Abstract
Onychomycosis, traditionally referred as a non-dermatophytic infection of the nail, is now used as a general term to denote any fungal nail infection. It is an important public health problem due to its increasing incidence and has significant clinical consequences in addition to serving as a reservoir of infection. We report a case of Onychomycosis in all 10 fingers of an immunocompetent male with no co-morbid conditions caused by a non-dermatophytic fungus, Aspergillus niger. To the best of our knowledge, this is the first case of its kind to be reported.
Keywords: Aspergillus niger, onychomycosis, superficial infections
INTRODUCTION
Onychomycosis is a fungal infection of nails caused by dermatophytes, yeasts, and non-dermatophytic molds where dermatophytes account for nearly 70% of the cases.[1] It accounts for upto 50% of nail disorders[2] and 1.5-15% of patients presenting to the dermatologist.[1] Several factors have been implicated in the increase in disease incidence such as reduced peripheral circulation, diabetes, nail trauma, and poor nail hygiene. Although not life-threatening, it may have significant clinical consequences such as secondary bacterial infections, chronicity, therapeutic difficulties, and disfigurement apart from serving as a reservoir of infection.[2]
Dermatophytes are the most frequently implicated causative agents in onychomycosis (nearly 90% in toenail and at least 50% in fingernail infections). Previously regarded as contaminants, yeasts are now increasingly recognized as pathogens in fingernail infections, as are some non-dermatophytic molds.[3] Here, we report a case of onychomycosis due to Aspergillus niger. To the best of our knowledge, this is the first report of a non-dermatophytic mold infecting all 10 fingers of an immunocompetent male.
CASE REPORT
A 42-year-old male vegetable vendor by occupation, presented to the Dermatology out-patient department of our hospital with brownish black discoloration and disfigurement of all the nails of 8 months duration. The patient first noticed it on the nail of the right index and middle finger which gradually spread to the other fingers and all the fingers of the left hand. On examination, all fingernails were brownish black in color with loss of texture and there were dystrophic changes as well as onycholysis in most of the fingers [Figure 1].
Figure 1.
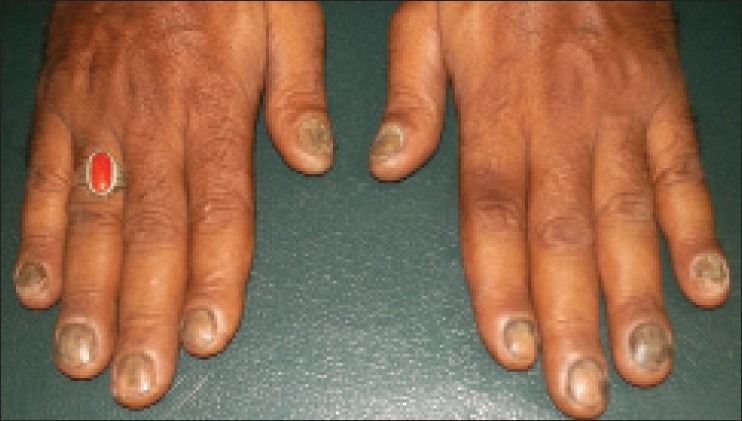
All 10 fingernails showing brownish black discoloration and dystrophic changes
Multiple specimens of nail scrapings and clippings from different parts of the affected nails were collected from all fingers after cleaning the area with 70% alcohol. The samples were sent to the Microbiology department where direct examination by 40% Potassium hydroxide mount was done that showed branched septate hyphae. The clippings were cultured on two sets of Sabouraud's dextrose agar with and without antibiotics and cycloheximide which were incubated at room temperature and 37°C.
After 3 days, growth was seen in all the tubes in the form of cottony white mycelium growth, which was soon covered with abundant black spores [Figure 2]. No bacterial growth was detected in the culture. Microscopic characterization of the fungal isolate was carried out by preparing a lactophenol cotton blue mount from the growth. They mostly consisted of erect conidiophores. The conidiophores terminated in a vesicle covered with phialides (biseriate). The secondary phialide bore chains of globose conidia were dark and covered the entire surface. The conidial head was large, black, and radiate. The fungal isolate was confirmed to be Aspergillus niger by these features. Repeat culture of the nail samples yielded the same organism.
Figure 2.
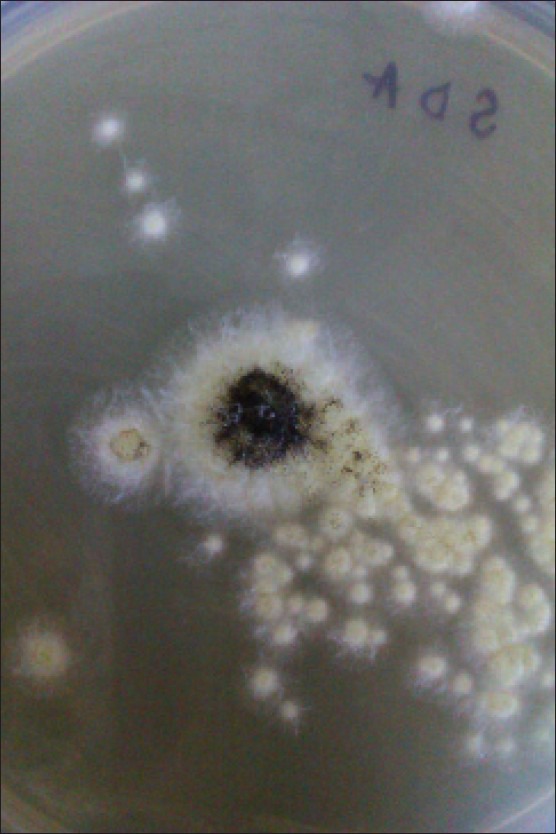
Colonies of Aspergillus niger growing on Sabouraud's dextrose agar with antibiotics at 25°C
Complete blood count was normal except for a slight anemia with a hemoglobin of 11 g/dl. The fasting and postprandial blood sugar levels were normal; the tests for Human Immunodeficiency Virus(HIV) and Hepatitis B surface antigen (HBsAg) were negative. The patient gave no history of alcohol consumption, smoking, or any drug addiction. The patient was started on oral itraconazole 200 mg BD for 7 days for 2 consecutive months (oral Itraconazole pulse therapy). After 2 months, his nails did not show much improvement in the appearance, though the microscopy and culture of the nail scrapings were negative for fungal elements. So he was given pulse therapy of itraconazole for a further 2 months and was reassured that the nail color and texture will take some time to regain normalcy.
DISCUSSION
Factors such as ageing, immunodeficiency, trauma, hyperhidrosis, socio-economic status, climatic conditions, and paronychia predispose to onychomycosis. Non-dermatophytic molds cause 1.5-6% of onychomycosis.[1] This is seen most frequently in elderly, in patients with skin diseases that affect the nails, and in the immunocompromised patients. Also, toenails are 25 times more likely to be infected than finger nails as the causative molds are ubiquitous fungi seen in soil, water, and decaying vegetations.[3] However, in our case, though the patient was middle aged and immunocompetent, his occupation involved handling fruits without wearing gloves which could be the predisposing factor.
Aspergillus onychomycosis is generally a distal subungual infection: It starts under the nail near the tip of the finger, where spores may have lodged or at the sides where the nail creases the skin. Once the fungus starts to grow, the infection spreads back toward the cuticle. It looks much the same as any fungal nail infection, discoloring the nail, causing it to become thick, distorted, and flaky. The fungus will not, however, spread to the surrounding skin like some other fungal causes of nail infection. Aspergillus species growing in nature often produce colorful pigments; therefore, an aspergillus nail infection may well appear greenish, black, brown, or various other shades.
It is not possible for a dermatologist to diagnose aspergillus onychomycosis just by looking at the affected nail. The proof of the ability of the organism to produce the nail infection depends on the direct demonstration of the fungus in the infected nail and its culture on artificial media. The need for meticulous examination of these preparations should be emphasized since it is quite possible that they may be overlooked in too cursory an examination.
Treatment of non-dermatophytic onychomycosis is not well standardized and can be difficult due to hardness of nail plate and location of infection between the nail bed and plate. However, aspergillus onychomycosis is shown to respond well to therapy.[4] Systemic antifungals such as itraconazole have been used successfully. The duration of therapy depends on the nails that are affected and the extent of infection. Affected fingernails typically require 3 months of therapy and toenails 6 months.[5] However, in our case, the patient was given itraconazole pulse therapy for 4 months because even though the microscopy and culture were negative after 2 months, the discoloration of the nails failed to improve indicating that there might be a persistent reservoir of infection.
Onychomycosis is a condition which may create a number of clinical and occupational complications which can significantly impair the quality of life of a patient. Thus, isolation of the causative fungi is essential in all suspected cases of onychomycosis and the type and duration of treatment varies according to the pathogen isolated. Though aspergillus specieshave been implicated in causation of onychomycosis, to the best of our knowledge, this is the first case of Aspergillus niger causing infection in all 10 fingernails in an immunocompetent patient.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Sageerabanoo, Malini A, Oudeacoumar P, Udayashankar C. Onychomycosis due to Trichosporonmucoides. Indian J Dermatol Venereol Leprol. 2011;77:76–7. doi: 10.4103/0378-6323.75001. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja S, Malhotra S, Charoo H. Etiological agents of onychomycosis from a tertiary care hospital in central Delhi, India. Indian J Fundam Appl Life Sci. 2011;1:11–4. [Google Scholar]
- 3.Kaur R, Kashyap B, Bhalla P. Onychomycosis-Epidemiology, diagnosis and management. Indian J Med Microbiol. 2008;26:108–16. doi: 10.4103/0255-0857.40522. [DOI] [PubMed] [Google Scholar]
- 4.Kristensen L, Stenderup J, Otkjaer A. Onychomycosis due to Aspergillustamarii in a 3-year-old boy. ActaDermVenereol. 2005;85:261–2. doi: 10.1080/00015550510025605. [DOI] [PubMed] [Google Scholar]
- 5.Denning DW, Evans EG, Kibbler CC, Richardson MD, Roberts MM, Rogers TR, et al. Fungal nail disease: A guide to good practice (report of a Working Group of the British Society for Medical Mycology) BMJ. 1995;311:1277–81. doi: 10.1136/bmj.311.7015.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]


