Introduction and biological activities of polyvalent immunoglobulins
Over the past 3 decades, administration of pooled human immunoglobulins (IVIGs) which contain a broad range of antibody specificities, originally in use for antibody replacement therapy, has been extended to other clinical conditions due to their anti-inflammatory and immunomodulatory effects, which were not anticipated when these polyclonal preparations were first developed1. Currently available IVIGs preparations are produced human pools of plasma obtained from several thousand healthy blood donors by using a number of preparatory steps. The large donor pool ensures the diversity of antibody repertoires similar to those present in normal human serum with antibody specificities to a wide spectrum of antigens. Moreover, immunoglobulin preparations contain natural antibodies (NAb), occurring independently of antigenic stimulation and representing a first-line defence against pathogens2–3. The distribution of IgG subclasses in IVIGs is comparable with that of IgG in normal human serum, however, unlike IgG purified from a single individual, therapeutic IVIGs preparations contain substantial amounts of IgG dimers and traces of multimers, due to the idiotype-anti-idiotype complex formation between IgG molecules from different individuals.
IVIGs is administered at distinct doses in the clinical settings4–5: whereas patients affected by Primary Immune Deficiencies are treated with replacement levels of IVIGs, patients with autoimmune and inflammatory diseases are treated with high dose of IVIGs. In autoimmune and inflammatory diseases, several mutually non-exclusive mechanisms have been put forward to explain the immunomodulatory effects6–8: IVIGs exerts immunoregulatory functions at multiple levels (Table I), implicating modulation of expression and function of Fc receptors, interference with complement activation and the cytokine profiles, modulation of idiotype network and cell proliferation. While some of these effects may explain the rapid and passive neutralisation of pathogenic autoantibodies, clinically the observed beneficial effects of IVIGs are well beyond the half-life of infused IgG, suggesting that the effect may not be due merely to a passive clearance or competition with pathogenic autoantibodies. Moreover, also at replacement dosages and beside their mere substitutive role, IVIGs have an active role and modulates the function of several cell types of the immune system, including dendritic cells, B lymphocytes, macrophages and T lymphocytes9.
Table I.
Biologic activity and mediators involved in mechanisms Fab-mediated, Fc-mediated, Complement-mediated, Sialylated Fc-mediated, and protein involved in the immune system homeostasis through intravenous immunoglobulins.
| Biologic activity | Mediators |
|---|---|
| Fab-mediated: | |
|
|
|
| |
| Fc-mediated: | |
|
|
|
| |
| Complement binding | Inhibition of deposition of activated complement components on target tissues |
|
| |
| Sialylated Fc | Activation of effector macrophages to increase the expression of inhibitory FcγR IIB |
|
| |
| Other soluble proteins contained in IVIG | Cytokines, chemochines, soluble cytokine receptors and receptor antagonists |
Legend IVIGs: intravenous immunoglobulins.
Replacement treatment: type, dose and timing of treatment
Immunoglobulin replacement is the standard therapy for primary antibody deficiencies (PAD) aiming to replace the missing antibodies and thereby to prevent recurrent infections. A number of Ig products are available and deciding which one to use, and in what dose are the points to consider.
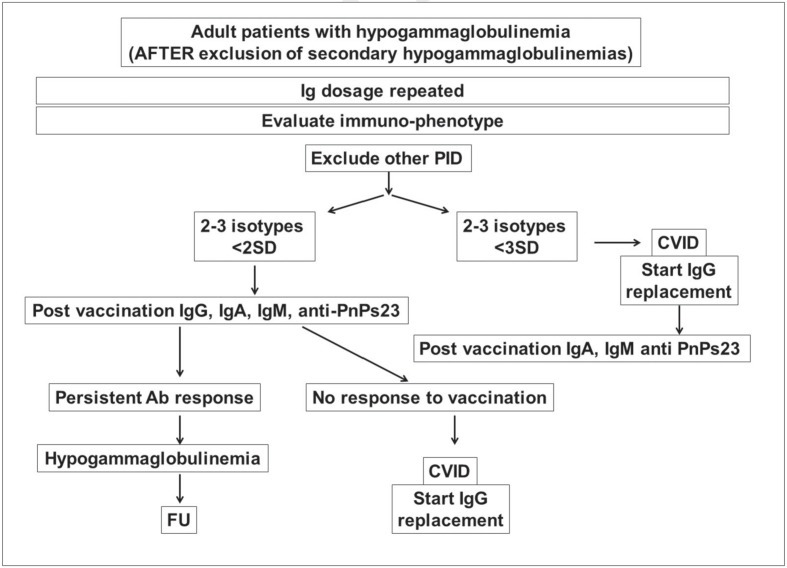
For PAD patients, a thorough evaluation of immune function before deciding on Ig replacement is important10. We have recently developed an algorithm that might help physicians in the diagnostic process and in the decision on the immunoglobulin replacement starting (Figure 1).
Figure 1.
Algorithm for diagnosis in patients with hypogammaglobulinemias.
Legend Ig: immunoglobulins; PID: Primary Immune Deficiencies; Ab: antibodies; PnPs23: 23-valent pneumococcal polysaccharides; FU: follow up; SD: standard deviation.
After diagnosis, the optimal immunoglobulin replacement dosages required over time to minimise infection risks are not yet definitely established with a consequent wide variation in treatment practices11–14. Debate continues regarding the exact timing and the optimal prophylaxis regimen, knowing that the system of care is itself an important determinant of patient’s outcome. Individualised medicine and personalised health research presents methodological challenges. Different options have been explored to establish how we should individualise immunoglobulin replacement therapy. As with many interventions, there may be specific subgroups of patients who are more likely to benefit from treatment with higher or lower dosages of immunoglobulins.
Clinical PAD phenotypes are quite variable within these diseases and patients are susceptible to a wide range of complications other than infections15. In PAD we have identified a clinical phenotype characterised by a high pneumonia risk16: patients who had low IgG and IgA levels at diagnosis; patients who had IgA level <7 mg/dL and who had bronchiectasis and absence of protective IgA and IgM anti-polysaccharide after pneumococcal immunisation, confirming previous data that demonstrate that the loss of function of memory B cells seems to represent the major cause of PAD-associated infectious diseases.
We also demonstrated that better patient’s outcome could be achieved by shorting the administration intervals from 3–4 to 2-1 weeks without the need to greatly increase the monthly cumulative Ig dosage in those PAD patients: 1) who present an infectious risk profile, 2) who are affected by bronchiectasis and/or enteropathy; 3) who continue to had adverse events despite pre-medication.
On the other hand, in patients with fewer disease-associated complications the immunoglobulin replacement could be done with the widely used interval of 3 or 4 weeks, even administering a low immunoglobulin replacement dosage. Also the adverse events can be greatly reduced by administrating low dosages in a single setting and by shortening the administration intervals17. Thus, the suggested protective high trough IgG level (1,000 mg/dL)18 to be maintained to avoid infections should not be considered a general goal and only large prospective multi-centre studies might help to define the prevalence of patients needing a higher or lower monthly Ig dosage and the best interval between administrations. These considerations have a vast potential to ameliorate the clinical practice of immunoglobulins replacement treatment.
Replacement treatment: setting of treatment
In the United States, immunologists have reported to the Immune Deficiency Foundation that their patients receive their IVIGs infusions in a hospital out-patient setting or at home by home infusion service19. About 20% of these practitioners have allowed the self-infusion of IVIG at home. In the United States, the increasing of subcutaneously immunoglobulins use (SCIGs) has also proved satisfactory with similar efficacy rates as for intravenous administration. This use appears to approach at 33% for immune deficient patients in the United States at this time.
In Italy, patients are treated only in a hospital setting as out-patients or in-patients. Home treatment with IVIGs is not allowed. A lower number, about 20%, of patients affected by Primary Immune Deficiencies receive SCIGs self-infused at home.
Anti-inflammatory and immuno-modulating treatment: mechanisms of action, dose and timing of treatment
IVIGs therapy is administered at high doses (1–2 grams per kilogram body weight) for the suppression of autoantibody-triggered inflammation in a variety of clinical settings20–22. However, dosages and timing of administration are not defined for most of the clinical conditions. Trials comparing different regimens are also lacking. Thus, there is a wide variation in treatment practice. Consequently, there is now urgent need to define when treatment should be started, at which dosages, and to define the maintenance dose and the intervals between administrations. In the trials aimed to define the efficacy, IVIG are usually administered as a 2 g/kg loading dose followed by a 1 g/kg maintenance dose every 3–4 weeks, for variable periods of time.
More information is available on the biological mechanisms of action23–26: IVIGs induce significant modifications in the innate and adaptive compartment of the immune system, including dendritic cells, the monocyte/macrophage system, granulocytes, natural killer cells, regulatory T cell subsets and B cells. Together, these findings may help to explain the beneficial effects of IVIGs in autoimmune and inflammatory disorders due to dysregulated cellular immunity. Despite the identification of immunomodulatory and antiinflammatory activities in various diseases, the benefits of immunoglobulins are not easily explained and probably cannot be explained by a single mechanism. This pleiotropic effect might provide advantages in treating the various inflammatory and autoimmune conditions, but only knowing that immune globulin can exert both antiinflammatory and proinflammatory effects, depending on the interacting partner27. Ultimately, the anti-inflammatory and immune modulating responses may be explained by genetic and functional variations in FcγR expression. They might also relate to differential antibody Fc glycosylation patterns28. The demonstration that minor variations in the IgG-attached sugar moiety can switch an antibody from a proinflammatory to an anti-inflammatory state was a great surprise, and initiated many studies directed at understanding the function of the complex array of glycovariants present in serum IgG. More recently, attempts to bioengineer a protein with immunomodulatory activities similar to those of native IgG have been going ahead. Delineation of the potential role of sialylated Fc in some of the immunomodulatory activities may be one important step if results similar to those shown in animals can be found in humans. If only a portion of the total intravenous immunoglobulins is effective, that would explain why the doses currently required are so high.
Prioritising IVIG indications
Intravenous immune globulin therapy, especially at doses of 2 g per kilogram per month, is expensive, and with expanding use there are concerns about present and future supplies, especially if the donor pool decreases or is limited by safety issues and increased pathogen screening of donors of the source plasma. Given the number of indications for therapeutic immunoglobulin and the diversity of related clinical fields, careful consideration of the usefulness of IVIG in each condition is warranted. There are no specific guidelines in place for this process and there is a tremendous variability amongst institutions and practices. Recently, evidence-based disease indications have been proposed20. The Department of Health, London, assigned to clinical condition with level of evidence 1A and 2A a high or a medium priority. In case of shortage the list of high priority includes: Primary Immune Deficiencies, specific antibody deficiencies, thymoma with immunodeficiency, alloimmune thrombocytopenia, chronic inflammatory demyelinating polyradiculoneuropathy, Guillain-Barré syndrome, haemolitic disease of the newborn, bone marrow transplantation in Primary Immune Deficiencies, immune thrombocytopenic purpura, Kawasaki disease, paraprotein-associated demyelinating neuropathy, toxic epidermal necrolysis, Stevens-Jonhson syndrome. Similarly, the American Academy for Allergy and Clinical Immunology updated the list of priorities, including primary immunodeficiencies with the exclusion of the selective IgA deficiency, toxic epidermal necrolysis, ITP, CIDP and Kawasaki disease. They eliminate from the list the following indications: autism and recurrent spontaneous abortion.
Adverse reactions: the need to monitor and to quantify adverse events (AE)
IVIGs is generally considered a safe and efficacious therapeutic modality. However, it is associated with adverse effects (Table II). It is very important for clinicians to have the knowledge of those AE profiles and strategies for their identification and management. Very few data are available on the incidence of serious and mild AE analysed and quantified in post-marketing surveys. More data are available on haematological toxicities29: multiple reports of red blood cell haemolysis after high doses and also when IVIGs are administered at low doses; thrombosis; stroke; pulmonary embolism; deep vein thrombosis and arterial thrombotic events including myocardial infarction. Leukopenia, neutropenia and monocytopenia are also associated with IVIGs therapy. Fortunately, those cytopenias usually appear to be self-limited and have not been correlated with increased infection incidence.
Table II.
Adverse reactions to polyvalent immunoglobulin treatment.
| Common toxicities of IVIGs |
|---|
|
Legend IVIGs: intravenous immunoglobulins.
Knowledge is progressing on the mechanisms underlying AE. Beside the old description of IgA-deficient patients who have IgG or IgE anti-IgA antibodies with consequent reactions caused by complement activation, more recently, a novel risk due to a splice variant, FcγRIIa(exon6*), was described. It is characterised as a low-frequency allele, coding for a gain-of-function receptor for IgG. In the presence of immune complexes, FcγRIIa(exon6*) can contribute to anaphylaxis in patients with Primary Immune Deficiencies30.
Conclusions
Polyvalent immunoglobulins exert an effect more than mere substitution of antibodies against pathogenic microbes; it rectifies the defective cellular compartment signalling thus re-establishing immune homeostasis. Only knowing these multiple and sometimes opposite effects exerted at different dosages it will be possible to introduce a rationale use of IVIGs.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Council of Europe. Human normal immunoglobulin for intravenous administration Monograph 01/2002:0918 European Pharmacopoeia. 4th ed. Strasbourg: Council of Europe Publishing; 2002. (4.02) [Google Scholar]
- 2.Ochsenbein AF, Fehr T, Lutz C, et al. Control of early viral and bacterial distribution and disease by natural antibodies. Science. 1999;286:2156–9. doi: 10.1126/science.286.5447.2156. [DOI] [PubMed] [Google Scholar]
- 3.Kaveri SV. Intravenous immunoglobulin: exploiting the potential of natural antibodies. Autoimmun Rev. 2012;11:792–4. doi: 10.1016/j.autrev.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Durandy A, Kaveri SV, Kuijpers TW, et al. Intravenous immunoglobulins - understanding properties and mechanisms. Clin Exp Immunol. 2009;158(Suppl 1):2–13. doi: 10.1111/j.1365-2249.2009.04022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaveri SV, Lacroix-Desmazes S, Bayry J. The anti-inflammatory IgG. N Engl J Med. 2008;359:307–9. doi: 10.1056/NEJMcibr0803649. [DOI] [PubMed] [Google Scholar]
- 6.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. 2013;13:176–89. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 7.Tha-In T, Bayry J, Metselaar HJ, et al. Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol. 2008;29:608–15. doi: 10.1016/j.it.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Gelfand E. Intravenous Immune Globulin in Autoimmune and Inflammatory Diseases. N Engl J Med. 2012;367:2015–25. doi: 10.1056/NEJMra1009433. [DOI] [PubMed] [Google Scholar]
- 9.Kaveri KS, Maddur MS, Hegde P, et al. Immunoglobulins in immunodeficiencies: more than mere replacement therapy. Clin and Exp Immunol. 2011;164:2–5. doi: 10.1111/j.1365-2249.2011.04387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cunningham Rundles C. Key aspects for successful immunoglobulin therapy of primary immunodeficiencies. Clinical and Experimental Immunology. 2011;164(Suppl 2):16–19. doi: 10.1111/j.1365-2249.2011.04390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ochs HD, Fischer SH, Wedgwood RJ. Comparison of high-dose and low-dose intravenous immunoglobulin therapy in patients with primary immunodeficiency diseases. Am J Med. 1984;76:78–82. doi: 10.1016/0002-9343(84)90324-3. [DOI] [PubMed] [Google Scholar]
- 12.Bonagura VR, Marchlewski R, Cox A, Rosenthal DW. Biologic IgG level in primary immunodeficiency disease: the IgG level that protects against recurrent infection. J Allergy Clin Immunol. 2008;122:210–2. doi: 10.1016/j.jaci.2008.04.044. [DOI] [PubMed] [Google Scholar]
- 13.Lucas M, Hugh-Jones K, Welby A, et al. Immunomodulatory therapy to achieve maximum efficacy: doses, monitoring, compliance, and self-infusion at home. J Clin Immunol. 2010;30:S84–9. doi: 10.1007/s10875-010-9400-y. [DOI] [PubMed] [Google Scholar]
- 14.Roifman CM, Berger M, Notarangelo LD. Management of primary antibody deficiency with replacement therapy: summary of guidelines. Immunol Allergy Clin North Am. 2008;28:875–6. doi: 10.1016/j.iac.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Chapel H, Lucas M, Lee M. Commonvariable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112:277–86. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 16.Quinti I, Soresina A, Guerra A, et al. IPINet Investigators. Effectiveness of Immunoglobulin Replacement Therapy on Clinical Outcome in Patients with Primary Antibody Deficiencies: Results from a Multicenter Prospective Cohort Study. J Clin Immunol. 2011;31:315–22. doi: 10.1007/s10875-011-9511-0. [DOI] [PubMed] [Google Scholar]
- 17.Quinti I, Milito C, Pulvirenti F, et al. Better patient’s outcome can be achieved by shortening the monthly replacement intervals in patients with severe primary antibody deficiencies and adverse reactions to immunoglobulins. J Biol Reg Homeos Ag. 2013 (in press) [Google Scholar]
- 18.Orange JS, Grossman WJ, Navickis RJ, Wilkes MM. Impact of trough IgG on pneumonia incidence in primary immunodeficiency: a meta-analysis of clinical studies. Clin Immunol. 2010;137:21–30. doi: 10.1016/j.clim.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 19.Orange JS, Ochs HD, Cunningham-Rundles C. Prioritization of Evidence-Based Indications for Intravenous Immunoglobulin. J Clin Immunol. 2013;33:1033–6. doi: 10.1007/s10875-013-9912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orange JS, Hossney EM, Weiler CR, et al. Use of intravenous immunoglobulin in human disease: a review of evidence by members of the Primary Immunodeficiency Committee of the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2006;117(Suppl):S525–53. doi: 10.1016/j.jaci.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 21.Newburger JW, Takahashi M, Burns JC, et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N Engl J Med. 1986;315:341–7. doi: 10.1056/NEJM198608073150601. [DOI] [PubMed] [Google Scholar]
- 22.Bayry J, Negi VS, Kaveri SV. Intravenous immunoglobulin therapy in rheumatic diseases. Nat Rev Rheumatol. 2011;7:349–59. doi: 10.1038/nrrheum.2011.61. [DOI] [PubMed] [Google Scholar]
- 23.Tha-In T, Bayry J, Metselaar HJ, et al. Modulation of the cellular immune system by intravenous immunoglobulin. Trends Immunol. 2008;29:608–15. doi: 10.1016/j.it.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 24.Nimmerjahn F, Ravetch JV. Anti-inflammatory actions of intravenous immunoglobulin. Annu Rev Immunol. 2008;26:51–33. doi: 10.1146/annurev.immunol.26.021607.090232. [DOI] [PubMed] [Google Scholar]
- 25.Seite JF, Shoenfeld Y, Youinou P, Hillion S. What is the contents of the magic draft IVIg? Autoimmun Rev. 2008;7:435–9. doi: 10.1016/j.autrev.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 26.Kaveri SV, Lacroix-Desmazes S, Bayry J. The anti-inflammatory IgG. N Engl J Med. 2008;359:307–9. doi: 10.1056/NEJMcibr0803649. [DOI] [PubMed] [Google Scholar]
- 27.Durandy A, Kaveri SV, Kuijpers TW, et al. Intravenous immunoglobulins - understanding properties and mechanisms. Clin Exp Immunol. 2009;158(Suppl 1):2–13. doi: 10.1111/j.1365-2249.2009.04022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anthony RM, Ravetch JV. A novel role for the IgG Fc glycan: the anti-inflammatory activity of sialylated IgG Fcs. J Clin Immunol. 2010;30(Suppl 1):S9–14. doi: 10.1007/s10875-010-9405-6. [DOI] [PubMed] [Google Scholar]
- 29.Baxley A, Akhtari M. Hematologic toxicities associated with intravenous immunoglobulin therapy. Int Immunopharmacol. 2011;11:1663–7. doi: 10.1016/j.intimp.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 30.Van der Heijden J, Geissler J, van Mirre E, et al. A novel splice variant of FcγRIIa: a risk factor for anaphylaxis in patients with hypogammaglobulinemia. J Allergy Clin Immunol. 2013;131:1408–16. doi: 10.1016/j.jaci.2013.02.009. [DOI] [PubMed] [Google Scholar]