Abstract
Background:
Androgenetic alopecia (AGA) or male pattern baldness (MPB) has been found to be associated with the risk of coronary artery disease (CAD). The well-known risk factors are family history of CAD, hypertension, increased body mass index (BMI), central obesity, hyperglycemia, and dyslipidemia. The newer risk factors are serum lipoprotein-a (SL-a), serum homocysteine (SH), and serum adiponectin (SA).
Aim:
Identifying individuals at risk of CAD at an early age might help in preventing CAD and save life. Hence, a comparative study of CAD risk factors was planned in 100 males of AGA between the age of 25 and 40 years with equal number of age- and sex-matched controls.
Materials and Methods:
Patients of AGA grade II or more of Hamilton and Norwood (HN) Scale and controls were examined clinically and advised blood test. The reports were available for fasting blood sugar (FBS), serum total serum cholesterol (SC) in 64 cases, 64 controls; lipoproteins (high, low, very low density, HDL, LDL, VLDL), serum triglycerides (ST) in 63 cases, 63 controls; SL-a in 63 cases, 74 controls; SH in 56 cases, 74 controls; and SA in 62 cases, 74 controls.
Results:
In these cases family history (FH) of AGA and CAD was significantly high. The blood pressure (BP) was also found to be significantly high in the cases. The difference of mean serum HDL, LDL, VLDL, ST, SH, and SL-a in cases and controls were statistically significant and with increasing grade of AGA, the risk factors also increased.
Conclusion:
Patients with AGA appear to be at an increased risk of developing CAD, therefore, clinical evaluation of cases with AGA of grade II and above may be of help in preventing CAD in future.
Keywords: Androgenetic alopecia and risk of coronary artery disease, androgenetic alopecia, coronary artery disease risk factors, male pattern baldness
INTRODUCTION
Androgenetic alopecia (AGA) or male pattern baldness (MBP), characterized by frontal and vertex scalp hair loss, commonly affects males from third decade onwards.[1,2] It can have a significant adverse effect on quality of life and its prevalence in Asian men is probably higher than often suspected.[3,4] AGA has been found to be associated with coronary artery disease (CAD) and enlargement of prostate.[5,6] The well-known risk factors of CAD are family history (FH) of CAD, hypertension, increased body mass index (BMI), central obesity, hyperglycemia, and dyslipidemia.[7,8,9,10,11] The newer risk factors are serum lipoprotein-a (SL-a) serum, homocysteine (SH), and serum adiponectin (SA).[12,13,14]
It was aimed to study above CAD risk factors in male patients with AGA in the age group of 25 to 40 years and to compare them with controls. This age group was chosen because usually CAD may not be present in this age but risk factors would have started developing. If resources are scarce and expensive screening tests for CAD are not easily available, it may help target a group of patients or identify individuals in which it may be possible to apply interventions to reduce the occurrence of CAD and save life.
MATERIALS AND METHODS
Patients were selected from Skin and Venereal Disease out-patient-department, S. S. Hospital, Banaras Hindu University, Varanasi, from January 2009 to June 2010. The study was done on 100 male patients presenting with AGA between the age of 25 and 40 years and on 100 age- and sex-matched controls (for other minor skin problems) after obtaining informed witnessed consent. This study was assessed and approved by the ethics committee and review board of Institute of Medical Sciences, BHU.
Cases of AGA grade II or more of Hamilton and Norwood (HN)[1,2] scale were included in the study and patients suffering from CAD, telogen effluvium, cicatricial alopecia, traction alopecia, and using any medication for alopecia were excluded. Details of hair loss, treatment taken, past history, FH of AGA, CAD, and disease of prostate and other chronic illnesses were noted.
General examination findings of pulse, blood pressure (BP) (120/80 mmHg, normal; >120-140/>80-90, borderline; >140/90, hypertension), BMI (weight in kg/height in meters square, 19-25, normal; 26-30, overweight; >30, obese), waist (abdominal circumference at umbilicus), and hip ratio (waist/hip ratio (WHR) >0.9, obese) of patients were recorded. Systemic examination, including cardiovascular, was done and grade of AGA was noted on proforma (printed with 24 scalp figures including vertex and temporal view of scalp of AGA grade I, II, IIa, III, III vertex, IIIa, IV, IVa, V, Va, VI, and VII).[1,2] Clinical examination of controls was performed similarly.
The blood samples (5 ml after 12-h fasting) of all cases and controls were collected in plain vials at the laboratory in the Department of Basic principles, IMS. Blood could be tested for fasting blood sugar (FBS), total serum cholesterol (SC) in 64 cases, 64 controls; HDL, LDL, VLDL, serum triglycerides (ST) in 63 cases, 63 controls; SL-a in 63 cases, 74 controls; SH in 56 cases, 74 controls, and SA in 62 cases and 74 controls. The values obtained were compared using Student's t-test for parametric variables and the Chi-square test for non-parametric variables. The difference was taken as significant if probability (P) was < 0.05.
RESULTS
About two third of the patients were in the age group of 25 to 30 years. The mean age of cases was 28.61 years ± 3.031 years and mean age in controls was 28.45 years ± 3.109. More than 40% of both cases and controls were students and the difference in occupation was not significant.
FH of AGA was found in 64% cases, 41% controls and CAD in 37% cases and 27% controls, which was significantly high in the cases (P < 0.002). FH of prostatic disease was positive in patients and controls.
The AGA was of II or III grade in 65% and IV or V in 31% of patients. The percentage of cases with normal, borderline, or high BP were 53, 37, and 10 as compared to 84, 15, and 1 of controls, respectively, which was significantly high in cases (P < 0.0001).
The difference in mean in cases and controls of BMI (23.108 ± 1.589 and 22.597 ± 1.599, P = 0.89), WHR (0.843 ± 0.091 and 0.836 ± 0.109, P = 0.12), FBS (102.9 mg/dl ± 0.21 and 103.87 ± 11.700, P = 0.125), SC (172.11 mg/dl ± 14.644 and 163.61 ± 11.619, P = 0.267), and SA (8.48 ± 2.44 and 12.50 ± 1.682, P = 0.134) were statistically insignificant.
The differences of mean in cases and controls of serum HDL (P < 0.002), LDL (P < 0.0001), VLDL (P < 0.0002), ST (P < 0.0001, Table 1), SH (P < 0.00001, Table 2), and SL-a (P < 0.00001, Table 3) were statistically significant. It was also observed that all the significant risk factors i.e., serum LDL, VLDL, ST, SL-a, and SH increased with increasing grade of AGA [Table 4, Figures 1 and 2].
Table 1.
HDL, LDL, VLDL, and ST level of 63 cases and 63 controls
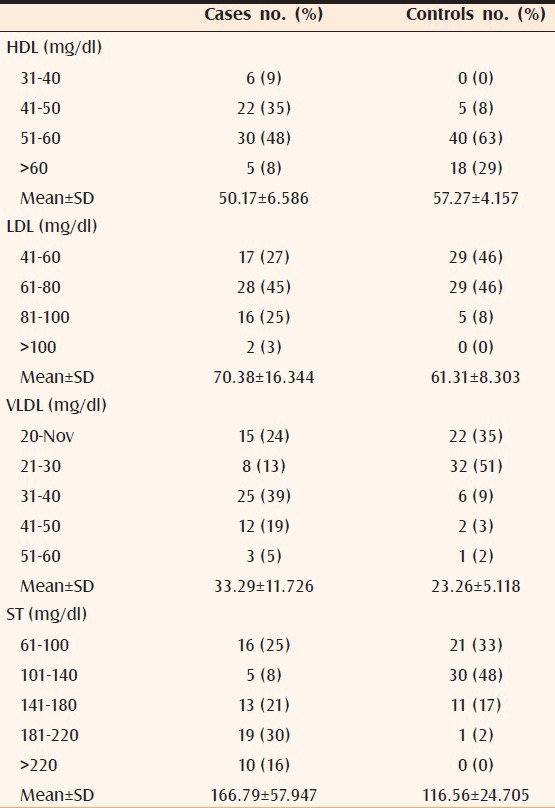
Table 2.
SH level of 56 patients and 74 controls
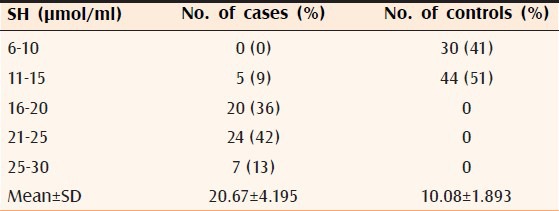
Table 3.
SL-a level of 63 cases and 74 controls
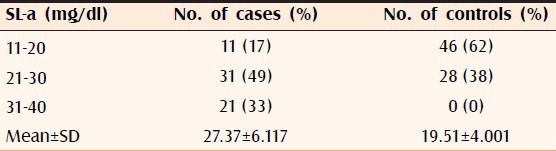
Table 4.
Relation of significant risk factors with grade of AGA
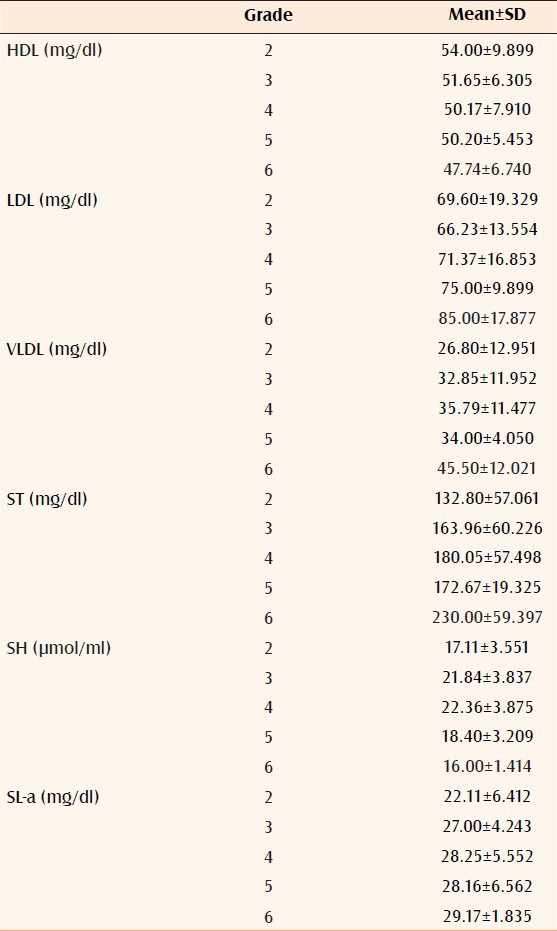
Figure 1.
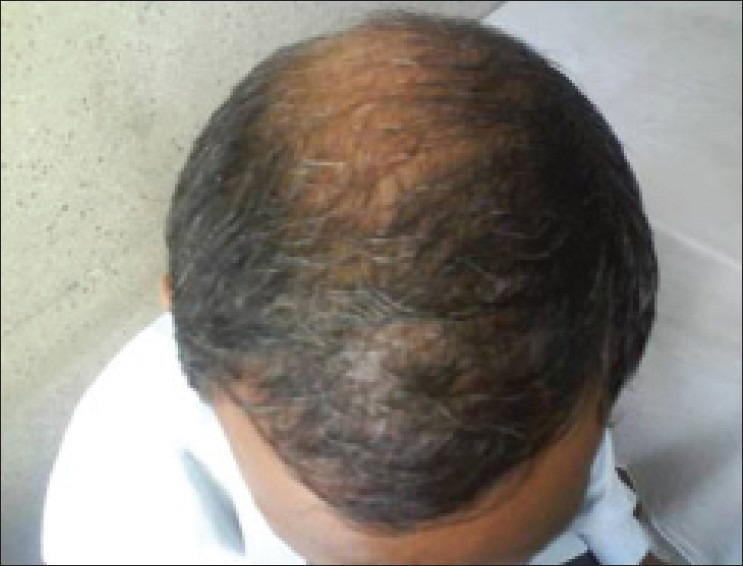
Male 35 year, AGA grade V, FH of CAD, BP borderline, low HDL, high TG and SH; so predisposed to CAD
Figure 2.
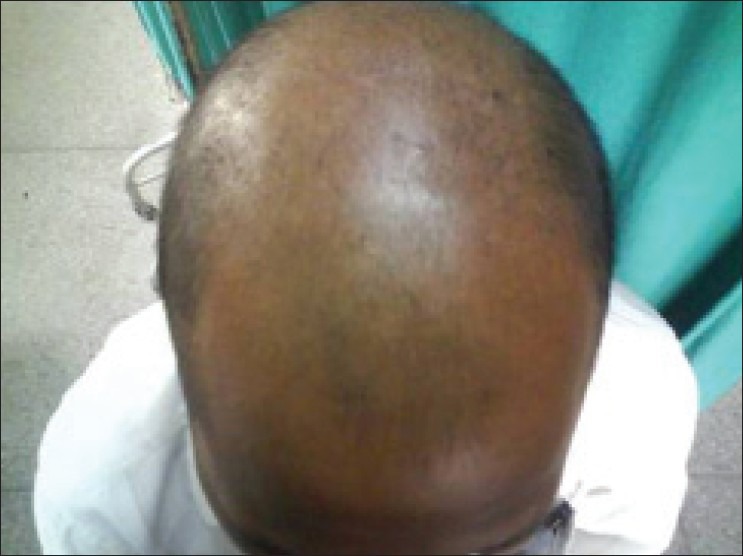
Male 36 year, AGA grade VI, low HDL, high LDL, VLDL, LP-a and SH and low SA; so at risk of CAD
DISCUSSION
AGA is said to progress in an orderly pattern with wide variation in individual patterns. Its clinical features have been described as recession of the frontal hair line leading to balding of the scalp vertex. The hair loss progresses up till only a rim of normal hair growth at the sides and back of the scalp remains. Gradual transition also occurs from large thick-pigmented terminal hairs to thinner, shorter, indeterminate hairs, and finally to non-pigmented vellus hairs in the involved areas when eventually hairs disappear completely. When hair loss is diffuse over the crown and frontal scalp with retention of the frontal hair line, it may resemble female pattern hair loss.[15] The HN classification is a method of clinically categorizing AGA.[1,2]
CAD is described as narrowing of coronary arteries supplying blood and oxygen to the heart which can slow down or stop due to plaque buildup of fatty material called atherosclerosis leading to chest pain, stable angina, shortness of breath, other symptoms, and heart attack.[16]
In this study, FH of both AGA and CAD was significantly high. The results were similar in previous studies.[5] There is higher frequency of balding in the fathers of bald men than in the fathers of non-bald men.[17] It is said that baldness has evolved in males through sexual selection as an enhanced signal of aging and social maturity. A variety of genetic and environmental factors are said to play a role in causing AGA. Androgen receptor gene which is X-linked recessive has been mentioned to be the cardinal prerequisite for balding but other genes are also involved. Genetic sensitivity of hair follicles to dihydrotestosterone (DHT) causes them to shrink when exposed to it.[18] 5-alphareductase is responsible for converting free testosterone (a major circulating androgen in men) into DHT.[15] Hair growth inhibitory factor-β, released from androgen-stimulated fibroblasts of the follicular dermal papilla may cause hair growth inhibition and hair follicle miniaturization, short lifespan, thereby preventing normal hair production.[19]
BP was significantly high in cases as compared to controls. High BP is known to accelerate atherosclerosis.[20] The differences in BMI and WHR, in cases and controls, were found to be statistically insignificant. This might be attributed to the relatively young age of cases, because the prevalence of obesity is strongly related to age and those between 25 and 34 years have the second lowest rate of obesity in adults.[8,9] Increased hip circumference is associated with increased hip subcutaneous fat, gluteal muscle, and total leg muscle mass. Leg muscle mass may represent an indirect measure of physical activity, which is inversely related to cardio-metabolic risk.[20]
There was a statistically significant difference in LDL, HDL, VLDL, and TG of cases and controls which have also been reported previously.[21] Elevated levels of LDL are thought to be a key determinant of CAD risk and that level of HDL is inversely related to risk. The protective effect of HDL is at least as strong as the atherogenic effect of LDL and is independent of lipids and other risk factors. Every change of 10 mg/dl in the HDL is associated with a 50% change in risk. At any level of SC, the risk varies widely depending on the LDL/HDL ratio. In SC, normally LDL (“bad”) cholesterol should be as low as possible, and HDL (“good”) cholesterol should be as high as possible.[11]
A significant elevation in SL-a was found in cases as compared to controls which has also been reported earlier.[21] Increased level of SL-a is said to be associated with endothelial dysfunction and heart disease as it has a structure similar to LDL but is attached to a glycoprotein called apolipoprotein-a. It has sticky adhesive nature and can easily attach to LDL, calcium, and other components in an atherosclerotic plaque on the blood vessel wall. Due to its similarity with plasminogen, it competes for binding with fibrin inhibiting its breakdown and may promote blood clot formation. It also activates immune cells including monocytes and macrophages which help in inducing inflammation. These effects may help in inducing plaque formation and promote clot formation after the plaque is ruptured.[14]
There was significant elevation of SH in cases. Homocysteine is a sulfur-containing amino acid derived from the metabolism of dietary methionine.[12] Its concentrations are determined by genetic and nutritional factors. There is an inverse relationship between SH and vascular endothelial function. Diet-related increments in SH may contribute to the development and progress of atherosclerosis and thereby CAD. An acute increase in SH is associated with rapid onset vascular endothelial dysfunction which is an early manifestation of atherosclerosis and this effect may be mediated by an increase in oxidation stress.[22]
SA level was not significantly reduced in cases as compared to controls. SA is a 244-amino acid protein that, despite being derived solely from adipose tissue, is paradoxically reduced in obesity. It is the most abundant adipocytokine of adipose tissue cells and has recently been found to be decreased in CAD.[23] It was also observed that all the significant risk factors i.e. low HDL, high LDL, VLDL, TG, SL-a, and SH increased with increasing grade of AGA [Table 4, Figures 1 and 2].
Patients with AGA might be at an increased risk of CAD which increases with increasing grade of AGA. Thus, clinical evaluation of cases of AGA for risk factors of CAD can help in preventing a dreaded disease.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hamilton JB. Male hormone stimulation is prerequisite and an incitant in common baldness. Am J Anat. 1942;71:451–80. [Google Scholar]
- 2.Norwood OT. Male pattern baldness: Classification and incidence. South Med J. 1975;68:1359–65. doi: 10.1097/00007611-197511000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Paus R, Olsen EA, Messenger AG. Hair Growth Disorders. In: Wolff K, Goldsmith LA, Katz SI, Gilchrest BA, Paller AS, Leffell DJ, editors. Fitzpatrick's Dermatology In General Medicine. 7th ed. New York: McGraw Hill; 2008. pp. 753–72. [Google Scholar]
- 4.Pathomvanich D, Pongratananukul S, Thienthaworn P, Manoshai S. A random study of Asian male androgenetic alopecia in Bangkok, Thailand. Dermatol Surg. 2002;28:804–7. doi: 10.1046/j.1524-4725.2002.02036.x. [DOI] [PubMed] [Google Scholar]
- 5.Trevisan M, Farinaro E, Krogh V, Josssa F, Giumetti D, Fusco G, et al. Baldness and coronary heart disease risk factors. J Clin Epidemiol. 1993;46:1213–8. doi: 10.1016/0895-4356(93)90121-g. [DOI] [PubMed] [Google Scholar]
- 6.Chen W, Yang CC, Chen GY. Patients with a large prostate show a higher prevalence of androgenetic alopecia. Arch Dermatol Res. 2004;296:245–9. doi: 10.1007/s00403-004-0514-z. [DOI] [PubMed] [Google Scholar]
- 7.Guttmacher AE, Collins FS, Carmona RH. The family history- more important than ever. N Engl J Med. 2004;351:2333–6. doi: 10.1056/NEJMsb042979. [DOI] [PubMed] [Google Scholar]
- 8.Borghans L, Golsteyn BH. Time Discounting and the Body Mass Index: Evidence from the Netherlands. Econ Hum Biol. 2006;4:39–61. doi: 10.1016/j.ehb.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Seidell JC, Pérusse L, Després JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: The Quebec Family Study. Am J Clin Nutr. 2001;74:315–21. doi: 10.1093/ajcn/74.3.315. [DOI] [PubMed] [Google Scholar]
- 10.Nielson C, Lange T, Hadjokas N. Blood Glucose and Coronary Artery Disease in Non diabetic Patients. Diabetes Care. 2006;29:998–1001. doi: 10.2337/diacare.295998. [DOI] [PubMed] [Google Scholar]
- 11.Wranicz JK, Cygankiewicz I, Rosiak M, Kula P, Kula K, Zareba W. The relationship between sex hormones and lipid profile in men with coronary artery disease. Int J Cardiol. 2005;101:105–10. doi: 10.1016/j.ijcard.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Hankey JH, Eikelboom JW. Homocysteine and vascular disease. Lancet. 1999;354:407–13. doi: 10.1016/S0140-6736(98)11058-9. [DOI] [PubMed] [Google Scholar]
- 13.Schnabel R, Messow CM, Lubos E, Espinola-Klein C, Rupprecht HJ, Bickel C, et al. Serum adiponectin levels in coronary artery disease patients results from the Atherogene study. Eur Heart J. 2008;29:649–57. doi: 10.1093/eurheartj/ehn009. [DOI] [PubMed] [Google Scholar]
- 14.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically Elevated Lipoprotein (a) and Increased Risk of Myocardial Infarction. JAMA. 2009;301:2331–9. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 15.Kaufman KD. Androgen metabolism as it affects hair growth in androgenetic alopecia. Dermatol Clin. 1996;14:697–711. doi: 10.1016/s0733-8635(05)70396-x. [DOI] [PubMed] [Google Scholar]
- 16.Cotton SG, Nixon JM, Carpenter RG, Evans DW. Factors discriminating men with coronary heart disease from healthy controls. Br Heart J. 1972;34:458–64. doi: 10.1136/hrt.34.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ellis JA, Stebbing M, Harrap SB. Genetic analysis of male pattern baldness and the 5- alpha reductase genes. J Invest Dermatol. 1998;110:849–53. doi: 10.1046/j.1523-1747.1998.00224.x. [DOI] [PubMed] [Google Scholar]
- 18.Nyholt DR, Gillespie NA, Heath AC, Martin NG. Genetic basis of male pattern baldness. J Invest Dermatol. 2003;121:1561–4. doi: 10.1111/j.1523-1747.2003.12615.x. [DOI] [PubMed] [Google Scholar]
- 19.Hamada K, Randall VA. Inhibitory autocrine factors produced by the mesenchyme-derived hair follicle dermal papilla may be a key to male pattern baldness. Br J Dermatol. 2006;154:609–18. doi: 10.1111/j.1365-2133.2006.07144.x. [DOI] [PubMed] [Google Scholar]
- 20.Chowdhury B, Lantz H, Sjostrom L. Computed tomography-determined body composition in relation to cardiovascular risk factors in Indian and matched Swedish males. Metabolism. 1996;45:634–44. doi: 10.1016/s0026-0495(96)90036-0. [DOI] [PubMed] [Google Scholar]
- 21.Sasmaz S, Senol M, Ozcan A, Dogan G, Tuncer C, Akyol O, et al. The risk of coronary heart disease in men with androgenetic alopecia. J Eur Acad Dermatol Venereol. 1999;12:123–5. [PubMed] [Google Scholar]
- 22.Jonasson T, Ohlin AK, Gottsäter A, Hultberg B, Ohlin H. Plasma homocysteine and markers for oxidative stress and inflammation in patients with coronary artery disease: A prospective randomized study of vitamin supplementation. Clin Chem Lab Med. 2005;43:628–34. doi: 10.1515/CCLM.2005.108. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura Y, Shimada K, Fukuda D, Shimada Y, Ehara S, Hirose M, et al. Implications of plasma concentrations of adiponectin in patients with coronary artery disease. Heart. 2004;90:528–33. doi: 10.1136/hrt.2003.011114. [DOI] [PMC free article] [PubMed] [Google Scholar]


