Abstract
Autoimmune sensorineural hearing loss (ASNHL) is characterized typically by bilateral, rapidly progressive hearing loss that responds therapeutically to corticosteroid treatment. Despite its name, data implicating autoimmunity in the etiopathogenesis of ASNHL have been limited, and targeted self-antigens have not been identified. In the current study we show that the inner ear–specific proteins cochlin and β-tectorin are capable of targeting experimental autoimmune hearing loss (EAHL) in mice. Five weeks after immunization of SWXJ mice with either Coch 131–150 or β-tectorin 71–90, auditory brainstem responses (ABR) showed significant hearing loss at all frequencies tested. Flow cytometry analysis showed that each peptide selectively activated CD4+ T cells with a proinflammatory Th1-like phenotype. T cell mediation of EAHL was determined by showing significantly increased ABR thresholds 6 weeks after adoptive transfer of peptide-activated CD4+ T cells into naive SWXJ recipients. Immunocytochemical analysis showed that leukocytic infiltration of inner ear tissues coincided with onset of hearing loss. Our study provides a contemporary mouse model for clarifying our understanding of ASNHL and facilitating the development of novel effective treatments for this clinical entity. Moreover, our data provide experimental confirmation that ASNHL may be a T cell–mediated organ-specific autoimmune disorder of the inner ear.
Introduction
Autoimmune sensorineural hearing loss (ASNHL) is a clinical entity that typically involves bilateral, rapidly progressive hearing loss, usually in people between the ages of 30 and 60 years (1). ASNHL is diagnosed by exclusion of other disorders that mimic ASNHL, including Meniere disease, retrocochlear disorders, ototoxicity, and other systemic diseases, and by showing a therapeutic response to corticosteroid treatment (2). Although its name implies an autoimmune etiopathogenesis, data implicating self-recognition events in the development and progression of ASNHL have been limited to a small but growing number of studies that provide predominantly circumstantial evidence.
Autoantibodies have long been suspected of mediating ASNHL. Sera from ASNHL patients frequently contain Ab’s capable of immunostaining human inner ear tissues (3–7). The specificity of the autoantibodies, however, and their pathogenic potential is currently unclear. Several studies have shown that ASNHL patients often have elevated serum Ab titers directed against a 68-kDa inner ear protein believed to be heat shock protein 70 (HSP-70) (8–12). Immunization of BALB/c or CBA/J mice with bovine HSP-70 failed to mediate hearing loss, despite inducing high-titer Ab responses to HSP-70 (13). Moreover, HSP-70 is expressed in a variety of non-inner ear tissues, including kidney and intestine, that do not show any reported pathologic changes linked to ASNHL (8, 9). Thus, there is a conspicuous lack of direct evidence that HSP-70 Ab’s are autonomously pathogenic in ASNHL. Although other studies have shown hearing loss in guinea pigs injected with the KHRI-3 IgG1 mAb generated against cochlear hair cells, the identity of the recognized antigen is currently unknown (14–16).
Autoreactive T cells have also been implicated in ASNHL. PBMCs from ASNHL patients have been shown to elicit increased inhibition of leukocyte migration (17), increased proliferation (18), and have increased frequencies of IFN-γ–secreting T cells in response to human inner ear homogenate (19). The involvement of T cells in ASNHL is supported by animal studies showing cochlear pathology and/or hearing loss following immunization of guinea pigs with inner ear homogenate (7, 20–22) and following adoptive transfer of Lewis rats with T cell lines specific for inner ear homogenate (23, 24). Most studies to date, however, have involved the use of bovine or human inner ear homogenate, thereby precluding characterization of the specific self-antigens as well as the autoreactive T cell repertoires involved in disease initiation and progression.
Our goal in the current study was to determine whether defined inner ear–specific proteins were capable of targeting T cell–mediated autoimmune hearing loss in mice. We selected cochlin (formerly known as Coch-5B2) as a candidate autoimmune target protein because it is highly expressed in inner ear tissues. Cochlin is a product of the COCH gene mapped in humans to chromosome 14q12-q13, and its mutations are associated with an autosomal dominant, nonsyndromic, progressive sensorineural hearing loss with vestibular pathology (DFNA9) (25–28). Cochlin is an integral part of the inner ear ECM and is expressed in the regions of the fibrocytes of the spiral limbus and of the spiral ligament, inner ear regions showing histologic abnormalities in humans with COCH mutations (28, 29). In adult humans, cochlin expression is substantially confined to the cochlear and vestibular labyrinth, whereas in the mouse, cochlin is also expressed in the spleen, cerebrum, cerebellum/medulla, thymus, and retina (26). Nevertheless, cochlin appears to be the most abundant protein expressed in inner ear tissues (30) and, as such, is likely to have a high level of constitutive presentation by local professional APCs. In addition, antigenicity to cochlin has been documented in ASNHL, with many patients showing elevated serum levels of cochlin Ab’s (31). We selected β-tectorin as an inner ear target protein because it has been isolated exclusively from inner ear tissues. β-tectorin, derived from the TECTB gene, has a sequence domain found in matrix-forming proteins involved in the formation of filaments, and its expression is closely associated with hair cells in the basal region of the hair bundle (32, 33).
In the present study we have identified cochlin and β-tectorin peptides that are capable of mediating experimental autoimmune hearing loss (EAHL) following immunization of autoimmune-susceptible SWXJ mice or following transfer of peptide-activated CD4+ Th1 cells into naive SWXJ recipients. Thus, our study provides a contemporary mouse model for ASNHL and indicates that proinflammatory CD4+ T cells specific for inner ear peptides may be sufficient for mediating the hearing loss observed in ASNHL.
Results
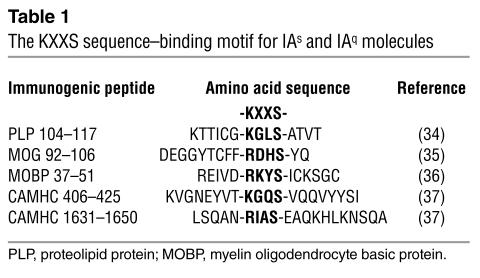
The KXXS MHC class II–binding motif.
To identify inner ear–specific T cell determinants, we exploited the KXXS sequence motif associated with IAq- and IAs-restricted CD4+ immunogenicity. Peptides containing a sequence with a lysine or conservatively substituted arginine residue separated by two irrelevant amino acids from a serine residue have been shown to be immunogenic and capable of inducing CD4+ T cell–mediated experimental autoimmune encephalomyelitis (EAE) in SWXJ (H-2q,s), SWR/J (H-2q), and SJL/J (H-2s) mice (34–36; Table 1). More recently, we have successfully applied the KXXS motif selection process to identify multiple immunogenic peptides of cardiac α-myosin heavy chain (CAMHC) capable of inducing CD4+ T cell–mediated experimental autoimmune myocarditis (EAMC) in SWXJ, SWR/J, and SJL/J mice (37). Thus, the KXXS MHC class II–binding motif provides a useful and efficient way to identify immunogenic T cell epitopes capable of inducing autoimmune disease.
Table 1.
The KXXS sequence–binding motif for IAs and IAq molecules
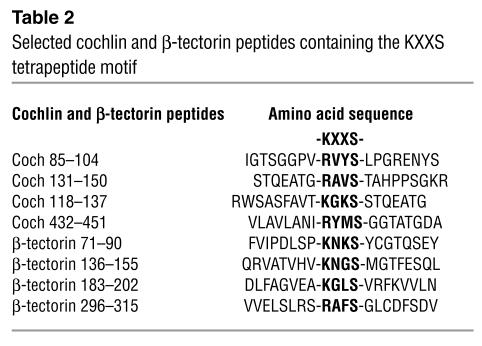
Identification of CD4+ T cell epitopes of inner ear–specific proteins.
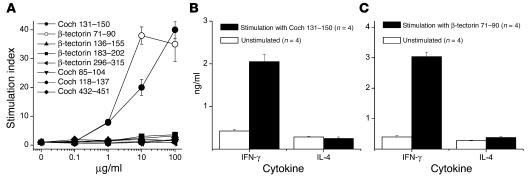
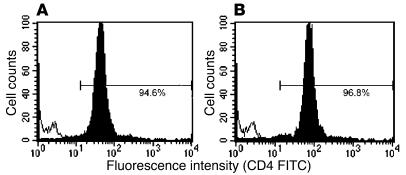
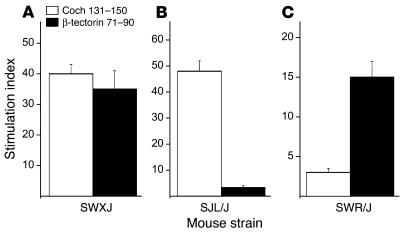
To develop an inner ear–specific autoimmune disease, we selected several KXXS-containing peptides from cochlin (26, 28) and β-tectorin (32, 33), proteins known to be highly expressed in inner ear tissues. Eight peptides were synthesized and used for immunization of SWXJ female mice (Table 2). Two of the peptides, Coch 131–150 and β-tectorin 71–90, were able to elicit substantial recall proliferative responses from 8- to 10-day primed LN cells, whereas the remaining six peptides were relatively nonimmunogenic (Figure 1A). ELISA analysis of 48-hour culture supernatants showed that recall responses to both Coch 131–150 (Figure 1B) and β-tectorin 71–90 (Figure 1C) involved the proinflammatory Th1-like phenotype with elevated production of IFN-γ and virtually no production of IL-4. Flow-cytometry analysis of peptide-activated LN cells showed that both Coch 131–150 (Figure 2A) and β-tectorin 71–90 (Figure 2B) preferentially activated CD4+ T cells.
Table 2.
Selected cochlin and β-tectorin peptides containing the KXXS tetrapeptide motif
Figure 1.
Immunogenicity of cochlin and β-tectorin peptides containing the KXXS MHC class II–binding motif. Female SWXJ mice were immunized with selected peptides containing the KXXS motif and derived from the sequences of inner ear–specific proteins. Eight to 10 days after immunization, LN cells were tested for recall proliferative responses to each peptide immunogen. (A) Coch 131–150 and β-tectorin 71–90 elicited marked dose-response reactivity compared with several other cochlin and β-tectorin peptides that were relatively nonimmunogenic. (B) ELISA analysis of 48-hour culture supernatants showed that recall responses to Coch 131–150 involved the proinflammatory Th1-like phenotype with elevated production of IFN-γ and virtually no production of IL-4. (C) Recall responses to β-tectorin 71–90 showed a similar Th1-like cytokine profile. Error bars show ± SE.
Figure 2.
CD4+ T cells respond to Coch 131–150 and β-tectorin 71–90. LN cells from mice primed to (A) Coch 131–150 and (B) β-tectorin 71–90 were stimulated in vitro for 3–4 days with 100 μg/ml peptide, washed repeatedly, and examined by flow cytometry after staining with FITC-conjugated mAb to mouse CD4 or CD8. The gated lymphocyte populations identified by intermediate size (forward scatter) and intermediate granularity (side scatter) consisted of predominantly CD4+ T cells responding to each peptide. The hollow unshifted peak shows superimposed staining with isotype control Ab’s.
MHC class II molecules restricting responses to Coch 131–150 and β-tectorin 71–90.
To determine which MHC class II molecules were involved in the restriction of the responses to each peptide, recall proliferative responses were measured in 8- to 10-day primed LN cells from SWXJ, SWR/J, and SJL/J mice immunized with each peptide. As expected, both Coch 131–150 and β-tectorin 71–90 were able to elicit recall responses from SWXJ-primed LN cells (Figure 3a). Coch 131–150, but not β-tectorin 71–90, elicited recall responses from SJL/J mice (Figure 3B), whereas β-tectorin 71–90, but not Coch 131–150, elicited recall responses from SWR/J mice (Figure 3C). Thus, the CD4+ T cell response to Coch 131–150 showed restriction by IAs, whereas the response to β-tectorin 71–90 showed restriction by IAq.
Figure 3.
T cell responses to Coch 131–150 and β-tectorin 71–90 are restricted by IAs and IAq, respectively. Eight to 10 days after immunization of female SWXJ (H-2q,s), SWR/J (H-2q), and SJL/J (H-2s) mice with either Coch 131–150 or β-tectorin 71–90, LN cells were tested for elicitation of recall proliferative responses to 100 μg/ml of each peptide. (A) LN cells from primed SWXJ mice responded to each peptide. (B) LN cells from primed SJL/J mice responded to Coch 131–150 but did not respond to β-tectorin 71–90, whereas (C) LN cells from primed SWR/J mice responded to β-tectorin 71–90 but did not respond to Coch 131–150. Thus, responses to Coch 131–150 showed restriction by IAs, whereas responses to β-tectorin 71–90 showed restriction by IAq. Error bars show ± SE.
Hearing loss following active immunization with Coch 131–150 and β-tectorin 71–90.
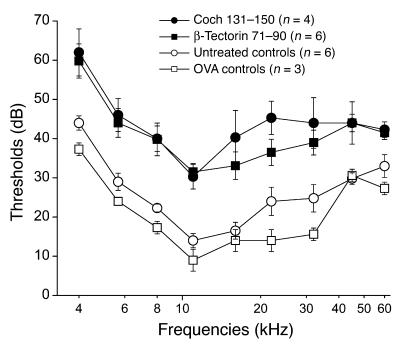
To determine whether Coch 131–150 and β-tectorin 71–90 were capable of targeting autoimmune hearing loss, female SWXJ mice were immunized with each peptide, and auditory brain stem responses (ABRs) were tested 5 weeks later. Figure 4 shows that a significant increase in ABR thresholds occurred at all frequencies tested from 4 to 60 kHz in mice immunized with either Coch 131–150 or β-tectorin 71–90 in comparison with either untreated age- and sex-matched controls (P = 0.01 and P = 0.024, respectively) or OVA-immunized age- and sex-matched controls (P = 0.012 and P = 0.017, respectively). Thus, mice immunized with either peptide developed substantial hearing loss within 5 weeks.
Figure 4.
Increased ABR thresholds show hearing loss in SWXJ mice immunized with Coch 131–150 or β-tectorin 71–90. Five weeks after immunization with either Coch 131–150 or β-tectorin 71–90, female SWXJ mice underwent ABR testing for determining hearing loss. Significant hearing loss was detected at all thresholds from 4 to 60 kHz in mice immunized with Coch 131–150 and β-tectorin 71–90 when compared with untreated age- and sex-matched controls (P = 0.01 and P = 0.024, respectively) and OVA-immunized controls (P = 0.012 and P = 0.017, respectively). Error bars show ± SE.
Hearing loss following adoptive transfer of activated T cells specific for either Coch 131–150 or β-tectorin 71–90.
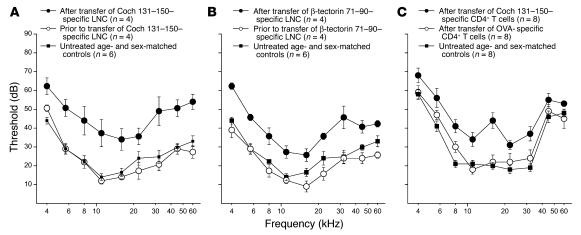
We next determined whether T cells specifically activated with Coch 131–150 and β-tectorin 71–90 were capable of passively transferring EAHL into naive recipients. Ten days after immunization of female SWXJ mice with either Coch 131–150 or β-tectorin 71–90, LN cells were activated in vitro with 100 μg/ml peptide and transferred (107 cells) into γ-irradiated (4.5 Gy) 8-week-old naive female recipients that had undergone ABR testing 1 day prior to cell transfer. Six weeks after the transfer, ABR thresholds were significantly increased at all frequencies tested from 4 to 60 kHz in mice transferred with primed LN cells activated with either Coch 131–150 (P = 0.018; Figure 5A) or β-tectorin 71–90 (P = 0.002; Figure 5B). Untreated 14-week-old female SWXJ mice showed ABR thresholds similar to those of 8-week-old recipients, indicating that no significant spontaneous age-related hearing loss occurred in SWXJ mice between 8 and 14 weeks of age, the ages at which cell transfer and final ABR testing, respectively, occurred.
Figure 5.
Hearing loss occurs in SWXJ mice following adoptive transfer of activated T cells specific for Coch 131–150 or β-tectorin 71–90. Ten days after immunization of female SWXJ mice with either Coch 131–150 or β-tectorin 71–90, LN cells were activated in vitro and transferred (107 cells) into γ-irradiated (4.5 Gy) 8-week-old naive female recipients that had undergone ABR testing 1 day prior to cell transfer. Six weeks later, ABR testing showed significantly increased thresholds and hearing deficit in mice that had received activated T cells specific for either (A) Coch 131–150 (P = 0.018) or (B) β-tectorin 71–90 (P = 0.002). Untreated 14-week-old female SWXJ mice showed no spontaneous hearing loss and are provided in each figure as reference. Eight to 10 days after immunization with either Coch 131–150 or OVA, splenocytes were activated in vitro with 100 μg/ml immunogen, and CD4+ T cells were purified more than 95% by magnetic bead separation. CD4+ T cells (2 × 107) were injected i.v. into γ-irradiated (4.5 Gy) 8-week-old naive female recipients. (C) Six weeks after transfer of purified CD4+ T cells, ABR testing showed significantly increased thresholds and hearing deficit in recipients of Coch 131–150–specific CD4+ T cells when compared with either age- and sex-matched controls (P = 0.001) or with recipients of OVA-specific CD4+ T cells (P = 0.001). Age- and sex-matched control mice and mice transferred with CD4+ T cells specific for OVA showed no differences in ABR thresholds (P = 0.39). Thus, purified CD4+ T cells specific for Coch 131–150 are capable of mediating EAHL. Error bars show ± SE. LNC, LN cells.
We next determined whether purified CD4+ T cells specifically activated with Coch 131–150 were capable of passively transferring EAHL. Eight to 10 days after immunization with Coch 131–150 or OVA in CFA emulsion, splenocytes were activated in vitro with 100 μg/ml of immunogen. After 3–4 days, CD4+ T cells were purified greater than 95% from activated cultures by magnetic bead separation and injected i.v. into naive γ-irradiated (4.5 Gy) SWXJ female recipients at 2 × 107 cells/mouse. Six weeks after transfer, ABR thresholds were significantly increased at all frequencies tested from 4 to 60 kHz in mice transferred with CD4+ T cells specific for Coch 131–150 when compared with age- and sex-matched controls (P = 0.001) and mice transferred with CD4+ T cells specific for OVA (P = 0.001; Figure 5C). Age- and sex-matched control mice and mice transferred with CD4+ T cells specific for OVA showed no differences in ABR thresholds (P = 0.39). Thus, purified CD4+ T cells specific for Coch 131–150 are capable of mediating EAHL.
EAHL is accompanied by inner ear inflammation.
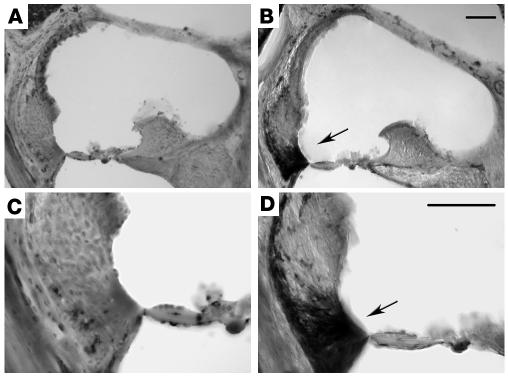
To determine whether inner ear inflammation occurred during the development of EAHL, cochleas were immunostained with CD45 Ab at 2, 3, 4, and 5 weeks after immunization of SWXJ mice with Coch 131–150. Figure 6, B and D, show marked CD45+ immunostaining in the spiral ligament of a mouse immunized 5 weeks earlier with Coch 131–150. CD45+ immunostaining was also prominent in the scala tympani at 5 weeks (data not shown). CD45+ immunostaining was not observed in untreated age- and sex-matched control mice (Figure 6, A and C) or in Coch 131–150–primed mice prior to 5 weeks after immunization. Thus, the development of EAHL is associated with the appearance of inner ear inflammation.
Figure 6.
Inner ear inflammatory infiltrates are associated with onset of EAHL. Cochleas from SWXJ mice were immunostained with CD45 Ab at 2, 3, 4, and 5 weeks after immunization with Coch 131–150. (A) Photomicrograph under ×10 magnification showing the organ of Corti from an untreated age- and sex-matched control SWXJ mouse. (B) Photomicrograph under ×10 magnification showing the organ of Corti from an SWXJ mouse 5 weeks after immunization with Coch 131–150, the earliest time of detection of hearing loss. Arrow shows prominent CD45+ immunostaining in the spiral ligament. (C) Photomicrograph at ×20 magnification showing the organ of Corti from an untreated age- and sex-matched control SWXJ mouse. (D) Photomicrograph at ×20 magnification showing the organ of Corti from an SWXJ mouse 5 weeks after immunization with Coch 131–150. Arrow shows prominent CD45+ immunostaining concentrated in the lower spiral ligament. Scale bars: 50 μm.
Discussion
Our study shows that CD4+ T cells specific for inner ear peptides are capable of mediating EAHL in mice. Thus, in addition to generating a contemporary mouse model for human ASNHL, our data provide compelling experimental support for the view that ASNHL may be a T cell–dependent autoimmune disorder of the inner ear. This view does not preclude a role for autoantibodies in ASNHL. HSP-70 autoantibodies frequently occur in ASNHL, but have not been shown to induce hearing loss in animals. It is likely that the ubiquitous expression of HSP-70 may preclude its ability to target organ-specific autoimmunity (8, 9). Although the pathogenic role of HSP-70 Ab’s may be questionable, hearing loss has been induced in animals with other experimentally generated Ab’s. The KHRI-3 mouse IgG1 mAb generated against guinea pig cochlear hair cells induces hearing loss in guinea pigs and in mice carrying the hybridoma, but the antigen it recognizes is currently unknown (14–16). Although KHRI-3 prominently stains cochlear cells bordering the tunnel of Corti and supporting cells of the second and third row of outer hair cells, it also reacts with several non-inner ear tissues including optic nerve, pia mater, and peripheral nerve fibers in muscle, tongue, lip, intestine, and eye (14–16).
It is possible that autoantibodies may contribute to or exacerbate preexisting inner ear pathology, as has been proposed (8, 9, 38). Such an auxilliary role for autoantibodies has been implicated in EAE where Ab’s to myelin oligodendrocyte glycoprotein (MOG) are not pathogenic per se when injected separately into naive mice but are capable of augmenting the severity of T cell–mediated disease onset and relapse (39, 40). Thus, while there is no evidence that an Ab response to HSP-70 autonomously initiates ASNHL, it remains to be determined whether HSP-70 Ab’s serve to enhance the underlying pathogenic process that leads to ASNHL or whether such autoantibody production simply represents a nonpathogenic epiphenomenon generated as a late inflammatory event.
There are a number of experimental animal models currently used in ASNHL studies, but none resembles the traditional antigen- and organ-specific mouse model system that has provided useful platforms for understanding and treating several autoimmune diseases, including multiple sclerosis (41), insulin-dependent diabetes mellitus (42), and rheumatoid arthritis (43). The keyhole limpet hemocyanin (KLH) guinea pig model involves a “pseudoautoimmune” attack of the inner ear by intracochlear KLH inoculation in KLH-primed guinea pigs (44). Thus, KLH presumably acts as an artificial “self” and directs targeted immunoreactivity against the inner ear. Although this model has been instrumental in discrediting long-held dogma regarding the inner ear as an immunologically privileged site (45), the KLH model is inherently flawed by the nonself features of focal distribution of injected KLH and by the intensity of immunoreactivity directed against such a phylogenetically distant and powerful antigen.
Collagen type II (CII) has been proposed as a potential target antigen in ASNHL (46, 47). Its ubiquitous expression precludes targeting of inner ear–specific autoimmunity, however. Moreover, the reported hearing loss associated with CII immunization has not been reproduced consistently (48). MRL/MpJ–lpr/lpr and C3H/lpr mice have been used recently in ASNHL studies because they develop spontaneous hearing loss (49–51). Their hearing deficiency, however, is due to a systemic lymphoproliferative disorder resulting from the autosomal-recessive lpr Fas deletion (52). Thus, the hearing loss observed in these lpr mice is part of a multiorgan global autoimmunity and bears little resemblance to the organ-specific features of ASNHL. A similar lack of organ-specificity occurs in the NZB/kl substrain of autoimmune-prone NZB mice that have inner ear immune complex deposition with high-frequency hearing loss (53, 54). These mice also have a systemic immune disorder with an accompanying renal pathology, however (55).
The lack of linkage with other inflammatory abnormalities strongly suggests that ASNHL is mediated by autoreactivity targeted against antigens that are inner ear–specific rather than systemically expressed. Several reports have shown that hearing loss and/or cochlear pathology occurs when guinea pigs are immunized with inner ear homogenate (7, 20–22). More recently, Gloddek et al. (23, 24) showed labyrinthitis but not hearing loss in naive Lewis rats adoptively transferred with activated T cells specific for inner ear homogenate. The predominant use of the guinea pig model with its limited availability of reagents and the exclusive use of crude antigen homogenate in prior studies preclude thorough immunologic characterization identification of target antigens and development of contemporary immunomodulatory treatment strategies.
In the current study we found that inner ear inflammation accompanied the appearance of hearing loss, with both occurring within 5–6 weeks after immunization with Coch 131–150. Leukocytic infiltration was most notable in the regions of the spiral ligament and scala tympani of the inner ear. Thus, the inflammation occurred where cochlin is highly expressed, and it affected inner ear regions showing histologic abnormalities in humans with COCH mutations (28, 29).
These findings, along with the fact that cochlin is substantially inner ear specific in humans (26) and is the most abundant protein found in inner ear tissues (30), make a compelling argument for cochlin as a candidate autoimmune target in ASNHL. Indeed, elevated serum levels of cochlin IgG Ab’s occur in many ASNHL patients (31), so it would not be particularly startling to find increased frequencies of cochlin-specific T cells in ASNHL patients. The exquisite inner ear specificity of β-tectorin (32, 33) also makes it a potential candidate for targeting inner ear–specific autoreactivity in ASNHL. Indeed, both proteins may be implicated in the pathogenesis of ASNHL as either primary targets initiating disease onset or secondary epitope spreading determinants, as has been shown in other putative human autoimmune diseases (56). Studies to determine frequencies of T cells specific for inner ear proteins are currently ongoing.
In summary, the identification of inner ear–specific autoimmune peptide targets firmly establishes the pivotal role of CD4+ T cells in mediating autoimmune hearing loss and provides a most useful model platform for clarifying our understanding of ASNHL and facilitating the development of novel effective treatments for this debilitating clinical disorder.
Methods
Mice and immunization.
All mice were purchased from The Jackson Laboratory (Bar Harbor, Maine, USA). SWXJ (H-2q,s) mice were generated at The Jackson Laboratory by mating SJL/J (H-2s) males with SWR/J (H-2q) females. At 6–8 weeks of age mice were injected subcutaneously in the abdominal flank with 200 μg of antigen in 200 μl of an emulsion of equal volumes of water and complete CFA containing 400 μg Mycobacteria tuberculosis H37RA (Difco Laboratories, Detroit, Michigan, USA). In experiments involving disease induction, each mouse was injected i.v. on days 0, 3, and 7 with 0.2 μg of purified Bordetella pertussis toxin (List Biological Laboratories Inc., Campbell, California, USA). The adjuvant effects of B. pertussis toxin are due presumably to its ability to increase vascular permeability, activate APCs, and promote the production of inflammatory Th1-like T cells (57–59). Mice were euthanized either by anesthesia overdose or by asphyxiation with CO2, followed by cervical dislocation. All protocols were approved by the Institutional Animal Care and Use Committee in compliance with the Public Health Service policy on humane care and use of laboratory animals.
Peptides.
Peptides selected for synthesis were derived from the known sequences of the inner ear proteins, cochlin (26, 28) and β-tectorin (32, 33). Peptides were selected based on those containing the KXXS-binding motif for IAs and IAq MHC class II molecules expressed in SWXJ mice (37). All peptides were synthesized by the Molecular Biotechnology Core Facility of the Lerner Research Institute using standard solid-phase methodology and 9-fluorenylmethoxycarbonyl (FMOC) side chain–protected amino acids. Peptides were purified greater than 97% by reverse-phase HPLC, and amino acid composition was confirmed by mass spectrometry.
Cell culture and proliferation assays.
To determine peptide immunogenicity, inguinal and axillary LN cells were teased into single-cell suspensions 8–10 days after immunization, washed thoroughly in HBSS (Invitrogen Corp., Carlsbad, California, USA), and cultured in 96-well flat-bottomed microtiter Falcon plates (BD Biosciences, San Jose, California, USA) at 3 × 105 cells/microtiter well in DMEM (CellGro; Mediatech Inc., Herndon, Virginia, USA) supplemented with 10% FBS (HyClone Laboratories, Logan, Utah, USA). Culture media also contained final concentrations of 2 mM fresh L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 30 mM HEPES buffer (Invitrogen Corp.). Synthetic peptides were added in serial 10-fold dilutions to triplicate wells with positive control wells containing 2 μg/ml anti-mouse CD3 (BD Biosciences) and negative control wells containing no antigen. Cells were cultured at a final volume of 200 μl/well at 37°C in humidified air containing 5% CO2. After 96 hours of culture, wells were pulsed with [methyl-3H] thymidine (l μCi/well, specific activity 6.7 Ci/mmol; New England Nuclear Corp., Boston, Massachusetts, USA). Sixteen hours after pulsing, cultures were harvested by aspiration onto glass fiber filters. Levels of incorporated radioactivity were determined by scintillation spectrometry. Results were expressed as mean counts per minute of triplicate experimental cultures, with antigen divided by mean counts per minute of cultures without antigen (stimulation index).
Passive transfer of EAHL.
Eight to ten days after immunization of female SWXJ mice with 200 μg Coch 131–150 or 200 μg β-tectorin 71–90 in CFA, LN cells were teased into single-cell suspensions and activated in vitro with 100 μg/ml peptide at 5 × 106 cells/ml in 24-well flat-bottomed Falcon plates (BD Biosciences) in a total volume of 2 ml/well in DMEM supplemented as described above. After 3–4 days, cells were harvested, washed thoroughly, and 107 cells were injected i.v. into naive γ-irradiated (4.5 Gy) SWXJ female recipients. In related experiments, 8–10 days after immunization with either 200 μg Coch 131–150 or 200 μg OVA (Sigma-Aldrich, St. Louis, Missouri, USA) in CFA emulsion, splenocytes were activated in vitro with 100 μg/ml of immunogen. After 3–4 days, CD4+ T cells were purified from activated cultures by incubation of cells with anti–CD4-coated magnetic beads and passage through a MACS LS column using a MidiMACS cell separator (Miltenyi Biotech, Auburn, California, USA). The positively selected CD4+ T cells (purified greater than 95%) were injected i.v. into naive γ-irradiated (4.5 Gy) SWXJ female recipients at 2 × 107 cells/mouse.
Hearing tests.
Auditory thresholds were measured by ABR testing. Tone pips were introduced in the right ear of anesthetized mice, and evoked potentials were recorded using needle electrodes inserted through the skin (vertex to pinna with a ground on the back near the tail). The auditory stimuli were 5-ms tone pips (0.5 ms rise-fall with a cos2 onset envelope) delivered at a rate of 40 per second. Responses were amplified ×10,000, filtered (100–3 Hz), and averaged using BioSig32 software (Tucker Davis Technologies Inc., Gainesville, Florida, USA). Sound-pressure levels (SPLs) were raised in 5-decibel (5-dB) steps from 5 dB SPL through 94 dB SPL. At each SPL, 1,024 responses were averaged with stimulus polarity alternated. The software included an “artifact reject” feature in which any single response waveform was discarded if the peak-to-peak voltage exceeded 15 μV. ABR thresholds, defined by the lowest SPL at which response peaks were clearly present, were read visually from stacked waveforms obtained at 5 dB sound-pressure intervals through 94 dB SPL. All ABR thresholds were conducted in a blinded manner by an investigator who was unaware of the treatment protocols.
ELISA assays.
Cytokine concentrations were determined by ELISA measurements of 48-hour supernatants of leukocyte cultures plated at 5 × 106 cells/well in 24-well flat-bottomed Falcon plates (BD Biosciences) in a total volume of 2 ml/well of DMEM supplemented as described above and containing 100 μg/ml of each peptide. Purified anti–mouse cytokine capture/detection Ab pairs were purchased commercially (BD Biosciences). The capture/detection Ab pairs used in the present study included anti-mouse IFN-γ (R4-6A2 and biotin-XMG1.2) and anti-mouse IL-4 (BVD4-1D11 and biotin BVD6-24G2). Fifty microliters of coating buffer (0.1 M NaHCO3, pH 8.2) containing capture Ab at 4 μl/ml were added to each microtiter well in 96-well enhanced protein-binding ELISA plates (Corning Inc., Corning, New York, USA). After overnight incubation at 4°C, plates were washed with 0.5% PBS/Tween and blocked by adding 200 μl/well of PBS containing 3% BSA. Plates were incubated for 2 hours at room temperature and washed. One hundred microliters of recombinant cytokine standards (BD Biosciences) at multiple concentrations and 100 μl of each test sample were added to duplicate wells. After incubation for 4 hours at room temperature and a thorough washing, 100 μl of appropriate detection Ab at 2 μl/ml were added to duplicate wells for 45 minutes at room temperature. After washing, 100 μl of a 1:400 dilution of avidin peroxidase (Sigma-Aldrich) were added to each well and incubated for 30 minutes at room temperature. Plates were washed, and each well received 100 μl of fresh substrate buffer containing 30% 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) substrate (Sigma-Aldrich) and 0.03% H2O2 in 0.1 M citric acid at pH 4.35. After 12 minutes the absorbance at 405 nm was measured using a model 550 ELISA microplate reader (Bio-Rad Laboratories Inc., Hercules, California, USA). Standard values were plotted as absorbance (optical density) versus cytokine concentration, and sample cytokine concentrations were determined as values within the linear part of the standard curve.
Flow cytometry.
Eight to 10 days after immunization of female SWXJ mice with 200 μg Coch 131–150 or 200 μg β-tectorin 71–90 in CFA, LN cells were mechanically teased into single-cell suspensions and reactivated in vitro with 100 μg/ml peptide for 3–4 days as described above. Cultured cells were washed thoroughly and centrifuged on density-gradient medium Lympholyte-M (Accurate Chemical and Scientific Corp., Westbury, New York, USA). Cells collected from the interface were washed repeatedly and resuspended in staining buffer consisting of PBS supplemented with 1% BSA and 0.1% NaN3). To minimize Fc-mediated binding, sample aliquots containing 106 cells were preincubated in purified anti–mouse CD16/CD32 (BD Biosciences) at a 1:50 dilution for 15 minutes at 4°C. After multiple washes, cells were incubated in the dark for 30 minutes at 4°C with FITC-conjugated Ab’s specific for mouse CD4 or CD8 (BD Biosciences) at concentrations previously determined for each Ab to provide optimum staining with minimal nonspecific binding. Unstained cells and cells stained with isotype-matched FITC-conjugated mAb were used as controls for determining background and thresholds for positive staining. Cells were washed thoroughly in staining buffer and fixed in 1% paraformaldehyde for processing on a FACScan flow cytometer (BD Biosciences). After collecting data on 20,000 events, the lymphocyte population was analyzed using CellQuest software (BD Biosciences) by gating on the intermediate size (forward scatter) and intermediate granularity (side scatter) population.
Immunocytochemistry.
At 2, 3, 4, and 5 weeks after immunization, anesthetized SWXJ mice were perfused intracardially with 4% paraformaldehyde. Cochleas were dissected and placed in 4% paraformaldehyde for 2 hours and thereafter in 120 mM EDTA. After 5 days, the cochleas were washed with PBS and cryoprotected overnight in 20% glycerol and 0.08 mM Sorenson’s buffer adjusted to a pH of 7.6. The cochleas were then infiltrated with 30% sucrose, frozen on dry ice, and serially sectioned at 30 μm with a sliding microtome (Leica Microsystems, Nussloch, Germany). Each section was placed in an individual well in 24-well plates (Corning Inc.) with cryostorage solution that contained final concentrations of 1% PVP, 30% sucrose, 30% ethylene glycol, and 0.2 M sodium phosphate. Free-floating sections of cochlea were washed with PBS and dried onto Superfrost PLUS slides (Fisher Scientific Co., Pittsburgh, Pennsylvania, USA). Once dry, sections were incubated in 1% H2O2 in 0.1% PBS/Triton X for 30 minutes. Subsequently, sections were washed thoroughly with 0.1% PBS/Triton X at 5-minute intervals and then incubated in 3% normal goat serum (Vector Laboratories, Burlingame, California, USA) for 30 minutes. Sections were then incubated overnight with rat anti-mouse CD45 (Serotec Ltd., Oxford, United Kingdom) in a 1:6,000 dilution, washed thoroughly with PBS at 5-minute intervals, and then incubated with biotinylated goat anti-rat Ab (Jackson ImmunoResearch Laboratories Inc., West Grove, Pennsylvania, USA) at a 1:1,000 dilution. After 1 hour, sections were washed thoroughly with PBS, incubated with avidin-biotinylated peroxidase complex (ABC elite kit; Vector Laboratories) for 1 hour, washed thoroughly with 0.1% PBS/Triton X at 5-minute intervals, and incubated with diaminobenzidine and 0.01% H2O2 for up to 8 minutes until color development occurred. Sections were then washed thoroughly with PBS, treated for 30 seconds with 0.04% OsO4, washed thoroughly one last time before being mounted in glycerol, and protected with a coverslip.
Statistical analysis.
Repeated measures ANOVA was used to compare differences between the treatment groups. The Tukey-Kramer multiple comparison procedure was used in pairwise analysis.
Acknowledgments
This work was supported primarily by grants from the Deafness Research Foundation, the Samuel Rosenthal Foundation, the Milton and Charlotte Kramer Foundation, and the Triple-T Foundation. Additional support came from NIH grants NS-37476, NS-36054, and AI-51837 (V.K. Tuohy).
Footnotes
See the related Commentary beginning on page 1114.
Nonstandard abbreviations used: auditory brain stem response (ABR); autoimmune sensorineural hearing loss (ASNHL); cardiac α-myosin heavy chain (CAMHC); collagen type II (CII); decibel (dB); experimental autoimmune encephalomyelitis (EAE); experimental autoimmune hearing loss (EAHL); experimental autoimmune myocarditis (EAMC); heat shock protein 70 (HSP-70); keyhole limpet hemocyanin (KLH); myelin oligodendrocyte glycoprotein (MOG); sound-pressure level (SPL).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.NICDC. 2001. Health information on sudden deafness. National Institute on Deafness and Other Communication Disorder (NIDCD). http://www.nidcd.nih.gov/health/hearing/sudden.asp.
- 2.Harris, J.P., Moscicki, R.A., and Hughes, G.B. 1997. Immunologic disorders of the inner ear. In Clinical otology. G.B. Hughes and M.L. Pensak, editors. Thieme Medical Publishers. New York, New York, USA. 381–391.
- 3.Arnold W, Pfaltz R, Altermatt HJ. Evidence of serum antibodies against inner ear tissues in the blood of patients with certain sensorineural hearing disorders. Acta Otolaryngol. 1985;99:437–444. doi: 10.3109/00016488509108935. [DOI] [PubMed] [Google Scholar]
- 4.Veldman, J.E., Hughes, G.B., and Roord, J.J. 1986. Immune-mediated inner ear disorders: immunological investigations in the neurotology clinic. In Handbook of neurotological diagnosis. J.W. House and A.F. Connor, editors. Marcel Dekker Inc. New York, New York, USA. 339–371.
- 5.Elies, W., and Plester, D. 1985. Sensorineural hearing loss and immunity. In Immunobiology, autoimmunity and transplantation in otorhinolaryngology. J.E. Veldman, B.F. McCabe, E.H. Huizing, and N. Mygind, editors. Kugler Publications. Amsterdam, Holland. 111–117.
- 6.Arnold W, Pfaltz CR. Critical evaluation of the immunofluorescence microscopic test for identification of serum antibodies against human inner ear tissue. Acta Otolaryngol. 1987;103:373–378. [PubMed] [Google Scholar]
- 7.Soliman AM. Experimental autoimmune inner ear disease. Laryngoscope. 1989;99:188–193. doi: 10.1288/00005537-198902000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Billings PB, Keithley EM, Harris JP. Evidence linking the 68 kilodalton antigen identified in progressive sensorineural hearing loss patient sera with heat shock protein 70. Ann. Otol. Rhinol. Laryngol. 1995;104:181–188. doi: 10.1177/000348949510400302. [DOI] [PubMed] [Google Scholar]
- 9.Billings PB, Shin SO, Harris JP. Assessing the role of anti-hsp70 in cochlear impairment. Hear. Res. 1998;126:210–213. doi: 10.1016/s0378-5955(98)00172-5. [DOI] [PubMed] [Google Scholar]
- 10.Bloch DB, San Martin JE, Rauch SD, Moscicki RA, Bloch KJ. Serum antibodies to heat shock protein 70 in sensorineural hearing loss. Arch. Otolaryngol. Head Neck Surg. 1995;121:1167–1171. doi: 10.1001/archotol.1995.01890100075013. [DOI] [PubMed] [Google Scholar]
- 11.Gottschlich S, Billings PB, Keithley EM, Weisman MH, Harris JP. Assessment of serum antibodies in patients with rapidly progressive sensorineural hearing loss and Meniere’s disease. Laryngoscope. 1995;105:1347–1352. doi: 10.1288/00005537-199512000-00016. [DOI] [PubMed] [Google Scholar]
- 12.Moscicki RA, et al. Serum antibody to inner ear proteins in patients with progressive hearing loss. Correlation with disease activity and response to corticosteroid treatment. JAMA. 1994;272:611–616. [PubMed] [Google Scholar]
- 13.Trune DR, Kempton JB, Mitchell CR, Hefeneider SH. Failure of elevated heat shock protein 70 antibodies to alter cochlear function in mice. Hear. Res. 1998;116:65–70. doi: 10.1016/s0378-5955(97)00198-6. [DOI] [PubMed] [Google Scholar]
- 14.Nair TS, et al. Monoclonal antibody induced hearing loss. Hear. Res. 1995;83:101–113. doi: 10.1016/0378-5955(94)00194-u. [DOI] [PubMed] [Google Scholar]
- 15.Nair TS, et al. KHRI-3 monoclonal antibody-induced damage to the inner ear: antibody staining of nascent scars. Hear. Res. 1999;129:50–60. doi: 10.1016/s0378-5955(98)00220-2. [DOI] [PubMed] [Google Scholar]
- 16.Disher MJ, et al. Human autoantibodies and monoclonal antibody KHRI-3 bind to a phylogenetically conserved inner-ear-supporting cell antigen. Ann. N.Y. Acad. Sci. 1997;830:253–265. doi: 10.1111/j.1749-6632.1997.tb51896.x. [DOI] [PubMed] [Google Scholar]
- 17.McCabe BF, McCormick KJ. Tests for autoimmune disease in otology. Am. J. Otol. 1984;5:447–449. [PubMed] [Google Scholar]
- 18.Hughes GB, Barna BP, Kinney SE, Calabrese LH, Nalepa NL. Predictive value of laboratory tests in “autoimmune” inner ear disease: preliminary report. Laryngoscope. 1986;96:502–505. doi: 10.1288/00005537-198605000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Lorenz RR, et al. Interferon-gamma production to inner ear antigens by T cells from patients with autoimmune sensorineural hearing loss. J. Neuroimmunol. 2002;130:173–178. doi: 10.1016/s0165-5728(02)00190-x. [DOI] [PubMed] [Google Scholar]
- 20.Harris JP. Experimental autoimmune sensorineural hearing loss. Laryngoscope. 1987;97:63–76. doi: 10.1288/00005537-198701000-00014. [DOI] [PubMed] [Google Scholar]
- 21.Soliman AM. The use of immunofluorescence in the non-decalcified frozen guinea pig cochlea to detect autoantibodies in inner ear disorders. Arch. Otorhinolaryngol. 1987;244:241–245. doi: 10.1007/BF00455313. [DOI] [PubMed] [Google Scholar]
- 22.Yamanobe S, Harris JP. Spontaneous remission in experimental autoimmune labyrinthitis. Ann. Otol. Rhinol. Laryngol. 1992;101:1007–1014. doi: 10.1177/000348949210101208. [DOI] [PubMed] [Google Scholar]
- 23.Gloddek B, Gloddek J, Arnold W. Induction of an inner-ear-specific autoreactive T-cell line for the diagnostic evaluation of an autoimmune disease of the inner ear. Ann. N. Y. Acad. Sci. 1997;830:266–276. doi: 10.1111/j.1749-6632.1997.tb51897.x. [DOI] [PubMed] [Google Scholar]
- 24.Gloddek B, Gloddek J, Arnold W. A rat T-cell line that mediates autoimmune disease of the inner ear in the Lewis rat. ORL J. Otorhinolaryngol. Relat. Spec. 1999;61:181–187. doi: 10.1159/000027668. [DOI] [PubMed] [Google Scholar]
- 25.Manolis EN, et al. A gene for non-syndromic autosomal dominant progressive postlingual sensorineural hearing loss maps to chromosome 14q12-13. Hum. Mol. Genet. 1996;5:1047–1050. doi: 10.1093/hmg/5.7.1047. [DOI] [PubMed] [Google Scholar]
- 26.Robertson NG, et al. Mapping and characterization of a novel cochlear gene in human and in mouse: a positional candidate gene for a deafness disorder, DFNA9. Genomics. 1997;46:345–354. doi: 10.1006/geno.1997.5067. [DOI] [PubMed] [Google Scholar]
- 27.Robertson NG, et al. Mutations in a novel cochlear gene cause DFNA9, a human nonsyndromic deafness with vestibular dysfunction. Nat. Genet. 1998;20:299–303. doi: 10.1038/3118. [DOI] [PubMed] [Google Scholar]
- 28.Robertson NG, et al. Inner ear localization of mRNA and protein products of COCH, mutated in the sensorineural deafness and vestibular disorder, DFNA9. Hum. Mol. Genet. 2001;10:2493–2500. doi: 10.1093/hmg/10.22.2493. [DOI] [PubMed] [Google Scholar]
- 29.Grabski R, et al. Mutations in COCH that result in non-syndromic autosomal dominant deafness (DFNA9) affect matrix deposition of cochlin. Hum. Genet. 2003;113:406–416. doi: 10.1007/s00439-003-0992-7. [DOI] [PubMed] [Google Scholar]
- 30.Ikezono T, et al. Identification of the protein product of the COCH gene (hereditary deafness gene) as the major component of bovine inner ear protein. . Biochim. Biophys. Acta. 2001; 1535:258–265. doi: 10.1016/s0925-4439(00)00101-0. [DOI] [PubMed] [Google Scholar]
- 31.Boulassel MR, Tomasi JP, Deggouj N, Gersdorff M. COCH5B2 is a target antigen of anti-inner ear antibodies in autoimmune inner ear diseases. Otol. Neurotol. 2001;22:614–618. doi: 10.1097/00129492-200109000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Killick R, Legan PK, Malenczak C, Richardson GP. Molecular cloning of chick beta-tectorin, an extracellular matrix molecule of the inner ear. J. Cell. Biol. 1995;129:535–547. doi: 10.1083/jcb.129.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Legan PK, Rau A, Keen JN, Richardson GP. The mouse tectorins. Modular matrix proteins of the inner ear homologous to components of the sperm-egg adhesion system. J. Biol. Chem. 1997;272:8791–8801. doi: 10.1074/jbc.272.13.8791. [DOI] [PubMed] [Google Scholar]
- 34.Tuohy VK, Thomas DM. Sequence 104-117 of myelin proteolipid protein is a cryptic encephalitogenic T cell determinant for SJL/J mice. J. Neuroimmunol. 1995;56:161–170. doi: 10.1016/0165-5728(94)00143-c. [DOI] [PubMed] [Google Scholar]
- 35.Amor S, et al. Identification of epitopes of myelin oligodendrocyte glycoprotein for the induction of experimental allergic encephalomyelitis in SJL and Biozzi AB/H mice. J. Immunol. 1994;153:4349–4356. [PubMed] [Google Scholar]
- 36.Holz A, Bielekova B, Martin R, Oldstone MB. Myelin-associated oligodendrocytic basic protein: identification of an encephalitogenic epitope and association with multiple sclerosis. J. Immunol. 2000;164:1103–1109. doi: 10.4049/jimmunol.164.2.1103. [DOI] [PubMed] [Google Scholar]
- 37.Jane-wit D, et al. A novel class II-binding motif selects peptides that mediate organ-specific autoimmune disease in SWXJ, SJL/J, and SWR/J mice. J. Immunol. 2002;169:6507–6514. doi: 10.4049/jimmunol.169.11.6507. [DOI] [PubMed] [Google Scholar]
- 38.Billings PB. Autoimmunogens, autoantigens, autoantibodies and autoimmune diseases: one-to-one or many-to-many relationships? Asian Pac. J. Allergy Immunol. 1987;5:67–79. [PubMed] [Google Scholar]
- 39.Schluesener HJ, Sobel RA, Linington C, Weiner HL. A monoclonal antibody against a myelin oligodendrocyte glycoprotein induces relapses and demyelination in central nervous system autoimmune disease. J. Immunol. 1987;139:4016–4021. [PubMed] [Google Scholar]
- 40.Linington C, Bradl M, Lassmann H, Brunner C, Vass K. Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am. J. Pathol. 1988;130:443–454. [PMC free article] [PubMed] [Google Scholar]
- 41.Swanborg RH. Experimental autoimmune encephalomyelitis in rodents as a model for human demyelinating disease. Clin. Immunol. Immunopathol. 1995;77:4–13. doi: 10.1016/0090-1229(95)90130-2. [DOI] [PubMed] [Google Scholar]
- 42.Bowman MA, Leiter EH, Atkinson MA. Prevention of diabetes in the NOD mouse: implications for therapeutic intervention in human disease. Immunol. Today. 1994;15:115–120. doi: 10.1016/0167-5699(94)90154-6. [DOI] [PubMed] [Google Scholar]
- 43.Anthony DD, Haqqi TM. Collagen-induced arthritis in mice: an animal model to study the pathogenesis of rheumatoid arthritis. Clin. Exp. Rheumatol. 1999;17:240–244. [PubMed] [Google Scholar]
- 44.Harris JP. Immunology of the inner ear: evidence of local antibody production. Ann. Otol. Rhinol. Laryngol. 1984;93:157–162. doi: 10.1177/000348948409300211. [DOI] [PubMed] [Google Scholar]
- 45.Woolf NK, Harris JP. Cochlear pathophysiology associated with inner ear immune responses. Acta Otolaryngol. 1986;102:353–364. doi: 10.3109/00016488609119418. [DOI] [PubMed] [Google Scholar]
- 46.Yoo TJ, et al. Type II collagen-induced autoimmune sensorineural hearing loss and vestibular dysfunction in rats. Ann. Otol. Rhinol. Laryngol. 1983;92:267–271. doi: 10.1177/000348948309200310. [DOI] [PubMed] [Google Scholar]
- 47.Yoo TJ, Yazawa Y, Tomoda K, Floyd R. Type II collagen-induced autoimmune endolymphatic hydrops in guinea pig. Science. 1983;222:65–67. doi: 10.1126/science.6623056. [DOI] [PubMed] [Google Scholar]
- 48.Harris JP, Woolf NK, Ryan AF. A reexamination of experimental type II collagen autoimmunity: middle and inner ear morphology and function. Ann. Otol. Rhinol. Laryngol. 1986;95:176–180. doi: 10.1177/000348948609500214. [DOI] [PubMed] [Google Scholar]
- 49.Kusakari C, Hozawa K, Koike S, Kyogoku M, Takasaka T. MRL/MP-lpr/lpr mouse as a model of immune-induced sensorineural hearing loss. Ann. Otol. Rhinol. Laryngol. Suppl. 1992;157:82–86. doi: 10.1177/0003489492101s1017. [DOI] [PubMed] [Google Scholar]
- 50.Trune DR. Cochlear immunoglobulin in the C3H/lpr mouse model for autoimmune hearing loss. Otolaryngol. Head Neck Surg. 1997;117:504–508. doi: 10.1016/s0194-5998(97)70022-6. [DOI] [PubMed] [Google Scholar]
- 51.Ruckenstein MJ, Milburn M, Hu L. Strial dysfunction in the MRL-Fas mouse. Otolaryngol. Head Neck Surg. 1999;121:452–456. doi: 10.1016/S0194-5998(99)70236-6. [DOI] [PubMed] [Google Scholar]
- 52.Nagata S, Suda T. Fas and Fas ligand: lpr and gld mutations. Immunol. Today. 1995;16:39–43. doi: 10.1016/0167-5699(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 53.Nariuchi H, Sone M, Tago C, Kurata T, Saito K. Mechanisms of hearing disturbance in an autoimmune model mouse NZB/kl. Acta Otolaryngol. Suppl. 1994;514:127–131. doi: 10.3109/00016489409127576. [DOI] [PubMed] [Google Scholar]
- 54.Sone M, Nariuchi H, Saito K, Yanagita N. A substrain of NZB mouse as an animal model of autoimmune inner ear disease. Hear. Res. 1995;83:26–36. doi: 10.1016/0378-5955(94)00189-w. [DOI] [PubMed] [Google Scholar]
- 55.Tago C, Yanagita N. Cochlear and renal pathology in the autoimmune strain mouse. Ann. Otol. Rhinol. Laryngol. Suppl. 1992;157:87–91. doi: 10.1177/0003489492101s1018. [DOI] [PubMed] [Google Scholar]
- 56.Tuohy VK, Yu M, Weinstock-Guttman B, Kinkel RP. Diversity and plasticity of self recognition during the development of multiple sclerosis. J. Clin. Invest. 1997;99:1682–1690. doi: 10.1172/JCI119331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Linthicum DS, Munoz JJ, Blaskett A. Acute experimental autoimmune encephalomyelitis in mice. I. Adjuvant action of Bordetella pertussis is due to vasoactive amine sensitization and increased vascular permeability of the central nervous system. Cell. Immunol. 1982;73:299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- 58.Patterson CE, Stasek JE, Schaphorst KL, Davis HW, Garcia JG. Mechanisms of pertussis toxin-induced barrier dysfunction in bovine pulmonary artery endothelial cell monolayers. Am. J. Physiol. 1995;268:L926–L934. doi: 10.1152/ajplung.1995.268.6.L926. [DOI] [PubMed] [Google Scholar]
- 59.Hou W, et al. Pertussis toxin enhances Th1 responses by stimulation of dendritic cells. J. Immunol. 2003;170:1728–1736. doi: 10.4049/jimmunol.170.4.1728. [DOI] [PubMed] [Google Scholar]