Abstract
C-type lysozyme genes (Lyzls) belong to the class of lysozymes and are highly expressed in the testis and epididymis. The members Lyzl4 and Spaca3 have been reported to play a role in sperm–egg binding and fertilisation in mice. However, the function of the remaining two mouse c-type lysozyme genes, Lyzl1 and Lyzl6, is still not clear. In the present study, we analysed the tissue expression and androgen-dependent expression of mouse c-type lysozyme genes and the possible contribution of human recombinant LYZL6 (rLYZL6) to immunity. The expression of Lyzls was detected by RT-PCR, Western blots, immunohistochemistry and immunofluorescence. The bacteriolytic activity of rLYZL6 was analysed by a colony-forming assay. In mice, the expression of Lyzl genes was mainly in the testis and epididymis in a developmentally regulated manner and androgen- or testicular factor-regulated manner. Immunodetection revealed the presence of LYZL6 protein in primary spermatocytes and round spermatids of the testis and on the post-acrosomal area and midpiece of mature epididymal spermatozoa. The rLYZL6 protein exhibited antibacterial activity. From the results, Lyzls may play a role in mitochondrial function of spermatozoa and LYZL6 may contribute to the innate immunity of the male genital tract.
Keywords: antibacterial properties, c-type lysozyme genes (Lyzls), expression characterisation, human LYZL6, testosterone
INTRODUCTION
Lysozyme, a bactericidal agent, was first discovered by Alexander Fleming in the 1920s.1 A group of lysosomal proteins with bacteriolytic activity has been found in mammals.2,3 From their amino-acid sequence and biological origin, lysozymes are divided into six families: chicken-type (c-type), goose-type (g-type), invertebrate-type (i-type), phage, bacterial and plant lysozymes.3 The c-type lysozyme genes LYZL2, LYZL4, LYZL6 and SPACA3 are all highly expressed in the testis and epididymis.4
Lysozymes (muramidase), as a class of enzymes, lyse the cell walls of certain Gram-positive bacteria by catalyzing the hydrolysis of the beta-1,4-glycosidic linkage between N-acetylmuramic acid and N-acetylglucosamine in cell wall peptidoglycan.2 The powerful antibacterial activity of lysozyme has been confirmed by Prager,5 in addition to the demonstration that lysozymes not only protect hosts against infection from invading microorganisms, but also play a role in releasing nutrients from some bacteria in the digestive tract.
In the human male reproductive system, four c-type lysozyme genes (LYZL2, SPACA3, LYZL4 and LYZL6) have been identified4,6 and are all highly expressed in the testis or epididymis. Mandal et al.6 showed that antisera to a sperm lysozyme-like protein (SLLP1), encoded by the SPACA3 gene, block sperm–egg binding in the hamster-oocyte penetration assay, indicating a possible role of SLLP1 in sperm–oocyte binding or penetration. There are four c-type lysozymes (Lyzl1, Lyzl3/Spaca3, Lyzl4 and Lyzl6) in mice; a fertilisation role has been shown for Lyzl4 and Spaca3 in mice,7,8 but the function of the remaining two mouse c-type lysozyme genes is not clear.
In the present study, we analysed the tissue expression and androgen-dependent expression of mouse c-type lysozyme genes and the possible contribution of human recombinant LYZL6 (rLYZL6) to immunity.
MATERIALS AND METHODS
Animals and sample preparation
C57BL/6J mice (7-week-old, male) were obtained from the Animal Research Center of the LuYe Pharmaceutical Company (Yantai, China) and housed in a pathogen-free facility as well as maintained in a controlled environment (21±2 °C, 12-h light–dark periods). The testis, epididymis, seminal vesicle, vas deferens, kidney, brain, heart, liver, spleen, stomach, lung, muscle, bladder, jejunum and adrenal were extracted for total RNA and protein. For analysis of the expression of c-lysozyme genes in the reproductive system of different ages, the animals (number) were killed on postnatal days 14 (5), 21 (5), 28 (3), 35 (3), 42 (3), 49 (3), 70 (3) and 90 (3) days. The time points chosen coincide with major developmental events.9 All animal experimental procedures were approved by the Ethics Committee of Shandong Research Center of Stem Cell Engineering (Yantai, China).
RNA isolation and semiquantitative RT-PCR
Total RNA was extracted by using RNAiso Plus reagent (TaKaRa, Dalian, China) according to the manufacturer's instructions. The concentration of RNA was determined by spectrophotometry (Nanodrop 2000/2000C; Thermo Scientific, Wilmington, MA, USA). Total RNA (1 µg) was reverse-transcribed with 2U AMV Reverse Transcriptase (Promega, Madison, WI, USA). Gene expression was detected by PCR with gene-specific primers (Table 1) in 20 µl reaction mixture containing 2 µl 10×PCR buffer (with MgCl2), 2 µl dNTP mix (10 mmol l−1), 0.5 µl of each primer (10 mmol l−1), 1 µl Taq DNA polymerase (2.5 U ml−1; TaKaRa), 12 µl deionized water and 2 µl cDNA template. Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) expression was used as the internal control. PCR was performed under the following conditions: a 10-min initial denaturation at 94 °C, followed by an appropriate number of cycles (number of cycles are shown in Table 1) of denaturing cycles at 94 °C for 30 s, annealing (annealing temperatures are shown in Table 1) for 30 s and extension at 72 °C for 30 s, and the final extension step at 72 °C for 10 min. Deionized water instead of cDNA templates was used for the negative controls for all the PCR reactions. All of the PCR products were analysed by electrophoresis in a 1.5% (w/v) agarose gel, the absorbance of each band was analysed with Gene Tools (product version: 4.02; Syngene, San Diego, CA, USA). The results were expressed as the quantity of target relative to that of Gapdh.
Table 1. Gene-specific primers used in this study.
| Gene | Direction | 5′–3′ | Tm (°C)a | Cycles |
|---|---|---|---|---|
| Lyzl1 | ForwardReverse | GCATAGTCGCAGAATCCACAGCCCTGCCAATAGTTC | 51 | 28 |
| Lyzl3 | ForwardReverse | GGCCAAGGTCTTCAGTCGACAGCCATCCACCCAGTC | 54 | 28 |
| Lyzl4 | ForwardReverse | ACCAGAGCTTGGCGACGATGTGGCCGCACCATTCATT | 53 | 26 |
| Lyzl6 | ForwardReverse | ATCCATCGCTGTAGTTTGTGAAATGAGGTTGGGTCT | 47 | 28 |
| Gapdh | ForwardReverse | TCAACGGCACAGTCAAGGACCAGTGGATGCAGGGAT | 56 | 26 |
Abbreviation: Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Annealing temperature.
Castration and testosterone supplementation
Twenty-four C57BL/6J mice were bilaterally orchiectomized through the scrotum under ketamine anesthesia (220 mg kg−1 body weight, i.p.) on an aseptic operating table. The sham-operated mice, whose scrota received the same incision but were then sutured, served as the control group. Mice were divided into eight groups (three mice per group) and killed on days 1, 3, 5 and 7 after castration as well as 1, 3, 5 and 7 days after the initial testosterone propionate injection. Testosterone propionate in tea seed oil (5 mg kg−1 body weight, i.m.; Shanghai General Pharmaceutical Co., Shanghai, China) was injected once on the seventh day after castration. The epididymides were excised and RNA was extracted. Pooled serum samples from the carotid artery from each group were measured for testosterone concentration by RIA as described previously.10
Western blot analysis
Tissue proteins were extracted with RIPA buffer (20 mmol l−1 Tris-HCl, 150 mmol l−1 NaCl, 5 mmol l−1 EDTA, 1% (v/v) NP-40, 5 mmol l−1 NaPPi, 1 mmol l−1 Na3VO4 and 1 mmol l−1 PMSF). The protein concentration was determined with the Bradford assay (Bio-Rad, Hercules, CA, USA).11 Protein of 30 µg per lane was loaded and separated on 12% (w/v) sodium dodecyl sulphate–polyacrylamide gels and transferred onto polyvinyl difluoride membranes by applying 200 mA for 1 h. The membranes were blocked with 5% (w/v) fat-free milk for 1 h at room temperature, and then incubated overnight at 4 °C with the primary antibody. After being washed with TBST (TBS with 0.05% (v/v) Tween-20), membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (ZB-2306: ZhongShan Biotechnology, Beijing, China) for 1 h at room temperature, followed by three washes with TBST. The peroxidase activity was visualized with DAB reagent (ZL-9018, ZhongShan Biotechnology). The primary antibodies used were LYZL6 (ARP-53746_P050, 1∶1000 dilution in phosphate-buffered saline (PBS); Aviva Biotechnology, Beijing, China) and Gapdh (sc-25778, 1∶2000 dilution in PBS; Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Immunolocalisation of LYZL6 in the testis and epididymis
Testicular and epididymal tissues for immunohistochemistry were immersed for 12 h in Bouin's solution immediately after removal from the body. The tissues were embedded in paraffin and 4-µm sections were deparaffinized in dimethylbenzene and rehydrated in a graded ethanol series. Antigens were unmasked by incubating sections in boiling 0.01 M sodium citrate buffer (pH 6.0) in a microwave oven. Sections were exposed to 3% (v/v) hydrogen peroxide for 10 min to inhibit endogenous peroxidase activity. After incubation in blocking buffer (PBS with 5% (w/v) non-fat powdered milk) for 1 h at room temperature, the slides were incubated with the anti-LYZL6 antibody (1∶80 dilution in blocking buffer) at 4 °C overnight in a humid chamber. Horseradish peroxidase-conjugated goat anti-rabbit IgG (1∶200 diluted in TBS) was applied to the sections for 1 h at 37 °C and the retained reaction product was detected with DAB reagent (ZL-9018, ZhongShan Biotechnology). After being counterstained with haematoxylin, the sections were examined with bright-field microscopy (DM LB2; Leica, Nussloch, Germany). The staining specificity was checked by incubation of the section with the respective host IgG.
Indirect immunofluorescence
Spermatozoa from the testis, caput, corpus, cauda epididymidis and vas deferens were separated by puncturing the ducts from each mouse and the first exuded drop was placed in tissue culture wells and incubated at 37 °C for 30 min with 5% (v/v) CO2 in mouse tubule fluid culture medium (90126; Irvine Scientific, Santa Ana, CA,USA). The spermatozoa were collected and washed with PBS, placed on 1% (w/v) gelatin-coated slides, air-dried and then fixed with cold methanol for 5 min. The slides were then blocked for 1 h at room temperature with 3% (w/v) bovine serum albumin) in PBS, and incubated overnight at 4 °C with rabbit anti-LYZL6 polyclonal antibody (diluted 1∶60 in PBS containing 3% bovine serum albumin). After three washes with PBST, the FITC-conjugated goat anti-rabbit IgG (1∶200 dilution in PBS containing 3% bovine serum albumin) was applied for 1 h at room temperature. The slides, which were washed three times with PBS, were counterstained by propidium iodide (0.01 mg ml−1; Invitrogen, Carlsbad, CA, USA) and mounted in 80% (w/v) glycerol after washing three times with PBS, and examined with a Meta 510 laser scanning microscope (Carl Zeiss, Jena, Germany).
Preparation and purification of rLYZL6
The human LYZL6 gene was amplified by PCR from the human epididymal cDNA library12 with the specific primers (F: 5′-ctaggatccctcatcagtcgctgtgactt-3′ R: 5′-cacctcgagtcatctcaggcggcatc-3′). The open reading frame was cloned into the pGEX-4T-1 expression vectors (TaKaRa) at the BamHI and XhoI sites. The resulting plasmid encoded a recombinant fusion protein (glutathione S-transferase (GST)-LYZL6) that contained the LYZL6 peptide from amino acids 20 to 148. The resulting plasmid of pGEX-4T-1/LYZL6 was transformed in Escherichia coli (E. coli) BL21 (DE3) strain according to the supplier's instructions. The transformed E. coli were grown to mid-log phase (OD600: 0.4–0.5) and the fusion protein was induced with 0.4 mM isopropyl-1-thio-𝒹-galactoside (Sigma, St Louis, MO, USA) for 5 h at 30 °C. Fractions were analysed on 15% (w/v) gradient polyacrylamide Tris-Tricine gels and stained with Coomassie blue G250. All of the experiments were performed according to standard procedures.13 The total protein was extracted after the cells were harvested by sonication. Then rLYZL6 was purified with Glutathione Sepharose 4B column according to the instructions of the manufacturer (17-0756-01; Amersham, Uppsala, Sweden).
Assay of bacteriolytic activity of rLYZL6
A colony-forming unit (CFU) assay was employed to test the antibacterial activity of rLYZL6 protein as described previously.14 Briefly, overnight cultures of Staphylococcus aureus (S. au ATCC 25923) were grown to mid-log phase (A600: 0.4–0.5) and diluted with 10 mM PBS (pH 7.4). Bacteria (approximately 2×106 CFU ml−1) were incubated at 37 °C with 12.5–100 µg ml−1 rLYZL6, and samples of the assay mixture were incubated for 15, 30, 60 and 120 min. At each time point, the samples were serially diluted with 10 mM PBS (pH 7.4) and 100 µl of each was spread on an LB agar plate and incubated at 37 °C overnight to allow colonies to form. The colonies were counted, and the antibacterial activity was expressed as the percentage of colonies surviving (survival (%)=(number of colonies after treatment with the antibacterial peptide/number of colonies surviving without the antibacterial peptide)×100).
RESULTS
Expression of c-type lysozyme genes in different tissues
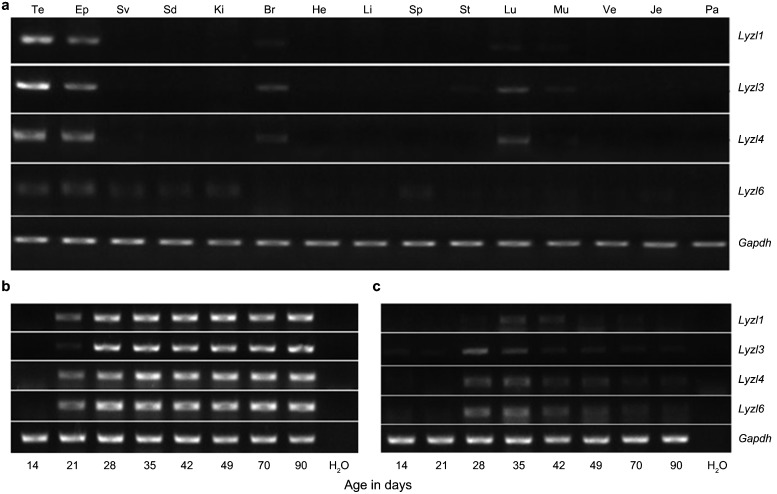
All Lyzls were expressed at a higher level in the testis and epididymis than in other tissues. There was a moderate expression of Lyzl1, Lyzl3 and Lyzl4 in brain and lung, and Lyzl6 was expressed slightly in the seminal vesicle, vas deferens, kidney and spleen (Figure 1a).
Figure 1.
Expression of Lyzls in mice. Tissue distribution of Lyzl1, 3, 4 and 6 in adult mice. (a) RT-PCR analysis was performed on total RNA isolated from Te (testis), Ep (epididymis), Sv (seminal vesicle), Vd (vas deferens), Ki (kidney), Br (brain), He (heart), Li (liver), Sp (spleen), St (stomach), Lu (lung), Mu (muscle), Bl (bladder), Je (jejunum) and Ad (adrenal). Developmental expression of Lyzls transcripts in the testis (b) and epididymis (c) of mice with postnatal age. RT-PCR for Lyzl1, 3, 4 and Lyzl6 was performed using RNA isolated from the testis and epididymis of 14- to 90-day-old mice (abscissa). Gapdh served as internal control. Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Postnatal expression of c-type lysozyme genes in the testis and epididymis
To elucidate the association of postnatal development and gene expression, Lyzls were analysed by RT-PCR of total RNA isolated from the testis and epididymis of 14- to 90-day-old mice. In the testis, all Lyzls were expressed from day 21 during postnatal development (Figure 1b). In the epididymis, a tenuous expression of Lyzl1 existed at day 28, and the highest expression was at day 35, then the expression decreased gradually. Lyzl3 was slightly expressed on the fourteenth and twenty-first post-natal days, and the expression sharply increased by the twenty-eighth day, then the expression decreased gradually. The expression of Lyzl4 and Lyzl6 showed the same tendency. On the twenty-eighth day, the expression of Lyzl4 and Lyzl6 were first detected, and high expression was maintained to the thirty-fifth day; from the forty-second to ninetieth days, the expression decreased gradually (Figure 1c).
Expression of c-type lysozyme genes in the epididymis of castrates with or without androgen supplementation
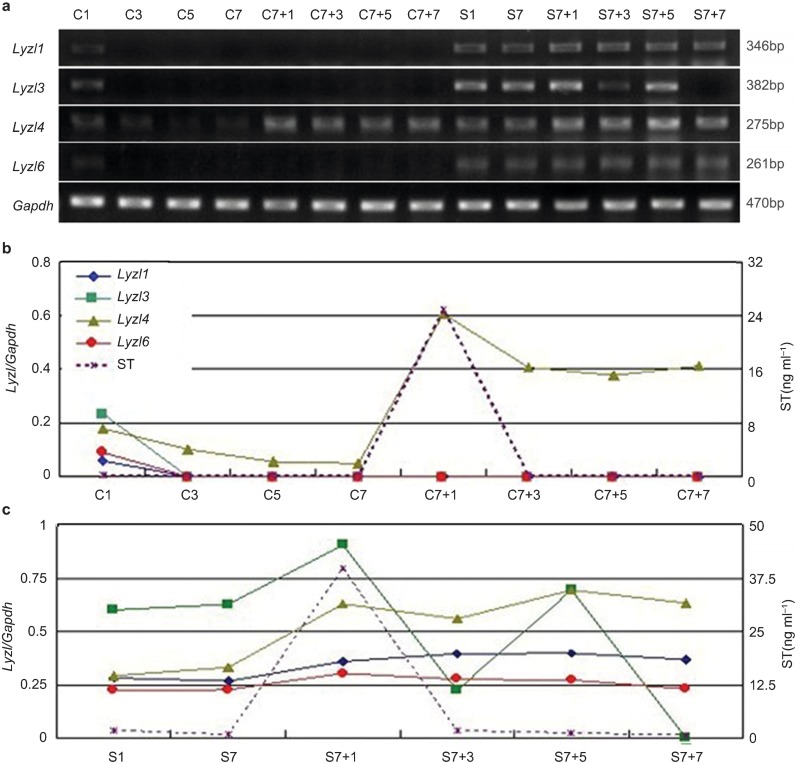
In the castrated animals, the serum testosterone level declined rapidly and was almost undetectable on postoperative day 3 (C3; Figure 2b). In parallel, a marked decrease was found in Lyzls 1,3,6 mRNA after surgery but a slower decline in Lyzl4 (Figure 2a). A sharp increase in transcriptional expression of Lyzl4 was detected one day after a single injection of testosterone propionate to the 7d-castrated mice (C7+1) and it then remained stable, whereas Lyzl1, Lyzl3 and Lyzl6 were undetectable at any time after androgen supplementation. It is noteworthy that Lyzl3 expression displayed dramatic fluctuation after testosterone supplementation in the sham-operated animals. At the last observation point (C7+7), Lyzl3 was undetectable (Figure 2c).
Figure 2.
Expression of Lyzls in mouse epididymides following castration and subsequent testosterone manipulation. (a) RT-PCR detection of adult mouse epididymal RNA from males bilaterally castrated for 1, 3, 5 and 7 days (C1, C3, C5, C7), sham-operated males for 1 and 7 days (S1, S7) and 1, 3, 5 and 7 days after a single injection of testosterone propionate applied to 7-day castrated mice (C7+1, C7+3, C7+5 and C7+7) and sham-operated mice (S7+1, S7+3, S7+7 and S7+7). Total RNA was pooled from three animals at each time-point in castrated animals (b) and shams (c). The relative expression of Lyzls mRNA (expression of Lyzls mRNA/Gapdh mRNA) (in different colours and symbols) in the epididymides was compared with the serum testosterone level during androgen manipulation (n=3). Gapdh, glyceraldehyde-3-phosphate dehydrogenase.
Expression of Lyzl6 in the testis and epididymis
RT-PCR revealed that the expression of Lyzl6 was relatively strong in the testis and cauda epididymidis, but moderate in the corpus and caput (Figure 3a). Western blots showed a good specificity of LYZL6 antibody (17 kDa) (Figure 3b). Immunohistochemistry revealed that LYZL6 was highly expressed in primary spermatocytes and round spermatids. Along the epididymal ducts, from the initial segment to the cauda, the expression of LYZL6 increased, and the positive staining was expressed in the principal and basal cells (Figure 3c).
Figure 3.
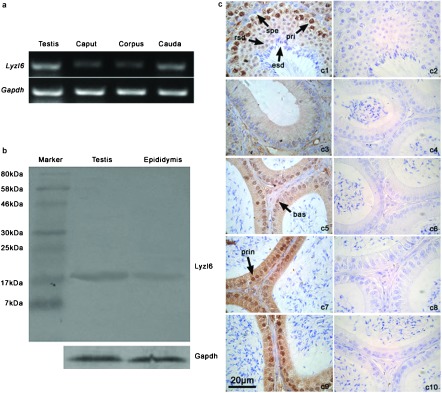
Expression of Lyzl6 gene and LYZL6 protein in the mouse testis and epididymis. (a) Total RNA isolated from the adult testis, caput, corpus and cauda was amplified by RT-PCR. (b) Western blot analysis of LYZL6 in the adult testis and epididymis. (c) Immunohistochemical localisation of LYZL6 in the adult mouse testis (c1), initial segment (c3), caput (c5), corpus (c7) and cauda (c9); c2, c4, c6, c8 and c10 are the corresponding negative controls. LYZL6 was mainly localized in the nuclei of primary spermatocytes (pri), round spermatids (rsd). The spermatogonia (spe) and elongated spermatids (esd) were all negative for LYZL6 staining. Basal (bas) and principal (prin) cells were positive in the epididymal sections. Scale bar=20 µm.
Immunofluorescence of LYZL6 on spermatozoa
Indirect immunofluorescence demonstrated that LYZL6 was located on the post-acrosomal area and the midpiece of mature spermatozoa (Figure 4d and 4e). The positive signal did not appear on the spermatozoa obtained from the testis, caput or corpus epididymidis (Figure 4a–4c).
Figure 4.
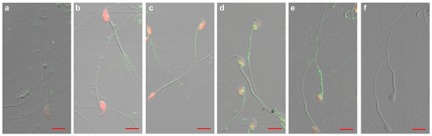
Immunofluorescence of LYZL6 on spermatozoa. Testicular spermatozoa (a); spermatozoa from the caput (b), corpus (c) and cauda (d) epididymidis, and vas deferens (e); phase contrast view of spermatozoa (f). Scale bars=10 µm.
Preparation of rLYZL6 protein
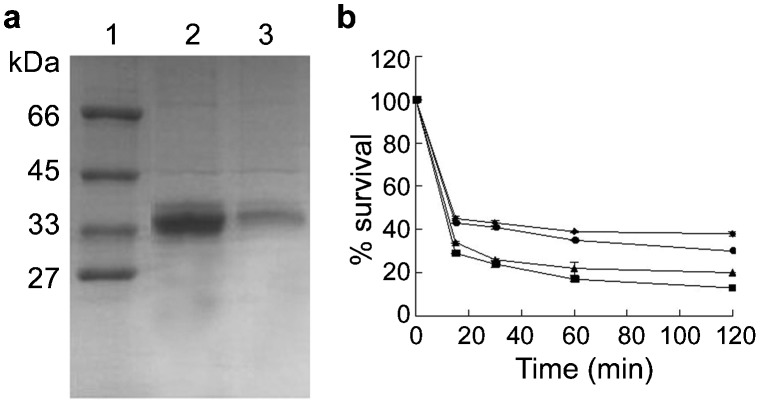
The cDNA fragment encoding LYZL6 between amino acids 20 and 148 fused with a GST tag was expressed in E. coli. A 39-kDa band (GST-LYZL6) was induced and existed in the supernatant of the bacterial lysate. As shown in Figure 5a, a prominent GST-LYZL6 band was observed after purification.
Figure 5.
(a) Purification of GST-LYZL6. Induction and purification of GST-LYZL6 in E. coli shown by Coomassie blue staining. Lane 1, molecular weight markers; lane 2, total protein of E. coli induced for 4 h; lane 3, total protein of E. coli induced 4 h with half concentration of the protein in lane 2. (b) Antibacterial activity of rLYZL6 by incubation of S.au ATCC25923 for 15–120 min at 37 °C with 12.5 µg ml−1 (diamonds), 25 µg ml−1 (circles), 50 µg ml−1 (triangles) and 100 µg ml−1 (squares) rLYZL6. To determine the number of CFUs, serial dilutions were plated and colonies were counted the following day. Data present means±s.e.m. of triplicate samples. CFU, colony-forming unit; GST, glutathione S-transferase; rLYZL6, recombinant LYZL6.
Antibacterial activity
When incubated for 15 min with 12.5 µg ml−1 rLYZL6, the survival rate of S. au was about 55%. At the concentration 100 µg ml−1, the survival rate was 30%. Following the extension of incubation time to 120 min, at the same concentration, the survival of S. au decreased to about 5%–15% of that after 15-min incubation (30% Figure 5b).
DISCUSSION
Mouse Lyzl3/Spaca3 and Lyzl4 can block sperm–egg binding or fusion in the hamster oocyte penetration assay, which indicates their possible role in fertilisation.7,8 To investigate further the role of the Lyzl family in reproduction further, we systematically report here the Lyzl family member mRNA expression in different murine tissues. Although Lyzls were expressed in other tissues, their expression was clearly higher in the testis and epididymis, which suggests a potential role in the male reproductive tract. Sun et al.7 showed that Lyzl4 mRNA was specifically expressed in the testis and epididymis in the mouse, but we showed that the expression of Lyzl4 mRNA was predominant in the testis and epididymis, while there was still moderate expression in the brain and lung.
During maturation, the production of testicular testosterone and possibly its availability to target organs, increases abruptly from 20 to 40 days of age in the mouse.15 By day 35, the principal cells of the epididymis attain adult-like structural features along with high levels of luminal androgens.16 In the present study of Lyzl expression during mouse development, the expression of Lyzl3 began from day 14 postnatal age and peaked at day 28. The other members' expression began from the twenty-eighth postnatal age and peaked at day 35, which parallels the histological development of the testis. This increase may be related to the entry of testicular fluid and spermatozoa into the epididymis that occurs around this time, but the subsequent decline with age as fertility reaches a maximum suggests more a role in the epididymal epithelial changes at this time than fertility-related phenomena.
The expression of most epididymal genes is the combined response to androgens and testicular factors.17,18,19,20 There are probably four types of regulation: (i) positive androgen control: the mRNAs decrease dramatically following bilateral castration and then recover to pre-orchidectomy levels following the administration of testosterone;21 (ii) negative androgen control: the mRNA levels increase following castration with a subsequent decrease in mRNA following testosterone replacement;22 (iii) testicular factor regulation: the mRNAs decrease dramatically following castration, but remain low to undetectable following testosterone supplementation;23 (iv) both androgen and testicular factor regulation: the mRNA levels decrease to undetectable following castration and then recover incompletely after androgen supplementation.24 On the basis of the castration experiment, in the present study, we presume that Lyzl1, Lyzl3 and Lyzl6 mRNA expression belongs to testicular factor regulation, since there is a post-castration decline and no response to testosterone. Lyzl4, on the other hand, belongs to positive androgen control type since a clear response to testosterone was observed. Lyzl4 appeared to increase with testosterone in the sham-operated controls, too. It is noteworthy that Lyzl3 mRNA expression fluctuated substantially following androgen supplement in the sham-operated group, a tendency different from that in the operated group, and declined after testosterone injection, findings that require future investigation.
Lyzl6 in the mouse reproductive tract has not been reported and, from sequence analysis, two essential catalytic residues are conserved in LYZL6.4 We focused on LYZL6 expression and localisation in the testis, epididymis and spermatozoa of mice, and then tested the antibacterial activity of rLYZL6. In the testis, LYZL6 was observed in primary spermatocytes and round spermatids, but not in elongated spermatids. In the epididymis, the principal cells outnumber all the other cell types combined by at least 3∶1.25,26 The principal cells in all regions exhibit the morphological features of cells actively involved in absorption and secretion.27 Basal cells have been proposed to play an active role in detoxification.28 Mouse LYZL6 expression showed principal and basal cell expression, but not all the principal and basal cells expressed LYZL. Most principal and basal cells in the corpus and cauda epididymidis expressed LYZL6 highly, with only a moderate expression in the caput, and a subset of cells expressed it in the initial segment. The gradual increase in expression of LYZL6 in the principal cells from the initial segment to the cauda may indicate that LYZL6 is a secretory protein, and combines with spermatozoa during sperm maturation. Determination of the functional differences in these LYZL6-expressing cells is an aspect for further study that could lead to a better understanding of the roles of the epididymis in sperm maturation.
In the mammalian testis, undifferentiated germ cells transform into spermatozoa through a complex series of events that include mitosis, meiosis and cellular differentiation.29 The ability to fertilize oocytes requires many proteins secreted by the epididymal epithelium cells, such as SLLP1.6 SLLP1 is located in acrosomal region of spermatozoa, involved in sperm–oocyte binding. The location on spermatozoa of LYZL6, another c-type lysozyme, was unknown before this study revealed it on the post-acrosomal area and midpiece of mature spermatozoa. However, there were not positive signals on immature spermatozoa. Mitochondrial respiration in the midpiece is a significant source of ATP required for mammalian sperm motility,30 but only by diffusion to other regions of the tail or by active transport along the flagellum can mitochondrial ATP be used for sperm motility. However, the diffusion of ATP to the principal piece is restricted because of the long flagellum of the mammalian spermatozoa.31 We detected LYZL6 in the midpiece of mature spermatozoa. From the result, LYZL6 may play an important role in mitochondrial functions of spermatozoa and sperm motility. In the future, we will produce lyzl6 gene knockout mice to study the specific roles.
As important enzymes related to antimicrobial activities, Lyzl family members might play vital roles in innate immune defense against pathogens invading the male tract. Lysozymes can hydrolyze the beta-1,4-glycosidic linkage between N-acetylmuramic acid and N-acetylglucosamine in bacterial cell walls.3 As LYZL6 is a member of the c-type lysozyme family, which is expected to exhibit hydrolytic activity against glycosyl bonds, we tested recombinant human LYZL6 protein antibacterial activity for its ability to kill S. au ATCC25923 in a CFU assay, and confirmed its bacteriolytic activity.
This activity might due to two essential catalytic residues, 35-Glu and 52-Asp, which are conserved in LYZL6.4 From the results of the present study LYZL6 may play a role in host defense against infection in the human male reproductive system. Recombinant c-type lysozyme is able to bind to bacterial cells and inhibit bacterial growth.32 A review of the literature reveals that two residues of human lysozyme are replaced in SLLP1, resulting in a loss of the bacteriolytic activity. Similar reports have also been found for mouse LYZL4,7 and rat LYZL4,33 because of the replacement of aspartate by glycine at the catalytic site. We therefore consider that the two catalytic residues are necessary for protein bacteriolytic activity. Together, these results indicate that LYZL6 is a functional lysozyme that is likely to participate in innate immune defense against bacterial pathogens, especially in the male reproductive tract.
In conclusion, we have systematically reported the expression of Lyzls 1,3,4,6 in several tissues of the mouse. They were expressed in the epididymis in a developmentally regulated manner and the expression of one (Lyzl4) was relative to androgen level. Immunodetection revealed the presence of LYZL6 protein in primary spermatocytes and round spermatids and on the post-acrosomal area and midpiece of mature spermatozoa. The rLYZL6 protein exhibited antibacterial activity against S. au. From these results, Lyzl6 may play reproductive and protective roles in the epididymis. Further studies are required to determine the molecular mechanisms that operate in controlling the expression of Lyzls during postnatal development and androgenic regulation.
AUTHOR CONTRIBUTIONS
JW, SJL, HS, HYW and JYL were responsible for the concept and framework of the paper. SJL, HS, JL and SHJ carried out RT-PCR, IHC, Western blot and IF. SJL and CTR carried out orchiectomy. SJL and PZ were responsible for preparation of recombinant proteins. SJL, HS and JW participated in the drafting and final editing. All authors read and approved the final manuscript.
Acknowledgments
This research was financially supported by the grants from the National Natural Science Foundation of China (No. 31071262) and the Shandong Science and Technology Department (No. 032050102).
The authors declare no competing financial interests.
References
- Fleming A. On a remarkable bacteriolytic element found in tissues and secretions. Proc R Soc Lond B Biol Sci. 1922;93:306–17. [Google Scholar]
- Jollès P, Jollès J. What's new in lysozyme research? Always a model system, today as yesterday. Mol Cell Biochem. 1984;63:165–89. doi: 10.1007/BF00285225. [DOI] [PubMed] [Google Scholar]
- Prager EM, Jollès P. Animal lysozymes c and g: an overview. EXS. 1996;75:9–31. doi: 10.1007/978-3-0348-9225-4_2. [DOI] [PubMed] [Google Scholar]
- Zhang K, Gao R, Zhang H, Cai X, Shen C, et al. Molecular cloning and characterization of three novel lysozyme-like genes, predominantly expressed in the male reproductive system of humans, belonging to the c-type lysozyme/alpha-lactalbumin family. Biol Reprod. 2005;73:1064–71. doi: 10.1095/biolreprod.105.041889. [DOI] [PubMed] [Google Scholar]
- Prager EM. Adaptive evolution of lysozyme: changes in amino acid sequence, regulation of expression and gene number. EXS. 1996;75:323–45. doi: 10.1007/978-3-0348-9225-4_17. [DOI] [PubMed] [Google Scholar]
- Mandal A, Klotz KL, Shetty J, Jayes FL, Wolkowicz MJ, et al. SLLP1, a unique, intra-acrosomal, non-bacteriolytic, c lysozyme-like protein of human spermatozoa. Biol Reprod. 2003;68:1525–37. doi: 10.1095/biolreprod.102.010108. [DOI] [PubMed] [Google Scholar]
- Sun R, Shen R, Li J, Xu G, Chi J, et al. Lyzl4, a novel mouse sperm-related protein, is involved in fertilization. Acta Biochim Biophys Sin (Shanghai) 2011;43:346–53. doi: 10.1093/abbs/gmr017. [DOI] [PubMed] [Google Scholar]
- Herrero MB, Mandal A, Digilio LC, Coonrod SA, Maier B. Mouse SLLP1, a sperm lysozyme-like protein involved in sperm–egg binding and fertilization. Dev Biol. 2005;284:126–42. doi: 10.1016/j.ydbio.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Li W, Zhang J, Liu X, Xu R, Zhang Y. Correlation of appearance of metastasis-associated protein1 (Mta1) with spermatogenesis in developing mouse testis. Cell Tissue Res. 2007;329:351–62. doi: 10.1007/s00441-007-0412-8. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Zheng M, Shi Q, Zhang L, Zhen W, et al. An epididymis-specific secretory protein HongrES1 critically regulates sperm capacitation and male fertility. PLoS ONE. 2008;3:e4106. doi: 10.1371/journal.pone.0004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Liu J, Li J, Wang H, Zhang C, Li N, et al. Cloning, expression and location of RNase9 in human epididymis. BMC Res Notes. 2008;1:111. doi: 10.1186/1756-0500-1-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell D. New York: Cold Spring Harbor Laboratory Press; 2011. Molecular Cloning: A Laboratory Manual. 3rd ed; pp. 304–31. [Google Scholar]
- Yenugu S, Hamil KG, Birse CE, Ruben SM, French FS, et al. Antibacterial properties of the sperm-binding proteins and peptides of human epididymis 2 (HE2) family; salt sensitivity, structural dependence and their interaction with outer and cytoplasmic membranes of Escherichia coli. Biochem J. 2003;372:473–83. doi: 10.1042/BJ20030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallon C, Veyssiere G, Berger M, Jean-Faucher C, de Turckheim M, et al. Age-related changes in the concentration of cytosolic androgen receptors in the epididymis, vas deferens and seminal vesicle of maturing male mice. J Androl. 1989;10:188–94. doi: 10.1002/j.1939-4640.1989.tb00085.x. [DOI] [PubMed] [Google Scholar]
- Robaire B, Seenundun S, Hamzeh M, Lamour SA. Androgenic regulation of novel genes in the epididymis. Asian J Androl. 2007;9:545–53. doi: 10.1111/j.1745-7262.2007.00316.x. [DOI] [PubMed] [Google Scholar]
- Cao SF, Xu C. Specialized gene expression and regulation in the epididymis. Zhonghua Nan Ke Xue. 2006;12:71–4. [PubMed] [Google Scholar]
- Cornwall GA, Hann SR. Specialized gene expression in the epididymis. J Androl. 1995;16:379–83. [PubMed] [Google Scholar]
- Hinton BT, Lan ZJ, Rudolph DB, Labus JC, Lye RJ. Testicular regulation of epididymal gene expression. J Reprod Fertil Suppl. 1998;53:47–57. [PubMed] [Google Scholar]
- Hu Y, Zhou Z, Xu C, Shang Q, Zhang YD, et al. Androgen down-regulated and region-specific expression of germ cell nuclear factor in mouse epididymis. Endocrinology. 2003;144:1612–9. doi: 10.1210/en.2002-220915. [DOI] [PubMed] [Google Scholar]
- Ghyselinck NB, Jimenez C, Lefrancois AM, Dufaure JP. Molecular cloning of a cDNA for androgen-regulated proteins secreted by the mouse epididymis. J Mol Endocrinol. 1990;4:5–12. doi: 10.1677/jme.0.0040005. [DOI] [PubMed] [Google Scholar]
- Cyr DG, Robaire B. Regulation of sulfated glycoprotein-2 (clusterin) messenger ribonucleic acid in the rat epididymis. Endocrinology. 1992;130:2160–6. doi: 10.1210/endo.130.4.1547732. [DOI] [PubMed] [Google Scholar]
- Penttinen J, Pujianto DA, Sipila P, Huhtaniemi I, Poutanen M. Discovery in silico and characterization in vitro of novel genes exclusively expressed in the mouse epididymis. Mol Endocrinol. 2003;17:2138–51. doi: 10.1210/me.2003-0008. [DOI] [PubMed] [Google Scholar]
- Walker JE, Jones R, Moore A, Hamilton DW, Hall L. Analysis of major androgen-regulated cDNA clones from the rat epididymis. Mol Cell Endocrinol. 1990;74:61–8. doi: 10.1016/0303-7207(90)90205-m. [DOI] [PubMed] [Google Scholar]
- Zhen W, Li P, He B, Guo J, Zhang YL. The novel epididymis-specific beta-galactosidase-like gene Glb1l4 is essential in epididymal development and sperm maturation in rats. Biol Reprod. 2009;80:696–706. doi: 10.1095/biolreprod.108.071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belleannée C, da Silva N, Shum WW, Marsolais M, Laprade R, et al. Segmental expression of the bradykinin type 2 receptor in rat efferent ducts and epididymis and its role in the regulation of aquaporin 9. Biol Reprod. 2009;80:134–43. doi: 10.1095/biolreprod.108.070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermo L, Schellenberg M, Liu LY, Dayanandan B, Zhang T, et al. Membrane domain specificity in the spatial distribution of aquaporins 5, 7, 9, and 11 in efferent ducts and epididymis of rats. J Histochem Cytochem. 2008;56:1121–35. doi: 10.1369/jhc.2008.951947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robaire B, Viger RS. Regulation of epididymal epithelial cell functions. Biol Reprod. 1995;52:226–36. doi: 10.1095/biolreprod52.2.226. [DOI] [PubMed] [Google Scholar]
- Abou-Haila A, Tulsiani DR. Mammalian sperm acrosome: formation, contents, and function. J Arch Biochem Biophys. 2000;379:173–82. doi: 10.1006/abbi.2000.1880. [DOI] [PubMed] [Google Scholar]
- Cao W, Haig-Ladewig L, Gerton GL, Moss SB. Adenylate kinases 1 and 2 are part of the accessory structures in the mouse sperm flagellum. Biol Reprod. 2006;75:492–500. doi: 10.1095/biolreprod.106.053512. [DOI] [PubMed] [Google Scholar]
- Ford WC. Glycolysis and sperm motility: does a spoonful of sugar help the flagellum go round. Hum Reprod Update. 2006;12:269–74. doi: 10.1093/humupd/dmi053. [DOI] [PubMed] [Google Scholar]
- Yu LP, Sun BG, Li J, Sun L. Characterization of a c-type lysozyme of Scophthalmus maximus: expression, activity and antibacterial effect. Fish Shellfish Immunol. 2013;34:46–54. doi: 10.1016/j.fsi.2012.10.007. [DOI] [PubMed] [Google Scholar]
- Narmadha G, Muneswararao K, Rajesh A, Yenugu S. Characterization of a novel lysozyme-like 4 gene in the rat. PLoS ONE. 2011;6:e27659. doi: 10.1371/journal.pone.0027659. [DOI] [PMC free article] [PubMed] [Google Scholar]