Abstract
Neurodegeneration occurs in the majority of the more than 40 known lysosomal storage diseases. Since the nervous system in these disorders can be globally affected, effective treatment would require persistent widespread correction. Biffi et al. show such correction is possible in a mouse model of metachromatic leukodystrophy by the transplantation of hematopoietic cells genetically modified to overexpress the missing lysosomal enzyme. The results reveal a nervous system damage-response pathway that can be harnessed to provide therapy to the nervous system in these serious disorders.
Although individual lysosomal storage diseases are rare, as a group they represent a significant fraction of severe inherited metabolic conditions (1). The more than 40 different storage disorders cumulatively affect 1 in 5,000 live births. Most of the disorders are caused by the deficiency of a lysosomal enzyme activity that results in the progressive accumulation of the enzyme’s undegraded substrates. Since lysosomal enzymes are expressed ubiquitously, many organ systems are often affected. Generally, the pathophysiology of a particular disorder is related to the degree of substrate accumulation in a cell or tissue type — determined by substrate synthesis and degradation rates — and the sensitivity of the cell or tissue type to the stored substrate. A case in point is metachromatic leukodystrophy (MLD), which is caused by the absence of arylsulfatase A. A major substrate for the enzyme is galactosyl-3-sulfate ceramide (sulfatide), a component of myelin sheaths produced by oligodendrocytes in the central nervous system and Schwann cells in the peripheral nervous system. In MLD, damage to oligodendrocytes and Schwann cells caused by storage of sulfatide results in demyelination, degeneration of the nervous system, and, in the most severe cases, death in childhood.
Neurodegeneration, a feature that MLD shares with the majority of lysosomal storage diseases, has made treatment of these diseases exceedingly difficult. Enzyme replacement therapies have been successful in preventing or reversing the somatic manifestations of some disorders by taking advantage of the cell’s efficient receptor-mediated uptake systems (2, 3). Lysosomal enzymes carry oligosaccharide structures that enable their recognition by plasma membrane receptors, which deliver the enzymes to lysosomes. Thus far, enzyme replacement therapy has proven unsuitable for treatment of the nervous system in storage diseases, because the blood-brain barrier precludes entry of proteins administered systemically. To circumvent this obstacle, strategies are being attempted that include direct introduction of gene transfer vectors or genetically modified cells into the nervous system (4–6). It is uncertain if such a highly invasive strategy would be suitable for the persistent and widespread correction needed for a globally affected nervous system found in many of the storage diseases. Another strategy utilizes small molecules that can pass through the blood-brain barrier to either partially inhibit substrate synthesis or stabilize the mutant enzyme (7, 8). While promising in concept, these approaches are in early stages.
Gene therapy for the nervous system via hematopoietic stem cells
Transplantation of hematopoietic stem cells, from either bone marrow or cord blood, has been utilized over the past several years as a potential treatment for lysosomal storage diseases. Significantly, it is the only treatment to date that has been shown to prevent or retard neurodegeneration in storage disease patients (9). A study in this issue of the JCI by Biffi et al. (10) reinforces the concept that hematopoietic stem cell transplantation, as a therapeutic platform, has several features well-suited for the treatment of a globally affected nervous system in lysosomal storage diseases. The authors show, in a mouse model of MLD, that transplantation of hematopoietic stem cells transduced with lentiviral vectors carrying arylsulfatase A prevents neurologic disease as determined by the absence of sulfatide storage and neuropathology, and the preservation of neurologic function. Importantly, a global correction was achieved that included both the central and the peripheral nervous systems. Although hematopoietic stem cells can potentially differentiate into multiple nervous system cell types (11–14), in this study the repopulating cells were almost exclusively macrophages and microglia. Because donor cells that were engineered to produce high levels of arylsulfatase A were more effective at preventing neurodegeneration than wild-type cells, therapeutic improvement was likely achieved by enzyme-deficient cells capturing arylsulfatase A secreted by donor microglia and endoneural macrophages.
Nervous system damage-response pathway
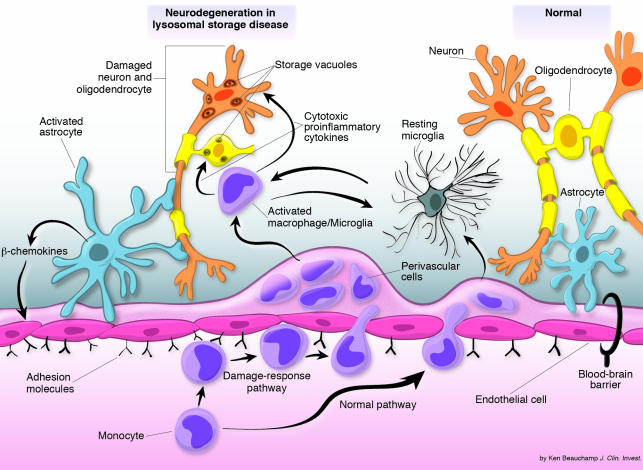
The findings in this study (10) help clarify a damage-response mechanism relevant to the pathogenesis of lysosomal storage diseases and illustrate how it can be exploited for nervous system therapy (Figure 1). Microglia, resident macrophages of the brain, are continuously replenished by blood-borne precursors, which cross the blood-brain barrier and migrate into the parenchyma of the nervous system. They take up residence as resting microglia to surveil and defend the nervous system against damaging agents or conditions. When the nervous system is injured, as in MLD (10), a damage-response mechanism is activated that results in the enhanced recruitment of macrophages/microglia. Most significantly, there is preferential recruitment of macrophages/microglia to sites of substrate storage and pathology (10, 14). Thus, if the hematopoietic stem cells have been corrected for the lysosomal enzyme deficiency, the macrophages/microglia may be able to deliver enzyme directly to sites of damage.
Figure 1.
Damage-response pathway in lysosomal storage disease. Normally, nervous system microglia and macrophages can be derived from blood-borne precursors; they pass through the blood-brain barrier into the perivascular regions and ultimately take up residence in the parenchyma as resting ramified cells. When nervous system damage occurs, cytokines and chemokines are generated from activated macrophages/microglia and astrocytes, which can stimulate the up-regulation of adhesion molecules on nervous system endothelial cells, resulting in enhanced transendothelial migration of monocytes from the blood into perivascular regions. These enzyme-deficient cells migrate into the parenchyma to sites of damage where they may secrete potentially cytotoxic proinflammatory cytokines, leading to further damage and cell death. If the hematopoietic precursors are corrected, the macrophages and microglia gain the ability to transfer the missing lysosomal enzyme to deficient cells in the nervous system at sites of damage.
The vigorous process of recruiting macrophages/microglia into the nervous system and their subsequent activation may actually hasten neurodegeneration in lysosomal storage diseases. Studies utilizing a mouse model of Sandhoff disease, another sphingolipid storage disease exhibiting profound neurodegeneration, have shown that the population of activated macrophages/microglia expands considerably in the central nervous system prior to neuronal apoptosis (15). Intriguingly, the transplantation of these mice with bone marrow–containing hematopoietic stem cells substantially reduces macrophage/microglia activation and neuronal cell death without reducing substrate storage. Furthermore, blocking monocyte recruitment into the nervous system reduces neuronal cell death (Y.-P. Wu and R.L. Proia, unpublished data). These results indicate that the activated macrophages/microglia can trigger cell death possibly through the elaboration of cytotoxic, inflammatory mediators as has been suggested for other neurodegenerative conditions like Alzheimer disease.
The work by Biffi et al. (10) highlights the concept that therapeutic hematopoietic stem cell transplantation actually corrects a nervous system damage-response pathway defective in lysosomal storage disorders. Ex vivo genetic modification of hematopoietic stem cells to heighten expression of the missing enzyme in macrophages/microglia serves to restore and enhance the corrective potential of this pathway and to dampen its destructive capacity. Harnessed for therapy, the damage-response pathway appears unique in its capacity to afford widespread and persistent correction of a globally damaged nervous system as well as to target specifically affected areas. The approach taken by Biffi et al. (10), with its remarkable therapeutic effect on MLD, is particularly important because it outlines a strategy that may ultimately herald effective treatment of the nervous system in lysosomal storage diseases.
Footnotes
See the related article beginning on page 1118.
Nonstandard abbreviations used: metachromatic leukodystrophy (MLD).
Conflict of interest: The authors have declared that no conflict of interest exists.
References
- 1.Scriver, C.R., et al., editors. 2001. In The metabolic and molecular bases of inherited disease. McGraw-Hill. New York, New York, USA. 3371–3896.
- 2.Brady RO. Enzyme replacement therapy: conception, chaos and culmination. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003;358:915–919. doi: 10.1098/rstb.2003.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kakkis ED, et al. Enzyme-replacement therapy in mucopolysaccharidosis I. N. Engl. J. Med. 2001;344:182–188. doi: 10.1056/NEJM200101183440304. [DOI] [PubMed] [Google Scholar]
- 4.Brooks AI, et al. Functional correction of established central nervous system deficits in an animal model of lysosomal storage disease with feline immunodeficiency virus-based vectors. Proc. Natl. Acad. Sci. U. S. A. 2002;99:6216–6221. doi: 10.1073/pnas.082011999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor RM, Wolfe JH. Decreased lysosomal storage in the adult MPS VII mouse brain in the vicinity of grafts of retroviral vector-corrected fibroblasts secreting high levels of beta-glucuronidase. Nat. Med. 1997;3:771–774. doi: 10.1038/nm0797-771. [DOI] [PubMed] [Google Scholar]
- 6.Snyder EY, Taylor RM, Wolfe JH. Neural progenitor cell engraftment corrects lysosomal storage throughout the MPS VII mouse brain. Nature. 1995;374:367–370. doi: 10.1038/374367a0. [DOI] [PubMed] [Google Scholar]
- 7.Platt FM, et al. Prevention of lysosomal storage in Tay-Sachs mice treated with N-butyldeoxynojirimycin. Science. 1997;276:428–431. doi: 10.1126/science.276.5311.428. [DOI] [PubMed] [Google Scholar]
- 8.Fan JQ, Ishii S, Asano N, Suzuki Y. Accelerated transport and maturation of lysosomal alpha-galactosidase A in Fabry lymphoblasts by an enzyme inhibitor. Nat. Med. 1999;5:112–115. doi: 10.1038/4801. [DOI] [PubMed] [Google Scholar]
- 9.Krivit W, et al. Hematopoietic stem-cell transplantation in globoid-cell leukodystrophy. N. Engl. J. Med. 1998;338:1119–1126. doi: 10.1056/NEJM199804163381605. [DOI] [PubMed] [Google Scholar]
- 10.Biffi A, et al. Correction of metachromatic leukodystrophy in the mouse model by transplantation of genetically modified hematopoietic stem cells. J. Clin. Invest. 2004;113:1118–1129. doi:10.1172/JCI200419205. doi: 10.1172/JCI19205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eglitis MA, Mezey E. Hematopoietic cells differentiate into both microglia and macroglia in the brains of adult mice. Proc. Natl. Acad. Sci. U. S. A. 1997;94:4080–4085. doi: 10.1073/pnas.94.8.4080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mezey E, Chandross KJ, Harta G, Maki RA, McKercher SR. Turning blood into brain: cells bearing neuronal antigens generated in vivo from bone marrow. Science. 2000;290:1779–1782. doi: 10.1126/science.290.5497.1779. [DOI] [PubMed] [Google Scholar]
- 13.Brazelton TR, Rossi FM, Keshet GI, Blau HM. From marrow to brain: expression of neuronal phenotypes in adult mice. Science. 2000;290:1775–1779. doi: 10.1126/science.290.5497.1775. [DOI] [PubMed] [Google Scholar]
- 14.Priller J, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat. Med. 2001;7:1356–1361. doi: 10.1038/nm1201-1356. [DOI] [PubMed] [Google Scholar]
- 15.Wada R, Tifft CJ, Proia RL. Microglial activation precedes acute neurodegeneration in Sandhoff disease and is suppressed by bone marrow transplantation. Proc. Natl. Acad. Sci. U. S. A. 2000;97:10954–10959. doi: 10.1073/pnas.97.20.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]