Introduction and notions of physiology
Antithrombin (AT) is a single-stranded glycoprotein synthesised in the liver with a plasma concentration of approximately 150 μg/mL and a molecular weight of approximately 59,000 Da1. It is a complex molecule with multiple biologically important properties. It is a serine protease inhibitor (serpin) that inactivates many enzymes in the coagulation cascade2,3. Indeed, it is the key inhibitor of the coagulation system and is estimated to provide 80% of the inhibitory activity against thrombin but it also inhibits activated factors X, IX, VII, XI, and XII. AT has a great affinity for thrombin and is also known as heparin cofactor as it is responsible for the anticoagulant effect of heparin. Heparins markedly accelerate the rate of complex formation between AT and the serine proteases thus increasing AT inhibitory activity 5,000–40,000-fold4. AT also has remarkable anti-inflammatory properties, several of which result from its actions in the coagulation cascade. Other anti-inflammatory properties of AT involve direct interactions with endothelial cells, leading to the release of prostacyclin, which also mediates its anti-platelet effect3. In addition, recent evidence of interactions of the endothelial cell growth factors bFGF (basic fibroblast growth factor) and VEGF (vascular endothelial cell growth factor) with a heparin-like molecule in matrix provide the rationale for further investigation into the possible role of AT as a potent anti-angiogenic factor5.
Normal values of AT activity in the plasma range from 80% to 120%. In normal conditions, its biological half-life is 1.5–2.5 days. In conditions of acquired deficiency and in the presence of heparin, the half-life of AT can be notably shorter, being reduced to only a few hours6.
Congenital AT deficiency
The estimated prevalence of congenital AT deficiency is 1/2,000–5,000 in the general population and 2–3% in a selected population of patients with thrombotic events1. Most cases are heterozygous7.
There are two different types of congenital AT deficiency4,7, which are inherited in an autosomal dominant manner:
- TYPE I (quantitative defect), in which there are proportional decreases in the concentration and, therefore, functional activity of AT.
- TYPE II (qualitative defect), characterised by normal levels of protein, but a reduction in its functional activity. Type II deficiencies can be further sub-classified into three types (IIa, IIb, IIc), depending on the location of the mutations and, consequently, the performance of different AT assays7,8.
The AT activity of patients suffering from this defect is reduced by 50% and they have frequent episodes of venous and9, less frequently, arterial thrombosis (e.g. myocardial infarction) or thromboembolism at a young age, even in the absence of risk factors.
Acquired AT deficiency
Various clinical conditions are associated with acquired AT deficiency. It can be caused by a reduced synthesis in the case of acute or chronic liver failure, usually accompanied by a parallel reduction of the synthesis of coagulation factors and natural inhibitors of the coagulation cascade. It can also be caused by an increased consumption [e.g. disseminated intravascular coagulation (DIC)] or increased excretion/loss (e.g. nephrotic syndrome, burns)6.
Indications for the clinical use of antithrombin
Patients with congenital AT deficiency
Although deficiencies of natural anticoagulants (and in particular, AT deficiency) are usually labeled as high-risk thrombophilias, this perception is based on older studies with important methodological limitations10. More rigorous recent studies have not confirmed a high risk of thrombosis recurrence11,12. Maintaining adequate levels of AT during high-risk periods is an important treatment goal. Long-term anticoagulant thromboprophylaxis is not recommended in asymptomatic patients with AT deficiency because of the increased risk of haemorrhage10,13. Evidence-based clinical practice guidelines from the American College of Chest Physicians recommend short-term thromboprophylaxis in high-risk clinical settings, including surgery, trauma, and management of pregnancy, labour and delivery10,13.
The goal of treatment for patients with hereditary AT deficiency is an initial increase in AT activity to ≥120% of normal levels followed by maintenance of activity at ≥80% of normal levels13.
However, no randomised clinical trials (RCTs) have been carried out to assess both the need and efficacy of AT replacement therapy7,14. Its use has been reported in case series only.
In conclusion, in the absence of symptoms or risk factors, congenital AT deficiency is not an indication for replacement therapy with AT concentrates, which should be reserved, on a temporary basis and in association with heparin therapy, to the following circumstances6: i) prophylaxis of deep vein thrombosis and thromboembolism in high-risk conditions such as major surgery, obstetric procedures, trauma, immobilisation; ii) treatment of ongoing thrombosis, until the indicated level of oral anticoagulation is reached.
Patients with acquired AT deficiency
The indications, efficacy and cost-effectiveness for the administration of AT concentrate in patients with acquired AT deficiency are not supported by adequately robust RCTs. Its controversial use in several settings of acquired deficiency has been further questioned by the recent publication of several guidelines15–17 and meta-analyses18–22.
According to the guidance for both diagnosis and treatment of DIC of the British Committee for Standards in Haematology (BCSH) (Grade A, level Ib) and the Società Italiana per lo studio dell’Emostasi e della Trombosi (SISET, Italian Society for Thrombosis and Hemostasis) (Grade D), the use of AT concentrates is not recommended15,16.
A similar recommendation against AT administration for the treatment of severe sepsis and septic shock has been endorsed by the 2012 international guidelines for management of severe sepsis and septic shock (Grade B)17.
Although acquired AT deficiency is a common and prognostically important finding in sick preterm infants with respiratory distress syndrome, those patients are unlikely to benefit from AT treatment and may be harmed18. There is also little evidence that AT administration reduces the risk of intracranial haemorrhage and the benefit of AT replacement in neonatal sepsis remains uncertain19.
AT also cannot be recommended for critically ill patients, based on the available evidence. In fact, it did not reduce the overall mortality compared with the control group [relative risk (RR): 0.96; 95% confidence interval (CI): 0.89 to 1.03; no heterogeneity between trials] or the incidence of increased bleeding events (RR: 1.52; 95% CI 1.30 to 1.78)20 in a statistically significant manner.
A recently published meta-analysis on haematological interventions for treating DIC during pregnancy and post-partum found no RCTs on the safety and efficacy of several haematological interventions, including the use of AT. Therefore, the authors concluded that such interventions need to be tested in RCTs assessing outcomes such as maternal death, perinatal death and safety21.
AT was evaluated in one study in children treated with L-asparaginase23 and had no significant effect on venous thromboembolism (RR: 0.84; 95% CI: 0.41 to 1.73) or major bleeding (RR: 0.78; 95% CI 0.03 to 18.57) when compared to controls22. Although the therapy with L-asparaginase causes an acquired AT deficiency, the concurrent depletion of other haemostatic proteins means that the exact mechanism leading to thrombosis has still to be identified. In fact, there is only limited evidence supporting the use of AT concentrates in either primary prevention or management of thrombotic events and evidence-based guidelines are lacking24.
A further setting where there is limited available clinical evidence for the use of AT is heparin resistance, which causes an increased risk of thromboembolic complications in patients undergoing cardiovascular surgery requiring cardiopulmonary bypass25. Although AT deficiency has been generally thought to be the primary mechanism of heparin resistance, the reasons for heparin resistance are both complex and multifactorial. Treatment options for heparin resistance include additional heparin or AT supplementation26.
Finally, AT concentrates are not indicated, even when AT levels are considerably below normal, in the following conditions: acute or chronic liver disease (where there is a parallel reduction of the synthesis of coagulation factors), nephrotic syndrome, protein-losing enteropathy, pre-eclampsia, multiple trauma and in the post-operative period6. In the setting of nephrotic syndrome, there is also an on-going debate regarding the appropriateness of prophylactic anticoagulation to prevent nephrotic syndrome-associated thromboembolism27,28. The cause of the controversy is the lack of any adequately robust RCT to establish the efficacy and safety of this approach.
An additional important issue exacerbating the argument on the use of AT is the required therapy duration with AT concentrate (for any indications) that is unknown14.
In conclusion, because of the high cost, AT should be administered “only when proven (by RCTs) rather than postulated (based on pathophysiology or on observations) benefit can be expected” from its infusion29.
Table I contains a brief fact sheet on AT with the indications for its clinical use generally reported within the Summary of Product Characteristics of the preparations containing AT currently available in Italy. However, as regards both acquired and congenital AT deficiencies, these indications do not appear to be completely in line with the scientific evidence we have at our disposal.
Table I.
Antithrombin, brief fact sheet.
| ATCa | B01AB02 |
| Definition | Antithrombin |
| U/Mb | International Units (I.U.) |
| Therapeutic indications | AT administration is used for prophylaxis and therapy for thromboembolic phenomena due to:
|
| NHS classc | H |
| Management information | - |
Anatomical Therapeutic Chemical Classification System. The World Health Organization system classifies all therapeutic medicinal products. The purpose is to serve as a tool for medicinal products utilisation research in order to improve the quality of their use. Medicinal products are classified into five different levels;
Unit of Measure;
National Health Service Class.
All medicinal products are divided in classes according to the level of reimbursement by the National Health Service. Class A includes all medicinal products funded by the National Health Service; class H includes all medicinal products distributed only by hospital pharmacies within the regional healthcare services; class C refers to all medicinal products sa paid for by private out-of-pocket expense.
Table II contains the brand names of the preparations containing AT currently available in Italy30, as well as the quantity of the active substance.
Table II.
Medicinal products containing antithrombin currently available on the Italian market.
Source: Farmadati (www.farmadati.it, accessed on 01/03/2012), processed and adapted by the Italian National Blood Centre.
| AIC codea | Name of medicinal product | I.U.b | Manufacturer | NHS classc |
|---|---|---|---|---|
| 025766039 | KYBERNIN P*IV FL 500UI+10ML+SE | 500 | CSL Behring SpA | H |
| 027113012 | ANTITROMBINA III 500UI*FL 10ML | 500 | Baxter SpA | H |
| 029378015 | AT III KED.*500UI+FL 10ML+SET | 500 | Kedrion SpA | H |
| 031118019 | ATENATIV*IV FL 500UI+FL 10ML | 500 | Octapharma Italy SpA | H |
| 034330035 | ANBINEX*FL 500UI+SIR 10ML+SET | 500 | Grifols Italia SpA | H |
| 025766027 | KYBERNIN P*IV FL 1000UI+F 20ML | 1,000 | CSL Behring SpA | H |
| 027113024 | ANTITROMBINA III 1000UI*FL20ML | 1,000 | Baxter SpA | H |
| 029378027 | AT III KED.*1000UI+FL 20ML+SET | 1,000 | Kedrion SpA | H |
| 031118021 | ATENATIV*IV FL 1000UI+FL 20ML | 1,000 | Octapharma Italy SpA | H |
| 034330047 | ANBINEX*FL 1000UI+SIR 20ML+SET | 1,000 | Grifols Italia SpA | H |
| 027113036 | ANTITROMBINA III 1500UI*FL30ML | 1,500 | Baxter SpA | H |
| 031118033 | ATENATIV*IV FL 1500UI+FL 30ML | 1,500 | Octapharma Italy SpA | H |
| 029378039 | AT III KED.*2000UI+FL 40ML+SET | 2,000 | Kedrion SpA | H |
AIC, Autorizzazione Immissione in Commercio, Marketing authorisation code. The Italian Medicines Agency (Agenzia Italiana del Farmaco, AIFA) is in charge of releasing the AIC code, which identifies each medicinal products package on the national market30;
International units of antithrombin contained in the medicinal product;
National Health Service Class.
All medicinal products are divided in classes according to the level of reimbursement by the National Health Service. Class A includes all medicinal products funded by the National Health Service; class H includes all medicinal products distributed only by hospital pharmacies within the regional healthcare Services; class C refers to all medicinal products as paid for by private out-of-pocket expense.
Quantification and characterisation of antithrombin demand
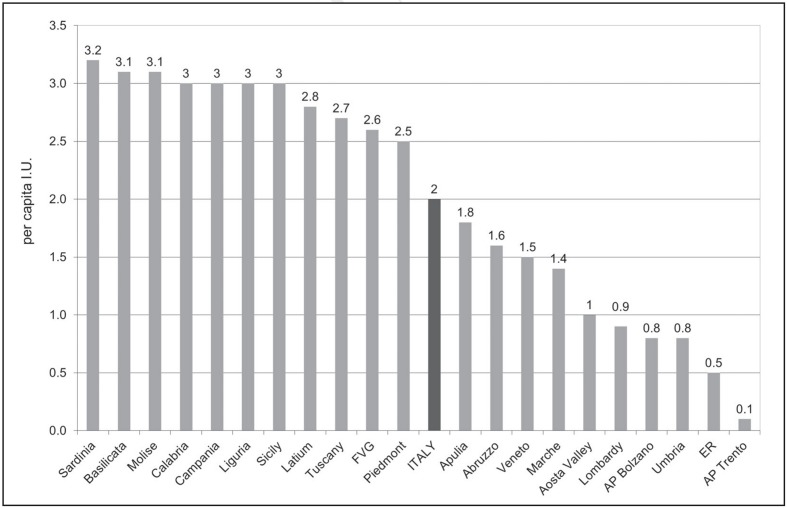
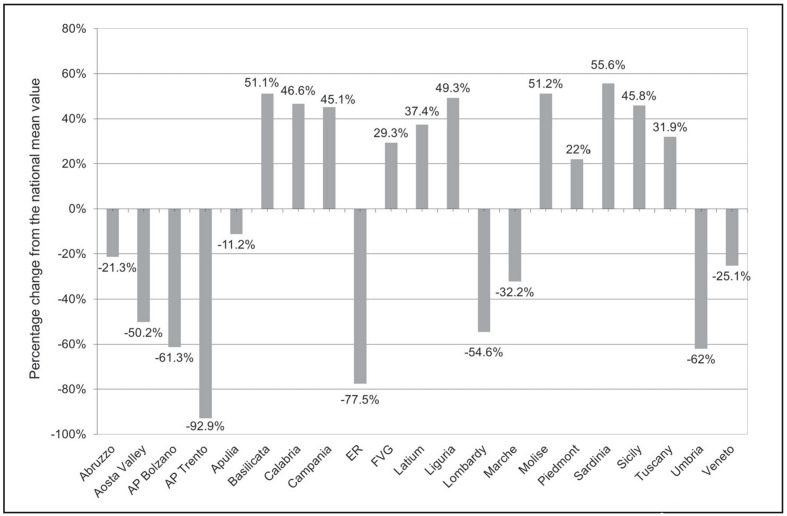
Table III and IV show the total (public and private) and total standardised demand for AT expressed in international units (I.U.) and in per capita I.U., respectively, in the period 2007–2011 at the national and regional levels, according to medicinal products traceability data31. In 2011, in Italy the total demand was 123,329,500 I.U. (Table III) while the total standardised demand was 2 per capita I.U. (Table IV). In the same year, the highest absolute demand was recorded in Campania, Latium and Sicily (Table III) representing 40% of national demand, although these regions have just over a quarter of the entire Italian population (26.4%). On the other hand, in 2011, the highest per capita demand was recorded in Sardinia with 3.2 I.U., Molise and Basilicata with 3.1 I.U. (Table IV, Figure 1). Figure 2 shows the 2011 deviation of per capita regional utilisation compared to the national mean value. The aforementioned Regions showed a percentage departure from the national average of +56% and +51%, respectively (Figure 2).
Table III.
Quantification of total (public and private) demand for antithrombin (expressed in international units) in Italy and Italian regions, from 2007 to 2011.
Source: medicinal product traceability, processed and adapted by the Italian National Blood Centre.
| Region | 2007 | 2008 | 2009 | 2010 | 2011 |
|---|---|---|---|---|---|
| Abruzzo | 2,344,000 | 2,785,500 | 2,397,000 | 2,065,500 | 2,149,500 |
| Aosta Valley | 183,000 | 228,500 | 218,000 | 118,500 | 130,000 |
| AP Bolzano | 175,000 | 430,000 | 200,000 | 275,000 | 400,000 |
| AP Trento | 247,000 | 245,000 | 153,000 | 131,000 | 77,000 |
| Apulia | 5,813,500 | 6,304,000 | 6,987,500 | 7,184,000 | 7,387,500 |
| Basilicata | 885,000 | 1,812,000 | 2,410,000 | 2,108,000 | 1,805,500 |
| Calabria | 5,308,500 | 7,061,500 | 7,018,500 | 5,216,500 | 5,996,500 |
| Campania | 10,662,500 | 10,225,500 | 11,152,500 | 17,428,500 | 17,222,500 |
| Emilia-Romagna | 1,799,000 | 2,285,000 | 2,376,000 | 2,577,000 | 2,031,000 |
| Friuli-Venezia Giulia | 2,009,500 | 3,238,000 | 3,249,000 | 2,740,000 | 3,250,000 |
| Latium | 15,976,500 | 14,182,000 | 14,289,500 | 14,180,000 | 16,017,000 |
| Liguria | 3,158,000 | 3,823,000 | 3,708,000 | 3,781,000 | 4,911,000 |
| Lombardy | 6,543,500 | 6,623,500 | 6,113,000 | 7,035,000 | 9,169,000 |
| Marche | 2,816,500 | 2,891,500 | 3,429,500 | 2,474,000 | 2,160,000 |
| Molise | 728,000 | 519,500 | 833,000 | 561,000 | 983,500 |
| Piedmont | 12,912,000 | 13,640,000 | 12,934,000 | 12,497,500 | 11,059,500 |
| Sardinia | 4,302,500 | 5,163,500 | 3,416,500 | 4,287,500 | 5,303,000 |
| Sicily | 13,385,000 | 12,351,500 | 12,826,500 | 9,719,000 | 14,978,000 |
| Tuscany | 9,414,500 | 7,684,000 | 10,124,500 | 8,916,000 | 10,064,000 |
| Umbria | 598,000 | 776,000 | 889,000 | 837,000 | 701,000 |
| Veneto | 6,182,000 | 4,933,000 | 5,313,000 | 6,000,500 | 7,524,000 |
| Other* | 1,296,000 | 673,000 | 558,500 | 292,000 | 10,000 |
|
| |||||
| Italy | 106,739,500 | 107,875,500 | 110,596,500 | 110,424,500 | 123,329,500 |
Legend AP: Autonomous Province; Other*: movements of medicinal products not univocally defined.
Table IV.
Quantification of total (public and private) standardised demand for antithrombin (expressed in per capita international units) in Italy and Italian regions, from 2007 to 2011.
Source: medicinal product traceability, processed and adapted by the Italian National Blood Centre.
| Region | 2007 | 2008 | 2009 | 2010 | 2011 |
|---|---|---|---|---|---|
| Abruzzo | 1.8 | 2.1 | 1.8 | 1.5 | 1.6 |
| Aosta Valley | 1.5 | 1.8 | 1.7 | 0.9 | 1 |
| AP Bolzano | 0.4 | 0.9 | 0.4 | 0.5 | 0.8 |
| AP Trento | 0.5 | 0.5 | 0.3 | 0.2 | 0.1 |
| Apulia | 1.4 | 1.5 | 1.7 | 1.8 | 1.8 |
| Basilicata | 1.5 | 3.1 | 4.1 | 3.6 | 3.1 |
| Calabria | 2.7 | 3.5 | 3.5 | 2.6 | 3 |
| Campania | 1.8 | 1.8 | 1.9 | 3 | 3 |
| Emilia-Romagna | 0.4 | 0.5 | 0.5 | 0.6 | 0.5 |
| Friuli-Venezia Giulia | 1.7 | 2.6 | 2.6 | 2.2 | 2.6 |
| Latium | 2.9 | 2.6 | 2.5 | 2.5 | 2.8 |
| Liguria | 2 | 2.4 | 2.3 | 2.3 | 3 |
| Lombardy | 0.7 | 0.7 | 0.6 | 0.7 | 0.9 |
| Marche | 1.8 | 1.9 | 2.2 | 1.6 | 1.4 |
| Molise | 2.3 | 1.6 | 2.6 | 1.8 | 3.1 |
| Piedmont | 3 | 3.1 | 2.9 | 2.8 | 2.5 |
| Sardinia | 2.6 | 3.1 | 2 | 2.6 | 3.2 |
| Sicily | 2.7 | 2.5 | 2.5 | 1.9 | 3 |
| Tuscany | 2.6 | 2.1 | 2.7 | 2.4 | 2.7 |
| Umbria | 0.7 | 0.9 | 1 | 0.9 | 0.8 |
| Veneto | 1.3 | 1 | 1.1 | 1.2 | 1.5 |
| Other* | na | na | na | na | na |
|
| |||||
| Italy | 1.8 | 1.8 | 1.8 | 1.8 | 2 |
Legend AP: Autonomous Province; Other*: movements of medicinal products not univocally defined; na: not assessable.
Figure 1.
Quantification of total (public and private) and total standardised demand for antithrombin (per capita international units) in Italy and Italian Regions, year 2011.
Source: medicinal product traceability, processed and adapted by the Italian National Blood Centre.
Legend AP: Autonomous Province; ER: Emilia-Romagna; FVG: Friuli-Venezia Giulia; I.U.: International Units.
Figure 2.
Percentage change from the national mean value of per capita regional utilisation of antithrombin in 2011.
Source: medicinal product traceability, processed and adapted by the Italian National Blood Centre.
Legend AP: Autonomous Province; ER: Emilia-Romagna; FVG: Friuli-Venezia Giulia.
In the same year, the lowest per capita demand was recorded in the Autonomous Province (AP) of Trento, in Emilia-Romagna and in Umbria with 0.1, 0.5, and 0.8 per capita I.U., respectively (Table IV, Figure 1); the same AP and Regions showed a negative percentage departure from the national mean value of 93%, 78%, and 62%, respectively (Figure 2).
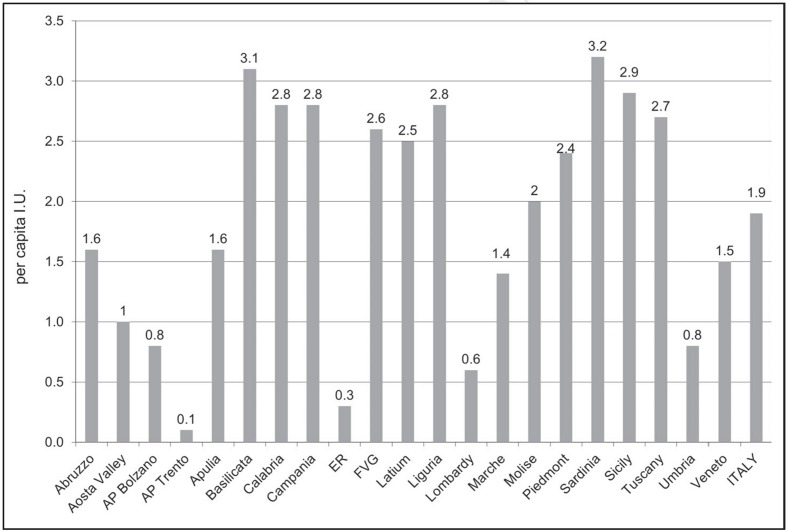
Although the hospital per capita AT utilisation does not show, neither per Region nor per type of facility (public or private), specific trends (Figures 3 and 4), as far as the public health facilities channel is concerned, the 2011 highest per capita demand was recorded in Sardinia, Basilicata and Sicily with about 3 I.U. each (Figure 3). On the contrary, the regional areas of lowest 2011 per capita AT demand were Emilia-Romagna and the AP of Trento with approximately 0.3 and 0.1 per capita I.U., respectively (Figure 3).
Figure 3.
Demand for antithrombin (per capita international units), in Italy and Italian regions, by public health facilities channels, year 2011.
Source: medicinal product traceability, processed and adapted by the Italian National Blood Centre.
Legend AP: Autonomous Province; ER: Emilia-Romagna; FVG: Friuli-Venezia Giulia; I.U.: International Units.
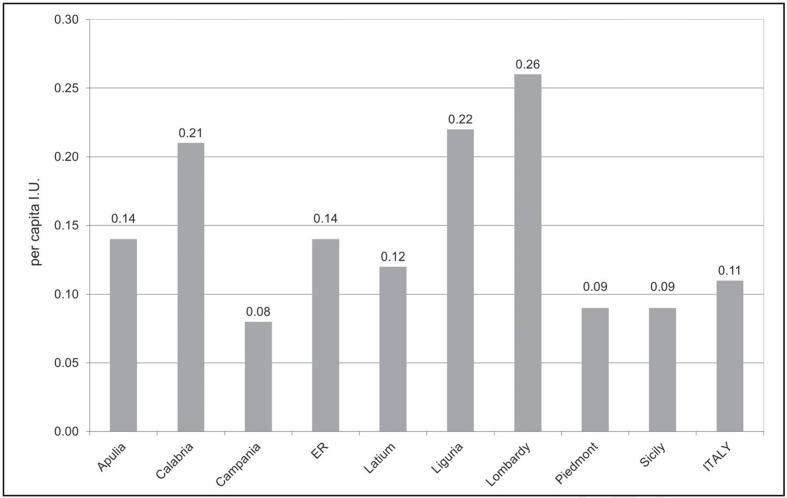
Figure 4.
Demand for antithrombin (per capita international units), in Italy and Italian regions, by private health facilities channels, year 2011.
Source: medicinal product traceability, processed and adapted by the Italian National Blood Centre.
Legend ER: Emilia-Romagna; I.U.: International Units.
In 2011, the highest distribution through private health facilities was recorded in Lombardy (0.3 per capita I.U.), Liguria and Calabria (0.2 per capita I.U., each) (Figure 4).
Furthermore, with respect to the world demand for AT, which is estimated to be about 700 million I.U., Italy (2 per capita I.U.) is the second country in the ranking, preceded only by Japan (about 3 per capita I.U.), and followed by Germany and France (1 and 0.3 per capita I.U., respectively32).
In addition, although at present there is a lack of unanimous international consensus on defined optimal per capita AT utilisation that would suggest an appropriate use of this plasma-derived medicinal product, benchmarking national data between Italy and other countries (i.e. Germany and France) provides a clear indication of the Italian tendency to over-use this medicinal product. This comparison should induce a careful consideration of its possible inappropriate usage, not only in Regions with a 2011 per capita demand higher than Japan (Sardinia, Molise and Basilicata), but also in all of the Italian Regions that recorded per capita AT usage much higher than Germany and France, which can be considered as comparable, in socio-economic terms, to Italy.
International recommendations for the appropriate use of antithrombin
At an international level, in addition to differences in utilisation, there are also differences in the recommendations for the appropriate use of AT concentrates. In fact, the guidelines recently published in the UK by the BCSH and in Italy by the SISET15,16, albeit with varying strength of the recommendation, do not advocate AT use in DIC. Contrary to these two European guidelines, those of the Japanese Society on Thrombosis Haemostasis/DIC subcommittee recommend AT use in the same condition with the following level of recommendation: B1 in general, B1 in organ dysfunction type, B2 in symptomatic/haemorrhagic/thrombotic type33. Therefore, a recent official communication by the active members of the subcommittee for DIC of the Scientific and Standardization Committee (SSC) of the International Society on Thrombosis Haemostasis (ISTH) has attempted to harmonise the three guidelines for DIC. The ITSH/SCC concluded that the use of AT concentrates in DIC patients is “potentially recommended” but needs further evidence34.
In the same way, with respect to supplement-doses of AT in patients with DIC, the DIC Committee of the Japanese Association for Acute Medicine (JAAM) has carried out a placebo-controlled RCT and reported its efficacy in septic DIC patients35. Contrary to the general trend in the Japanese scientific literature, the 2012 Surviving Sepsis Campaign Guidelines “recommend against antithrombin administration for the treatment of severe sepsis and septic shock (grade 1B)”17.
In addition, the 2009 recommendations on the use of AT by the Società Italiana di Medicina Trasfusionale e Immunoematologia (SIMTI, Italian Society of Transfusion Medicine and Immunohaematology) and the 2013 guidelines of the European Society of Anaesthesiology suggest that further studies are needed in patients undergoing extracorporeal circulation6,36. On the contrary, “AT concentrates are indicated to reduce plasma transfusion in patients with AT-mediated heparin resistance immediately before cardiopulmonary bypass” according to the 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines37.
Conclusions
The different recommendations for the clinical use of AT concentrates issued by scientific societies could, at least in part, be the reason for inconsistent and unstandardised therapeutic approaches and variability, also at international level, in the usage of this plasma-derived drug. At the moment, Italian data on AT utilisation show that there is substantial room for improving its appropriate use. Indeed, appropriateness and duration of AT therapy are topical and hot issues that still need the support of solid evidence. However, the evidence we need can only be obtained through adequately robust RCTs aimed at providing prospective validation of the use of a plasma-derived drug whose infusion, so far, is still being widely exploited in clinical practice due to postulated (based on pathophysiology or on observations) rather than really proven benefits29.
Footnotes
The Authors declare no conflicts of interest.
References
- 1.Hathaway WE, Goodnight SH., Jr . Malattie dell’Emostasi e Trombosi. Milan: McGraw-Hill Companies Italia; 1994. [Google Scholar]
- 2.Bauer KA. Selective inhibition of coagulation factors: advances in antithrombotic therapy. Semin Thromb Hemost. 2002;28(Suppl 2):15–24. doi: 10.1055/s-2002-32313. [DOI] [PubMed] [Google Scholar]
- 3.Roemisch J, Gray E, Hoffmann JN, et al. Antithrombin: a new look at the actions of a serine protease inhibitor. Blood Coagul Fibrinolysis. 2002;13:657–70. doi: 10.1097/00001721-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Haemostasis and Thrombosis Task Force, British Committee for Standards in Haemathology. Investigation and management of heritable thrombophilia. Br J Haematol. 2001;114:512–28. doi: 10.1046/j.1365-2141.2001.02981.x. [DOI] [PubMed] [Google Scholar]
- 5.Azhar A, Singh P, Rashid Q, et al. Antiangiogenic function of antithrombin is dependent on its conformational variation: implication for other serpins. Protein Pept Lett. 2013;20:403–11. [PubMed] [Google Scholar]
- 6.Liumbruno G, Bennardello F, Lattanzio A, et al. Italian Society of Transfusion Medicine and Immunohaematology (SIMTI) Working Party. Recommendations for the use of antithrombin concentrates and prothrombin complex concentrates. Blood Transfus. 2009;7:325–34. doi: 10.2450/2009.0116-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patnaik MM, Moll S. Inherited antithrombin deficiency: a review. Haemophilia. 2008;14:1229–39. doi: 10.1111/j.1365-2516.2008.01830.x. [DOI] [PubMed] [Google Scholar]
- 8.Lane DA, Bayston T, Olds RJ, et al. Antithrombin mutation database: 2nd (1997) update. For the Plasma Coagulation Inhibitors Subcommittee of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 1997;77:197–211. [PubMed] [Google Scholar]
- 9.Kottke-Marchant K, Duncan A. Antithrombin deficiency: issues in laboratory diagnosis. Arch Pathol Lab Med. 2002;126:1326–36. doi: 10.5858/2002-126-1326-AD. [DOI] [PubMed] [Google Scholar]
- 10.Bates SM, Greer IA, Middeldorp S, et al. American College of Chest Physicians. VTE, thrombophilia, antithrombotic therapy, and pregnancy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(Suppl 2):e691S–736S. doi: 10.1378/chest.11-2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson L, Wu O, Langhorne P, et al. Thrombosis Risk and Economic Assessment of Thrombophilia Screening (TREATS) Study. Thrombophilia in pregnancy: a systematic review. Br J Haematol. 2005;132:171–96. doi: 10.1111/j.1365-2141.2005.05847.x. [DOI] [PubMed] [Google Scholar]
- 12.Friederich PW, Sanson BJ, Simioni P, et al. Frequency of pregnancy-related venous thromboembolism in anticoagulant factor-deficient women: implications for prophylaxis. Ann Intern Med. 1996;125:955–60. doi: 10.7326/0003-4819-125-12-199612150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Rodgers GM. Role of antithrombin concentrate in treatment of hereditary antithrombin deficiency. An update. Thromb Haemost. 2009;101:806–12. [PubMed] [Google Scholar]
- 14.Sharpe CJ, Crowther MA, Webert KE, Donnery C. Cerebral venous thrombosis during pregnancy in the setting of type I antithrombin deficiency: case report and literature review. Transfus Med Rev. 2011;25:61–5. doi: 10.1016/j.tmrv.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145:24–33. doi: 10.1111/j.1365-2141.2009.07600.x. [DOI] [PubMed] [Google Scholar]
- 16.Di Nisio M, Baudo F, Cosmi B, et al. Italian Society for Thrombosis and Haemostasis. Diagnosis and treatment of disseminated intravascular coagulation: guidelines of the Italian Society for Haemostasis and Thrombosis (SISET) Thromb Res. 2012;129:e177–84. doi: 10.1016/j.thromres.2011.08.028. [DOI] [PubMed] [Google Scholar]
- 17.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013;39:165–228. doi: 10.1007/s00134-012-2769-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bassler D, Millar D, Schmidt B. Antithrombin for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev. 2006;4:CD005383. doi: 10.1002/14651858.CD005383.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bassler D, Schmidt B. Antithrombin replacement in neonates: is there any indication? Thromb Res. 2006;118:107–11. doi: 10.1016/j.thromres.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 20.Afshari A, Wetterslev J, Brok J, Møller AM. Antithrombin III for critically ill patients. Cochrane Database Syst Rev. 2008;3:CD005370. doi: 10.1002/14651858.CD005370.pub2. [DOI] [PubMed] [Google Scholar]
- 21.Martí-Carvajal AJ, Comunián-Carrasco G, Peña-Martí GE. Haematological interventions for treating disseminated intravascular coagulation during pregnancy and postpartum. Cochrane Database Syst Rev. 2011;3:CD008577. doi: 10.1002/14651858.CD008577.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Nisio M, Porreca E, Ferrante N, et al. Primary prophylaxis for venous thromboembolism in ambulatory cancer patients receiving chemotherapy. Cochrane Database Syst Rev. 2012;2:CD008500. doi: 10.1002/14651858.CD008500.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell L, Andrew M, Hanna K, et al. Trend to efficacy and safety using antithrombin concentrate in prevention of thrombosis in children receiving l-asparaginase for acute lymphoblastic leukemia. Results of the PAARKA study. Thromb Haemost. 2003;90:235–44. doi: 10.1160/TH02-11-0283. [DOI] [PubMed] [Google Scholar]
- 24.Truelove E, Fielding AK, Hunt BJ. The coagulopathy and thrombotic risk associated with L-asparaginase treatment in adults with acute lymphoblastic leukaemia. Leukemia. 2013;27:553–9. doi: 10.1038/leu.2012.290. [DOI] [PubMed] [Google Scholar]
- 25.Spiess BD. Treating heparin resistance with antithrombin or fresh frozen plasma. Ann Thorac Surg. 2008;85:2153–60. doi: 10.1016/j.athoracsur.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 26.Finley A, Greenberg C. Review article: heparin sensitivity and resistance: management during cardiopulmonary bypass. Anesth Analg. 2013;116:1210–22. doi: 10.1213/ANE.0b013e31827e4e62. [DOI] [PubMed] [Google Scholar]
- 27.Glassock RJ. Prophylactic anticoagulation in nephrotic syndrome: A clinical conundrum. J AmSocNephrol. 2007;18:2221–5. doi: 10.1681/ASN.2006111300. [DOI] [PubMed] [Google Scholar]
- 28.Kerlin BA, Ayoob R, Smoyer WE. Epidemiology and pathophysiology of nephrotic syndrome-associated thromboembolic disease. Clin J Am Soc Nephrol. 2012;7:513–20. doi: 10.2215/CJN.10131011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vamvakas EC. Reasons for moving toward a patient-centric paradigm of clinical transfusion medicine practice. Transfusion. 2013;53:888–901. doi: 10.1111/j.1537-2995.2012.03825.x. [DOI] [PubMed] [Google Scholar]
- 30.Calizzani G, Lanzoni M, Candura F, et al. Anni 2007–2011. Roma: Istituto Superiore di Sanità; 2012. [Accessed on 24/08/2013]. Analisi della Domanda dei Principali Medicinali Plasmaderivati in Italia. (Rapporti ISTISAN 12/53). Available at: http://www.iss.it/binary/publ/cont/dodici53web.pdf. [Google Scholar]
- 31.Lanzoni M, Biffoli C, Candura F, et al. Plasma-derived medicinal products in Italy: information sources and flows. Blood Transfus. 2013;11(Suppl 4):s13–7. doi: 10.2450/2013.004s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Robert P The Marketing Research Bureau (MRB) The Worldwide Plasma Fractions Market 2008. Orange, CT: The Marketing Research Bureau, Inc; 2010. April 2010 Ed. [Google Scholar]
- 33.Wada H, Asakura H, Okamoto K, et al. Japanese Society of Thrombosis Hemostasis/DIC subcommittee. Expert consensus for the treatment of disseminated intravascular coagulation in Japan. Thromb Res. 2010;125:6–11. doi: 10.1016/j.thromres.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 34.Wada H, Thachil J, Di Nisio M, et al. Guidance for diagnosis and treatment of DIC from harmonization of the recommendations from three guidelines. J Thromb Haemost. 2013 doi: 10.1111/jth.12155. [DOI] [PubMed] [Google Scholar]
- 35.Iba T, Nagaoka I, Boulat M. The anticoagulant therapy for sepsis-associated disseminated intravascular coagulation. Thromb Res. 2013;131:383–9. doi: 10.1016/j.thromres.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 36.Kozek-Langenecker SA, Afshari A, Albaladejo P, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013;30:270–382. doi: 10.1097/EJA.0b013e32835f4d5b. [DOI] [PubMed] [Google Scholar]
- 37.Society of Thoracic Surgeons Blood Conservation Guideline Task Force. Ferraris VA, Brown JR, Despotis GJ, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]