;82:1043–55. doi: 10.1007/s00420-009-0458-x. [DOI] [PubMed] [Google Scholar]
- 7.Ulbrich W, Lamprecht A. Targeted drug-delivery approaches by nanoparticulate carriers in the therapy of inflammatory diseases. J R Soc Interface. 2010;7:S55–66. doi: 10.1098/rsif.2009.0285.focus. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lboutounne H, Faivre V, Falson F, Pirot F. Characterization of transport of chlorhexidine-loaded nanocapsules through hairless and wistar rat skin. Skin Pharmacol Physiol. 2004;17:176–82. doi: 10.1159/000078820. [DOI] [PubMed] [Google Scholar]
- 9.Degim IT. New tools and approaches for predicting skin permeability. Drug Discov Today. 2006;11:517–23. doi: 10.1016/j.drudis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Nasir A. Nanodermatology: A bright glimpse just beyond the horizon-part I. Skin Therapy Lett. 2010;15:1–4. [PubMed] [Google Scholar]
- 11.Bronaugh RL, Stewart RF. Methods for in vitro percutaneous absorption studies IV: The flow-through diffusion cell. J Pharm Sci. 1985;74:64–7. doi: 10.1002/jps.2600740117. [DOI] [PubMed] [Google Scholar]
- 12.Bronaugh RL, Collier SW. Preparation of human and animal skin. In: Bronaugh RL, Maibach HI, editors. In vitro Percutaneous Absorption: Principles, Fundamentals, and Applications. Vol. 1. Boca Raton: CRC Press; 1991. pp. 2–5. [Google Scholar]
- 13.Xu ZP. Inorganic nanoparticles as carriers for efficient cellular delivery. Chem Eng Sci. 2006;61:1027–40. [Google Scholar]
- 14.Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: Focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95–112. doi: 10.2147/NSA.S19419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pinheiro T, Pallon J, Alves LC, Verıssimo A, Filipe P, Silva JN, et al. The influence of corneocyte structure on the interpretation of permeation profiles of nanoparticles across skin. Nucl Instrum Methods Phys Res. 2007;B260:119–23. [Google Scholar]
- 16.Elkeeb R, AliKhan A, Elkeeb L, Hui X, Maibach HI. Transungual drug delivery: Current status. Int J Pharm. 2010;384:1–8. doi: 10.1016/j.ijpharm.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Crissey JT. Common dermatophyte infections. A simple diagnostic test and current management. (197-200).Postgrad Med. 1998;103:191–2. doi: 10.3810/pgm.1998.02.359. 205. [DOI] [PubMed] [Google Scholar]
- 18.Küchler S, Radowski MR, Blaschke T, Dathe M, Plendl J, Haag R, et al. Nanoparticles for skin penetration enhancement: A comparison of a dendritic core-multishell-nanotransporter and solid lipid nanoparticles. Eur J Pharm Biopharm. 2009;71:243–50. doi: 10.1016/j.ejpb.2008.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Trotta M, Peira E, Debernardi F, Gallarate M. Elastic liposomes for skin delivery of dipotassium glycyrrhizinate. Int J Pharm. 2002;241:319–27. doi: 10.1016/s0378-5173(02)00266-1. [DOI] [PubMed] [Google Scholar]
- 20.Junyaprasert VB, Teeranachaideekul V, Souto EB, Boonme P, Müller RH. Q10-loaded NLC versus nanoemulsions: Stability, rheology and in vitro skin permeation. Int J Pharm. 2009;377:207–14. doi: 10.1016/j.ijpharm.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 21.Dubey V, Mishra D, Jain NK. Melatonin loaded ethanolic liposomes: Physicochemical characterization and enhanced transdermal delivery. Eur J Pharm Biopharm. 2007;67:398–405. doi: 10.1016/j.ejpb.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Wissing SA, Kayser O, Müller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56:1257–72. doi: 10.1016/j.addr.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Wissing SA, Müller RH. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity: In vivo study. Eur J Pharm Biopharm. 2003;56:67–72. doi: 10.1016/s0939-6411(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 24.Olvera-Martínez BI, Cázares-Delgadillo J, Calderilla-Fajardo SB, Villalobos-García R, Ganem-Quintanar A, Quintanar-Guerrero D. Preparation of polymeric nanocapsules containing octyl methoxycinnamate by the emulsification-diffusion technique: Penetration across the stratum corneum. J Pharm Sci. 2005;94:1552–9. doi: 10.1002/jps.20352. [DOI] [PubMed] [Google Scholar]
- 25.Nel A, Xia T, Mädler L, Li N. Toxic potential of materials at the nanolevel. Science. 2006;311:622–7. doi: 10.1126/science.1114397. [DOI] [PubMed] [Google Scholar]
- 26.Nasir A. Nanodermatology: A glimpse of caution just beyond the horizon-part II. Skin Therapy Lett. 2010;15:4–7. [PubMed] [Google Scholar]


