Abstract
Background
Our objective was to evaluate changes in serum leptin levels during pregnancy in overweight/obese and non-obese women and to assess total and percent weight gain during pregnancy as possible factors that influence leptin levels.
Material/Methods
In a prospective study of 42 low-risk pregnant women receiving prenatal care, we assessed serum leptin levels at gestational weeks 9–12, 25–28, and 34–37. Based on their pre-pregnancy body mass indices (BMIs), the cohort was divided into: non-overweight (BMI <25 kg/m2) and overweight/obese (BMI ≥25 kg/m2) subjects.
Results
We found a progressive increase in maternal weight gain during pregnancy in both groups. There was also a progressive increase in leptin levels in the 2 strata; however, the increase was significantly higher in the non-overweight patient group. We found that non-overweight pregnant women had a noticeably larger total weight gain. When analyzing the percent weight gain during pregnancy compared to the pre-pregnancy weight, the non-overweight group had a significantly greater percent weight gain than the overweight/obese group.
Conclusions
Our results suggest that the greater increase in leptin levels in non-overweight pregnant women can be explained by the higher percent weight gain in this group compared to overweight/obese women. These findings suggest that controlling the percent weight gain may be an important preventive measure when controlling leptin levels during pregnancy and subsequent medical complications.
Keywords: body weight gain, leptin, pregnancy, obesity
Background
Obesity is currently considered to be one of the major public health problems worldwide [1]; consequently, the number of obese women who become pregnant increases each year [2]. This condition is related with several diseases, such as hypertension, type-2 diabetes mellitus, dyslipidemia, metabolic syndrome, cardiovascular disease, and hepatic steatosis [3–5].
Obesity is also associated with adverse pregnancy outcomes such as pre-eclampsia, gestational diabetes mellitus (GDM), caesarean delivery, macrosomia, neural tube defect, thromboembolism, postpartum hemorrhage, puerperal infection, and increased risk of maternal and perinatal mortality [6–15]. According to the National Health and Nutrition Examination Survey, in the United States, more than half of pregnant women are overweight or obese [16]. The risks of most of these complications are amplified by excessive weight gain during pregnancy, which proportionally increases the degree of obesity [12].
Normal pregnancy has been characterized as a “diabetogenic” state [17]. Changes in the metabolism of carbohydrates and lipids occur during normal pregnancy to ensure a continuous supply of nutrients to the fetus despite non-continuous food intake by pregnant women [17]. These changes are progressive and promote the growth of adipose tissue during early pregnancy, which is followed by the induction of insulin resistance (IR) and lipolysis during late pregnancy [17–19].
In humans, leptin is the protein product of the ob gene and is primarily synthesized in adipose tissue. It sends an afferent signal to the central nervous system, where it acts to control food intake, insulin secretion, glucose utilization, glycogen synthesis, fatty acid metabolism, and in the regulation of adipose tissue, energy expenditure, body weight, and appetite [20,21]. During pregnancy, leptin is also synthesized by the placenta [22] and plays an important role in regulating maternal energy metabolism and the induction of physiological insulin resistance [17,22,23]. Elevated levels of this adipocytokine are also associated with GDM [22,24–26], pre-eclampsia [11,22,27], and intrauterine growth restriction (IUGR) [28,29]. Leptin levels increase progressively during pregnancy, and this increase is related to body fat and to the regulation of placental growth, transfer of nutrients, angiogenesis, and trophoblastic invasion [30]. Elevated leptin serum levels have been associated with maternal BMI [17,25]. However, these relationships are complex and have yet to be fully clarified [22,25].
In this study, we evaluated changes in serum leptin levels during pregnancy in overweight/obese and normal-weight pregnant women. In addition, we evaluated the percent and total body weight gain as possible factors influencing the levels of this adipocytokine in obese and non-obese pregnant women.
Material and Methods
We conducted a prospective, longitudinal study in which included 42 pregnant women in low-risk prenatal care between September 2010 and June 2011 at the Teaching Hospital and Maternity in Juiz de Fora, Minas Gerais, Brazil.
In total, 72 pregnant women were included, but only 42 of them completed prenatal care. Thirty of them were excluded during the study for various reasons, including voluntary withdrawal from prenatal care in the institution (n=11), withdrawal of consent to participate in the study (n=3), referral to high-risk prenatal care (n=8), preterm delivery (n=4), and spontaneous abortion (n=4). Inclusion criteria were: age greater than or equal to 20 years, gestational age less than 12 weeks at the beginning of prenatal confirmed by ultrasonography, and singleton pregnancy. Exclusion criteria were: hypertension, type 1 or 2 diabetes, drug addiction, smoking, and chronic systemic diseases.
At the first prenatal visit, all participants read and signed the consent form according to Resolution 196/96 of the Brazilian National Health Council and the Helsinki Declaration of 1975, revised in 1983. The study was approved by the Ethics Committee of the Federal University of Juiz de Fora – UFJF, opinion nº 0240/2010. All data collection was conducted exclusively by the physician responsible for the study.
Apart from routine visits, the pregnant women underwent clinical and laboratory evaluations 3 times during pregnancy (gestational weeks 9–12, 25–28, and 34–37). Socio-demographic and clinical/obstetric characteristics were obtained by data collection form and included age, race, marital status, level of education, number of pregnancies, number of births, number of abortions, history of pre-eclampsia, GDM, fetal macrosomia, and any stillbirths from previous pregnancies. We also collected patients’ family histories of hypertension, pre-eclampsia, diabetes, dyslipidemia, and obesity.
Anthropometric evaluation
The anthropometric characteristics collected for the study group included the patients’ weights and heights. Weight was measured in kilograms (within 100 grams) using a Filizola calibrated electronic balance (Personal Line 200; Filizola S.A., São Paulo, Brazil). Height was measured in meters (to 2 decimal places). Pre-pregnancy weight was obtained by patient records, and was collected during the first prenatal visit. Pre-pregnancy BMI was calculated from the ratio of body mass (kg) to squared height (m) and classified into 2 groups – non-overweight (BMI <25 kg/m2) and overweight/obese (BMI ≥25kg/m2) – based on criteria from the World Health Organization [31]. Gestational ages were verified using the parameters collected from ultrasounds performed between weeks 11 and 13 of pregnancy. If necessary, the results were used to retrospectively correct the calculations made on the first visit, which were based on last menstrual period as determined by the patient. Total weight gain was calculated by subtracting the weight measured at the last prenatal visit before birth, performed between weeks 37 and 40, from the pre-pregnancy weight reported at the first visit. Based on the total weight gain and pre-pregnancy weight of the patient, we calculated the percent weight gain.
Leptin measurement
During each study visit for prenatal care, we obtained a maternal serum sample, which was preserved in triplicate in labeled 2-ml cryogenic tubes (CRAL, SP, Brazil) and stored in a Thermo Scientific Freezer (model 902; Thermo Electric Scientific, Winchester, VA, USA) at −80°C for subsequent analysis. Serum leptin levels (ng/ml) were analyzed with enzyme-linked immune sorbent assays (ELISA) using a human leptin ELISA kit (KAC2281; Invitrogen Corporation, Carlsbad, CA, USA) according to the manufacturer’s instructions. The detection limit of this assay is 3.5 pg/ml. The inter-assay coefficient of variation was 3.9% for 150.6 pg/ml and 5.3% for 240.7 pg/ml. The readings were taken using an ELISA microplate reader (Expert Plus; Asys Hitech, Eugendorf, Austria) at 450 nm. These measurements were performed by researchers in the Clinical Immunology and Immunopathology Laboratory at the Center for Reproductive Biology, Federal University of Juiz de Fora.
Statistical analyses
For the statistical analyses, pregnant women were divided into 2 groups according to their BMIs (<25 kg/m2 or ≥25 kg/m2). Next, linear regression analyses were performed using maternal weight vs. gestational age, and leptin levels vs. gestational age. To compare leptin levels across the 3 time periods defined in this study, a 1-sided ANOVA was performed on the 2 groups, which was followed by a post hoc Bonferroni test. Subsequently, we used the t test to compare absolute weight gain and percent weight gain between the 2 groups. All of the statistical analyses were performed using GraphPad Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). We adopted a level of significance of P<0.05.
Results
The socio-demographic and clinical/obstetric characteristics of the cohort are described in Table 1. We found that 95.2% (40/42) of the women were between 20 and 30 years of age, 66.7% (28/42) were nulliparous, 95.2% (40/42) had no history of pre-eclampsia during previous pregnancies, and 100% had no previous history of GDM, macrosomia, or stillbirths. As for family history (FH), 83.3% (35/42) did not have a FH of pre-eclampsia, 76.2% (32/42) did not have a FH of dyslipidemia, and 78.6% (33/42) did not have a FH of obesity.
Table 1.
A description of the socio-demographic and clinical/obstetric characteristics of the cohort study.
| Total pregnant women | BMI <25 Kg/m2 | BMI ≥25 kg/m2 | |
|---|---|---|---|
| N (%) | n (%) | n (%) | |
| Cohort size | 42 | 27 (64.2) | 15 (35.8) |
| Maternal age | |||
| 20–30 | 40 (95.2) | 25 (92.5) | 15 (100) |
| >30 | 2 (4.8) | 2 (7.5) | 0 (−) |
| Race | |||
| White | 25 (59.5) | 15 (55.5) | 10 (66.6) |
| Black | 7 (16.7) | 7 (25.9) | 0 (−) |
| Mixed | 10 (23.8) | 5 (18.6) | 5 (33.4) |
| Asian | 0 (−) | 0 (−) | 0 (−) |
| Marital status | |||
| Sigle | 20 (47.6) | 16 (59.2) | 4 (26.7) |
| Married | 14 (33.3) | 7 (25.9) | 7 (46.6) |
| Other | 8 (19.1) | 4 (14.9) | 4 (26.7) |
| Education level completed | |||
| None | 5 (11.9) | 4 (14.8) | 1 (6,7) |
| Fundamental | 19 (45.2) | 13 (48.2) | 6 (40) |
| Secundary | 17 (40.5) | 9 (33.3) | 8 (53.3) |
| Higher | 1 (2.4) | 1 (3.7) | 0 (−) |
| Pre-eclampsia previous pregnancy | |||
| Yes | 2 (4.8) | 1 (3.7) | 1 (6.7) |
| No | 40 (95.2) | 26 (96.3) | 14 (93.3) |
| GDM previous pregnancy | |||
| Yes | 0 (−) | 0 (−) | 0 (−) |
| No | 42 (100) | 27 (100) | 15 (100) |
| Macrosomia previous pregnancy | |||
| Yes | 0 (−) | 0 (−) | 0 (−) |
| No | 42 (100) | 27 (100) | 15 (100) |
| Stillbirth previous pregnancy | |||
| Yes | 0 (−) | 0 (−) | 0 (−) |
| No | 42 (100) | 27 (100) | 15 (100) |
| Family history hypertension | |||
| Yes | 19 (45.2) | 16 (59.3) | 13 (86.7) |
| No | 23 (54.8) | 11 (40.7) | 2 (13.3) |
| Family history pre-eclampsia | |||
| Yes | 7 (16.7) | 3 (11.1) | 4 (26.7) |
| No | 35 (83.3) | 24 (88.9) | 11 (73.3) |
| Family history diabetes | |||
| Yes | 24 (57.1) | 17 (62.9) | 7 (46.7) |
| No | 18 (42.9) | 10 (37.1) | 8 (53.3) |
| Family history dyslipidemia | |||
| Yes | 10 (23.8) | 5 (18.5) | 5 (33.4) |
| No | 32 (76.2) | 22 (81.5) | 10 (66.6) |
| Family history obesity | |||
| Yes | 9 (21.4) | 4 (14.8) | 5 (33.4) |
| No | 33 (78.6) | 23 (85.2) | 10 (66.6) |
| Parturitions | |||
| Nulliparous | 28 (66.7) | 19 (70.4) | 9 (60) |
| Multiparous | 14 (33.3) | 8 (29.6) | 6 (40) |
| Previous history of abortion | |||
| Yes | 6 (14.3) | 3 (11.1) | 3 (20) |
| No | 38 (85.7) | 24 (88.9) | 12 (80) |
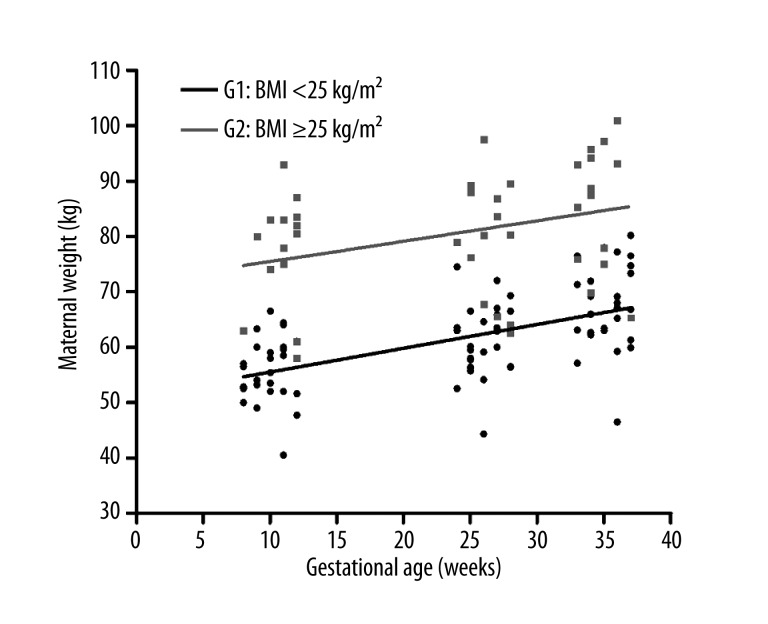
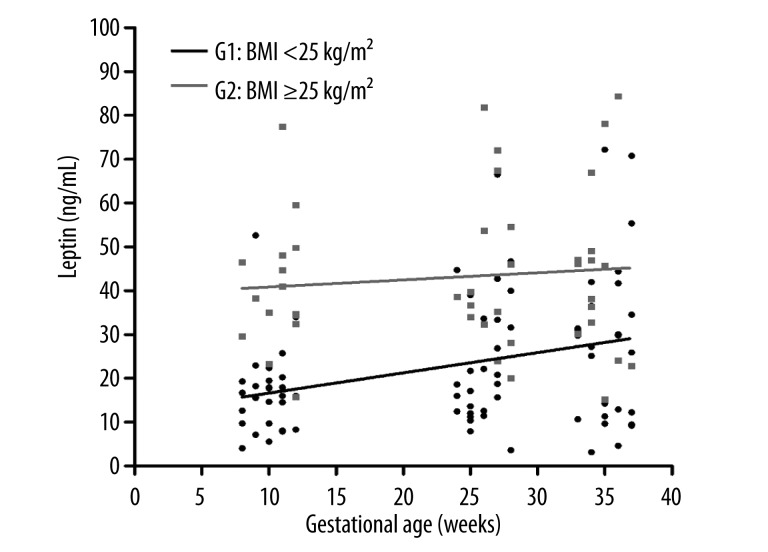
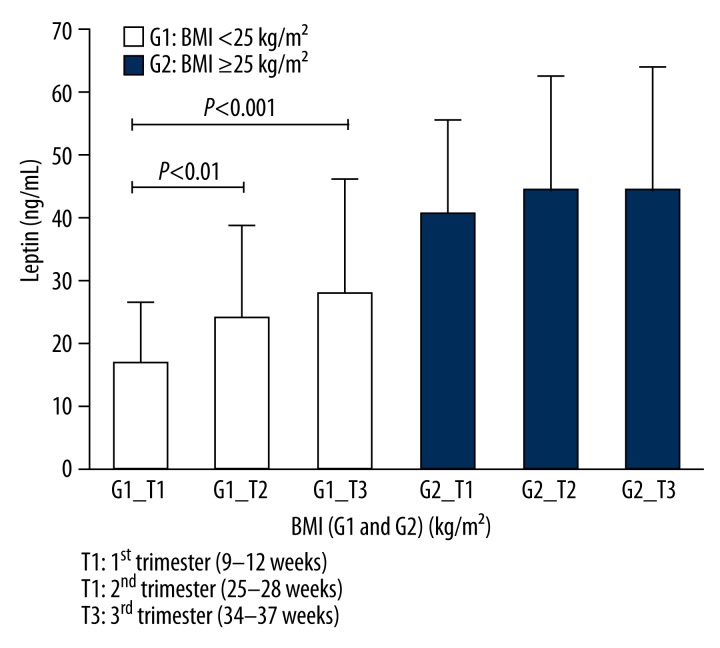
Figure 1 shows that, in the course of gestation, there was a progressive increase in maternal weight gain in both the non-overweight (BMI <25 kg/m2) and the overweight/obese (BMI ≥25 kg/m2) groups of pregnant women. There was a progressive increase in maternal serum levels of this adipocytokine in both groups. However, this increase was significantly greater in the non-overweight group (BMI <25 kg/m2) (Figure 2). Additionally, when the mean serum leptin levels were compared during the first (T1), second (T2), and third (T3) trimesters of pregnancy, we concluded that there were significant increases (T1 vs. T2, P<0.01 and T1 vs. T3, P<0.001) in leptin levels in Group 1 (non-overweight or G1), as seen in Figure 3. However, we did not observe a significant increase in leptin levels (P>0.05) during the 3 trimesters in Group 2 (overweight/obese or G2).
Figure 1.
Relationship between the increase in maternal weight and gestational age (weeks). Group 1: pre-gestational BMI <25 kg/m2 (non-overweight) is shown in black; Group 2: pre-gestational BMI ≥25 kg/m2 (overweight/obese) is shown in gray.
Figure 2.
Relationship between maternal serum leptin concentrations and gestational age (weeks). Group 1: pre-gestational BMI <25 kg/m2 (non-overweight) is shown in black; Group 2: pre-gestational BMI ≥25 kg/m2 (overweight/obese) is shown in gray.
Figure 3.
Maternal serum leptin concentrations (ng/ml) during the 1st, 2nd, and 3rd trimesters of gestation in the two patient groups characterized by pre-gestational BMI and gestational age (weeks). Group 1: BMI <25 kg/m2 (non-overweight) is shown in white; Group 2: pre-gestational BMI ≥25 kg/m2 (overweight/obese) is shown in gray.
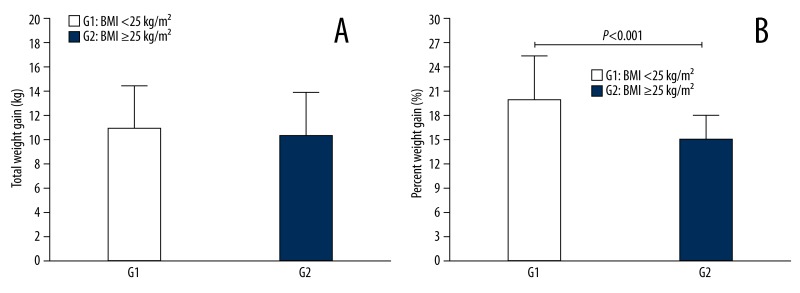
When we analyzed the total weight gain during pregnancy in the 2 groups, we found that G1 had a greater total weight gain than G2, although the difference between the 2 groups was not significant (P>0.05) (Figure 4A). However, when we compared the percent weight gain during pregnancy between G1 and G2, there was a significantly higher percent weight gain in G1 compared to G2 (P<0.001) (Figure 4B).
Figure 4.
Total and percent weight gain, according to BMI. (A) Total weight gain during gestation according to pre-gestational BMI for the two groups of pregnant women. Group 1 (G1) – non-overweight (BMI <25 kg/m2) and Group 2 (G2) – overweight/obese (BMI ≥25 kg/m2); (B) Percent weight gain during pregnancy according to G1 and G2.
Discussion
This study contributes to the body of knowledge available on the topic of evaluation of obesity in pregnancy and its association with serum leptin levels. Recently, obesity and leptin have been the subject of many studies [30–32], but there is still a significant gap in knowledge about the behavior of this hormone in women who were not overweight and those who were overweight/obese during pregnancy. From a methodological standpoint, this study provides adequate control of many intervening variables, such as the monitoring of all volunteers by a single physician, which minimizes the possible variations in data collection.
The changes in serum leptin levels during pregnancy have been well established through many studies for nearly 2 decades [32–34]. However, there are still many gaps in knowledge about the levels of this adipocytokine in pregnant women in relation to BMI and gestational weight gain.
We found that serum leptin levels in non-overweight (BMI <25 kg/m2) and overweight/obese (BMI ≥25 kg/m2) pregnant women increased progressively throughout pregnancy and the mean leptin concentrations were significantly higher in overweight/obese compared to normal-weight pregnant women. Similar findings were also reported by some recent studies that directly related leptin levels with groups of obese and non-obese pregnant women [25,35–38].
Our results also suggest that the leptin levels in non-overweight women were significantly higher (P<0.001) during pregnancy compared to overweight/obese women (Figures 2 and 3). This seems paradoxical, considering that probably the largest increase in leptin should have been detected in patients with overweight/obesity. Similar data were also observed by Misra [25] in the analysis of the serum leptin levels in non-overweight and in overweight/obese pregnant women. The authors concluded that the overweight/obese group had significantly higher serum leptin concentrations than the non-overweight group during pregnancy (P<0.01) and, although these concentrations increased significantly across gestation for both groups, the rate of increase was significantly smaller in overweight/obese women (P<0.05). They also concluded that factors other than fat mass alone can influence leptin concentrations in overweight/obese women compared to non-overweight women during pregnancy. This study suggests a hypothesis to explain this increase in leptin levels in the non-overweight group.
Analyzing the total weight gain and percent weight gain in both groups of pregnant women in our cohort, the results suggest that G1 had a greater total weight gain than G2, although the difference between the 2 groups was not significant (P>0.05) (Figure 4A). Furthermore, when we analyzed both groups for their percent weight gain during pregnancy compared to their pre-pregnancy weight, the non-overweight patients had a significantly greater percent weight gain (P<0.001) compared to the overweight/obese women (Figure 4B). Our finding suggests that the adipose tissue in women with a BMI <25 kg/m2 exhibits a higher percent increase during pregnancy compared to women with a BMI ≥25 kg/m2. This increase in adipose tissue can directly influence the production of adipocytokines and other inflammatory mediators by adipocytes. These findings suggest a hypothesis to explain the increase in leptin levels in non-overweight group
A limitation of this study was that the pre-pregnancy BMI was calculated using the pregnant woman’s self-reported weight at the first prenatal visit, and this information was subjective and prone to error. Another limitation is the small sample size, which may reduce the external validity of the research.
Conclusions
In summary, our results indicate that the significantly greater increase in serum leptin levels in non-overweight pregnant women may be explained by the significantly higher percent weight gain in this group compared to overweight/obese women. This study suggests that controlling both weight gain and the percent weight gain during the prenatal period may be an important preventive measure to control leptin levels during pregnancy and its subsequent medical complications.
Footnotes
Conflict of interest
None of the authors have personal financial interests related to this research.
Source of support: Departmental funds
References
- 1.WHO. World Health Organization. The WHO Global InfoBase. Prevalence of overweight & obesity map 2010. Sep 15, 2011. Available from: https://apps.who.int/infobase/Index.aspx.
- 2.Mandal D, Manda S, Rakshi A, et al. Maternal obesity and pregnancy outcome: a prospective analysis. J Assoc Physicians India. 2011;59:486–89. [PubMed] [Google Scholar]
- 3.Hurt RT, Frazier TH, McClave SA, Kaplan LM. Obesity epidemic: overview, pathophysiology, and the intensive care unit conundrum. JPEN J Parenter Enteral Nutr. 2011;35(5 Suppl):4S–13S. doi: 10.1177/0148607111415110. [DOI] [PubMed] [Google Scholar]
- 4.Kadakia MB, Fox CS, Scirica BM, et al. Central obesity and cardiovascular outcomes in patients with acute coronary syndrome: observations from the MERLIN-TIMI 36 trial. Heart. 2011;97(21):1782–87. doi: 10.1136/heartjnl-2011-300231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salazar MR, Carbajal HA, Espeche WG, et al. Relationships among insulin resistance, obesity, diagnosis of the metabolic syndrome and cardio-metabolic risk. Diab Vasc Dis Res. 2011;8(2):109–16. doi: 10.1177/1479164111403170. [DOI] [PubMed] [Google Scholar]
- 6.Murphy HR, Steel SA, Roland JM, et al. Obstetric and perinatal outcomes in pregnancies complicated by Type 1 and Type 2 diabetes: influences of glycaemic control, obesity and social disadvantage. Diabet Med. 2011;28(9):1060–67. doi: 10.1111/j.1464-5491.2011.03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagaria SJ, Bagaria VB. Strategies for Diagnosis and Prevention of Venous Thromboembolism during Pregnancy. J Pregnancy. 2011;2011:206858. doi: 10.1155/2011/206858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blomberg M. Maternal obesity and risk of postpartum hemorrhage. Obstet Gynecol. 2011;118(3):561–68. doi: 10.1097/AOG.0b013e31822a6c59. [DOI] [PubMed] [Google Scholar]
- 9.Davies GA, Maxwell C, McLeod L, et al. Obesity in pregnancy. J Obstet Gynaecol Can. 2010;32(2):165–73. doi: 10.1016/S1701-2163(16)34432-2. [DOI] [PubMed] [Google Scholar]
- 10.Einerson BD, Huffman JK, Istwan NB, et al. New gestational weight gain guidelines: an observational study of pregnancy outcomes in obese women. Obesity (Silver Spring) 2011;19(12):2361–64. doi: 10.1038/oby.2011.67. [DOI] [PubMed] [Google Scholar]
- 11.Mori T, Shinohara K, Wakatsuki A, et al. Adipocytokines and endothelial function in preeclamptic women. Hypertens Res. 2010;33(3):250–54. doi: 10.1038/hr.2009.222. [DOI] [PubMed] [Google Scholar]
- 12.Norman JE, Reynolds RM. The consequences of obesity and excess weight gain in pregnancy. Proc Nutr Soc. 2011;70(4):450–56. doi: 10.1017/S0029665111003077. [DOI] [PubMed] [Google Scholar]
- 13.Rasmussen SA, Chu SY, Kim SY, et al. Maternal obesity and risk of neural tube defects: a metaanalysis. Am J Obstet Gynecol. 2008;198(6):611–19. doi: 10.1016/j.ajog.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 14.Rytlewski K, Huras H, Kusmierska-Urban K, et al. Leptin and interferon-gamma as possible predictors of cesarean section among women with hypertensive disorders of pregnancy. Med Sci Monit. 2012;18(8):CR506–11. doi: 10.12659/MSM.883271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reardon DC, Coleman PK. Short and long term mortality rates associated with first pregnancy outcome: population register based study for Denmark 1980–2004. Med Sci Monit. 2012;18(9):PH71–76. doi: 10.12659/MSM.883338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flegal KM, Carroll MD, Kit BK, Ogden CL. Prevalence of obesity and trends in the distribution of body mass index among US adults, 1999–2010. JAMA. 2012;307(5):491–97. doi: 10.1001/jama.2012.39. [DOI] [PubMed] [Google Scholar]
- 17.Zavalza-Gomez AB, Anaya-Prado R, Rincon-Sanchez AR, Mora-Martinez JM. Adipokines and insulin resistance during pregnancy. Diabetes Res Clin Pract. 2008;80(1):8–15. doi: 10.1016/j.diabres.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 18.Castellano Filho DS, Aarestrup FM. Obesity, Adipocytokines and Pregnancy: An Update of the Literature. Interdisciplinary Journal of Experimental Studies. 2009;1(2):62–68. [Google Scholar]
- 19.Maghbooli Z, Hossein-Nezhad A, Rahmani M, et al. Relationship between leptin concentration and insulin resistance. Horm Metab Res. 2007;39(12):903–7. doi: 10.1055/s-2007-992812. [DOI] [PubMed] [Google Scholar]
- 20.Silveira MR, Frollini AB, Verlengia R, Cavaglieri CR. Correlation Between Obesity, Adipokines and the Imune System. Rev Bras Cineantropon Desempenho Hum. 2009;11:466–72. [Google Scholar]
- 21.Wauters M, Considine RV, Van Gaal LF. Human leptin: from an adipocyte hormone to an endocrine mediator. Eur J Endocrinol. 2000;143(3):293–311. doi: 10.1530/eje.0.1430293. [DOI] [PubMed] [Google Scholar]
- 22.Briana DD, Malamitsi-Puchner A. Reviews: adipocytokines in normal and complicated pregnancies. Reprod Sci. 2009;16(10):921–37. doi: 10.1177/1933719109336614. [DOI] [PubMed] [Google Scholar]
- 23.Eriksson B, Lof M, Olausson H, Forsum E. Body fat, insulin resistance, energy expenditure and serum concentrations of leptin, adiponectin and resistin before, during and after pregnancy in healthy Swedish women. Br J Nutr. 2010;103(1):50–57. doi: 10.1017/S0007114509991371. [DOI] [PubMed] [Google Scholar]
- 24.Gao XL, Yang HX, Zhao Y. Variations of tumor necrosis factor-alpha, leptin and adiponectin in mid-trimester of gestational diabetes mellitus. Chin Med J (Engl) 2008;121(8):701–5. [PubMed] [Google Scholar]
- 25.Misra VK, Trudeau S. The influence of overweight and obesity on longitudinal trends in maternal serum leptin levels during pregnancy. Obesity (Silver Spring) 2011;19(2):416–21. doi: 10.1038/oby.2010.172. [DOI] [PubMed] [Google Scholar]
- 26.Soheilykhah S, Mojibian M, Rahimi-Saghand S, et al. Maternal serum leptin concentration in gestational diabetes. Taiwan J Obstet Gynecol. 2011;50(2):149–53. doi: 10.1016/j.tjog.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 27.Montagnana M, Lippi G, Albiero A, et al. Serum pro-inflammatory cytokines in physiological and pre-eclamptic pregnancies. Gynecol Endocrinol. 2008;24(3):113–16. doi: 10.1080/09513590801895575. [DOI] [PubMed] [Google Scholar]
- 28.Briana DD, Malamitsi-Puchner A. Intrauterine growth restriction and adult disease: the role of adipocytokines. Eur J Endocrinol. 2009;160(3):337–47. doi: 10.1530/EJE-08-0621. [DOI] [PubMed] [Google Scholar]
- 29.Kyriakakou M, Malamitsi-Puchner A, Militsi H, et al. Leptin and adiponectin concentrations in intrauterine growth restricted and appropriate for gestational age fetuses, neonates, and their mothers. Eur J Endocrinol. 2008;158(3):343–48. doi: 10.1530/EJE-07-0692. [DOI] [PubMed] [Google Scholar]
- 30.Hauguel-de Mouzon S, Lepercq J, Catalano P. The known and unknown of leptin in pregnancy. Am J Obstet Gynecol. 2006;194(6):1537–45. doi: 10.1016/j.ajog.2005.06.064. [DOI] [PubMed] [Google Scholar]
- 31.WHO. World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:I–xii. 1–253. [PubMed] [Google Scholar]
- 32.Hardie L, Trayhurn P, Abramovich D, Fowler P. Circulating leptin in women: a longitudinal study in the menstrual cycle and during pregnancy. Clin Endocrinol (Oxf) 1997;47(1):101–6. doi: 10.1046/j.1365-2265.1997.2441017.x. [DOI] [PubMed] [Google Scholar]
- 33.Lage M, Garcia-Mayor RV, Tome MA, et al. Serum leptin levels in women throughout pregnancy and the postpartum period and in women suffering spontaneous abortion. Clin Endocrinol (Oxf) 1999;50(2):211–16. doi: 10.1046/j.1365-2265.1999.00637.x. [DOI] [PubMed] [Google Scholar]
- 34.Schubring C, Englaro P, Siebler T, et al. Longitudinal analysis of maternal serum leptin levels during pregnancy, at birth and up to six weeks after birth: relation to body mass index, skinfolds, sex steroids and umbilical cord blood leptin levels. Horm Res. 1998;50(5):276–83. doi: 10.1159/000023290. [DOI] [PubMed] [Google Scholar]
- 35.Misra VK, Straughen JK, Trudeau S. Maternal serum leptin during pregnancy and infant birth weight: The influence of maternal overweight and obesity. Obesity (Silver Spring) 2013;21(5):1064–69. doi: 10.1002/oby.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Misra VK, Trudeau S, Perni U. Maternal serum lipids during pregnancy and infant birth weight: the influence of prepregnancy BMI. Obesity (Silver Spring) 2011;19(7):1476–81. doi: 10.1038/oby.2011.43. [DOI] [PubMed] [Google Scholar]
- 37.Ostadrahimi A, Moradi T, Zarghami N, Shoja MM. Correlates of serum leptin and insulin-like growth factor-I concentrations in normal weight and overweight/obese Iranian women. J Womens Health (Larchmt) 2008;17(8):1389–97. doi: 10.1089/jwh.2007.0736. [DOI] [PubMed] [Google Scholar]
- 38.Straughen JK, Misra DP, Kumar P, Misra VK. The influence of overweight and obesity on maternal soluble fms-like tyrosine kinase 1 and its relationship with leptin during pregnancy. Reprod Sci. 2013;20(3):269–75. doi: 10.1177/1933719112452472. [DOI] [PMC free article] [PubMed] [Google Scholar]