Abstract
Understanding of autoimmune sensorineural hearing loss (ASNHL) has been hindered by the inaccessibility of the inner ear to biopsy and the lack of workable animal models. A report in this issue of the JCI describes a mouse model of CD4+ T cell–mediated ASNHL induced by immunization with peptides from the inner ear–specific proteins cochlin and β-tectorin.
The inner ear (IE), like most other specialized tissues and organs, can become the target of an autoimmune attack. Sensorineural hearing loss (SNHL) is often an early, although presumably secondary, complication of various non–organ-specific autoimmune diseases; however, the IE can also represent the primary focus of a unique disease entity, autoimmune IE disease (AIED) (1). Fortunately the disease is rare, but the small population size of affected individuals and the inaccessibility of the IE during an acute attack have hindered progress in our understanding of the etiology, diagnosis, and treatment of this disease. AIED is diagnosed by exclusion of other disorders that mimic it. The hearing loss is typically bilateral, asymmetric, and fluctuating and deteriorates rapidly over weeks or months; balance and equilibrium may or may not be affected. Diagnosis of AIED is tentatively confirmed if there is a positive response to trial corticosteroid therapy, although some individuals experience spontaneous recovery. Therapy must be initiated rapidly, since unchecked inflammatory damage to the fragile and irreplaceable sensory structures of the IE may easily lead to irreversible SNHL. On the other hand, systemic immunosuppressive drugs may increase susceptibility to infection, and steroids may induce water retention; hence the urgent need for better supporting diagnostic procedures for idiopathic SNHL, as well as more locally focused treatments. In general, we need a great deal more fundamental information about AIED before substantial progress can be made in its diagnosis and treatment.
Current aids in the diagnosis of AIED
Understanding of AIED has been hampered most by the inability to monitor events occurring within the bony confines of the IE during the course of the disease. We do not know what cells, in what proportions and with what antigen (Ag) specificities, are involved; nor do we know the relative role(s), if any, of the array of associated autoantibodies. The few human temporal bones derive from late- or end-stage AIED and show a marked osteoneogenic response to chronic IE inflammation (2). Given the inaccessibility of this area for biopsy, noninvasive procedures have been used for diagnostic testing. One such test measures recall responses of T cells to IE Ag (3), but it is inherently difficult and not readily available, and it only measures the levels of circulating, not nodal or IE-sequestered, lymphocytes. Tests for autoantibodies have identified multiple Ag’s associated with AIED, but there remains considerable controversy in the literature over which Ag’s are recognized and by what fraction of patients. Differences in disease-associated Ag’s reported between laboratories may derive in part from the use of different Ag sources, methods of Ag extraction, immunoblotting procedures, and relative molecular mass standards, as well as patient-selection criteria (see refs. 4 and 5), but as yet, there has been no exchange of sera between laboratories using different assays. Even with a single assay, identification of different Ag’s may reflect useful information regarding patient heterogeneity, e.g., disease stage, severity, activity, prognosis, susceptibility to intervention, etc.; however, confirmation must await much larger patient study groups. To date, no single Ag has been shown to be recognized by a high proportion of patient but not control sera. A battery of such Ag’s may eventually emerge. Of the Ag’s that have been biochemically characterized, most have been ubiquitous or common to non–IE-specific tissues and have therefore lacked logical or compelling association with pathology focused on the IE (6–8). When tested as immunogens with strong adjuvants in animals, the better-characterized putative autoantigens, such as collagen II, Hsp70, and myelin P0, have given inconsistent results or failed to elicit SNHL or IE inflammatory infiltration (6–8).
Animal models of autoimmune SNHL
Attempts to develop an animal model of AIED by immunization with relatively crude IE homogenates have met with limited success; either some animals were unresponsive or the model was underdeveloped and therefore unsuitable for determining the mechanism(s) of IE impairment, the immunologically active components involved, and their location (9–11). Progress is slowly being made in fractionation of the IE immunogen (12), but active fractions may still contain numerous components. Reproducing the exact model in other laboratories and overcoming animal-to-animal variation may be difficult until effective recombinant Ag’s are available. Experimental autoimmune SNHL (ASNHL) has been passively transferred in some animal models by T cell transfer (10, 13), demonstrating the critical role of T cells in the induction of ASNHL. On the other hand, there is also precedent for a critical role of autoantibodies in ASNHL. Administration of the IgG1 mAb KHRI-3 induced SNHL and loss of hair cells (14). KHRI-3 reacts with a glycoprotein Ag homologous to the human choline transporter CTL-2 (15), expressed on supporting cells in the organ of Corti. Patient Ab’s or mAb’s against other Ag’s or better epitopes of the same Ag may be effective at more physiologically relevant titers. Thus, there is ample evidence to support that experimental ASNHL is either T cell– or autoantibody-mediated, and human AIED may be mediated by either mechanism, or both, with some patients polarized into T cell–mediated disease and others into autoantibody-mediated disease and reactive to different Ag’s. A reproducible, easy-to-establish animal model of AIED would provide a very useful system in which to address questions regarding the early progression and final resolution of an IE autoimmune attack, how and which cells access the IE, and what constitutes a potent IE autoantigen.
Induction of ASNHL by immunization with peptides from IE proteins
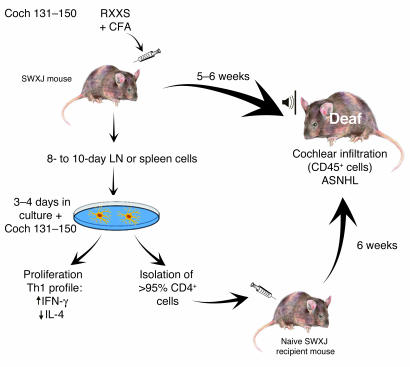
Previous models of experimental ASNHL established by immunization with a complex mixture of IE proteins have been highly erratic. This is understandable, since only some of the proteins were IE-specific, present in immunogenic quantity, and able to sufficiently break self-tolerance in such a manner as to affect hearing in even a fraction of the recipients. In this issue of the JCI, Solares, Tuohy, and colleagues (16) present a novel, straightforward approach to the development of experimental animal models of AIED. Reasoning that the best candidate Ag’s would be abundant, IE-specific proteins, the authors restricted their study to just two such proteins, cochlin and β-tectorin. Since neither protein was available in purified or recombinant form, they immunized autoimmune-prone SWXJ mice with peptides selected from the known sequences of these two extracellular proteins. The peptides contained a lysine or arginine residue separated from a serine by two amino acids, i.e., KXXS or RXXS. Tuohy and coworkers have previously shown that MHC class II molecules on APCs in the SWXJ hybrid mouse strain and in the parental SJL/J and SWR/J mice bind and present peptides bearing the KXXS or RXXS motif (17). Furthermore, in models of other autoimmune diseases, immunization of these mice with KXXS or RXXS peptides derived from known major autoantigens was shown to mediate the corresponding organ-specific autoimmune disease (17). In the present experiments immunizing with cochlin (Figure 1) and β-tectorin peptides emulsified with CFA, not all peptides with this motif stimulated an immune response as monitored by T cell proliferation upon re-exposure of lymph node cells to peptide in vitro (16). However, two peptides, Coch 131–150, containing RXXS, and β-tectorin 71–90, containing KXXS, stimulated strong recall responses. The lymph node cells that responded were shown by flow cytometry to be CD4+ T cells and produced high IFN-γ and low IL-4 levels characteristic of a Th1 response. The recall response to Coch 131–150 was restricted to I-As occurring in both SWXJ hybrid (I-Aq,s) mice and the SJL/J (I-As) parental strain, while the response to β-tectorin 71–90 was restricted to I-Aq occurring in the hybrid and the SWR/J (I-Aq) parent. Most importantly, mice immunized with either peptide demonstrated significant broad-frequency SNHL 5 weeks after immunization. Infiltration of the cochlea by CD45+ leukocytes was coincident with induction of experimental ASNHL after immunization with Coch 131–150. ASNHL was passively transferred to irradiated naive mice by transfer of lymph node cells or spleen CD4+ T cells, which had been removed from donor mice 10 days after immunization and restimulated in vitro with Coch 131–150 or β-tectorin 71–90.
Figure 1.
Systemic immunization of autoimmune-prone SWXJ mice with Coch 131–150, an RXXS peptide from the abundant IE extracellular protein cochlin, induced CD45+ cell cochlear infiltration and ASNHL in mice within 5–6 weeks. Lymph node (LN) or spleen cells placed in culture 8–10 days after immunization proliferated, as evidenced by 3H-thymidine incorporation, and showed a Th1 cytokine profile (high levels of IFN-γ and low levels of IL-4 production) after restimulation in vitro with Coch 131–150. Flow cytometry confirmed preferential reactivation of CD4+ T cells. After restimulation in vitro with peptide, the total population or the CD4+-enriched population (>95%) of cells induced ASNHL 6 weeks after passive transfer to naive, irradiated, histocompatible recipient mice. The same results were obtained after priming, restimulation, and transfer of CD4+ T cells specific for a KXXS peptide from a second IE protein, β-tectorin 71–90, but not with ovalbumin as Ag (16).
The ASNHL induced by immunization with Coch 131–150 or β-tectorin 71–90 in this model demonstrates that autoimmune responses to quite different cochlear Ag’s may adversely affect the IE (16). It is tempting to speculate that multiple autoantigens, perhaps including cochlin and β-tectorin, are also implicated in AIED in humans; however, it is not yet clear how accurately this model reflects events occurring in the spontaneous idiopathic disease or how heterogeneous AIED is with respect to autoantigens. Multiple autoantigens would certainly be consistent with the diverse array of putative autoantibodies reported to be associated with AIED (see refs. 4 and 5). Autoantibodies to cochlin have been identified in individuals with AIED (18), but a prior study reported a correlation between AIED and these autoantibodies in only 10% of patients (4 of 40; ref. 5). If cochlin and β-tectorin are involved in AIED, are they functioning as T cell or B cell Ag’s? Could the present model be extended to almost any IE-specific Ag, or, as is the case with the extracellular Ag’s cochlin and β-tectorin, is induction of ASNHL dependent on a highly abundant target Ag that is readily accessibility to T cells? Peptides from other IE proteins and non–IE-specific proteins will undoubtedly be tested in this model. With a list of autoantigens capable of inducing experimental ASNHL disease in animal models, AIED patients could be screened for T and B cell reactivities against those same Ag’s to determine whether these Ag’s are involved in the human disease. A number of questions still remain. What are the exact nature and kinetics of the IE damage resulting from immunization with IE Ag’s; do they differ with different Ag’s? Is the damage reversible? Is pathology dependent on the identity and location of the target Ag, or is it in part a bystander effect? What cells are infiltrating the cochlea? Is it mainly CD4+ cells? What cells do they recruit? How do cells and Ab’s cross the so-called blood-labyrinth barrier, analogous to the blood-brain barrier in the CNS? These questions, and many others as yet unasked, may be amenable to investigation by many laboratories using this simple and reproducible animal model. Questions regarding the etiology and self-perpetuation of spontaneous AIED may not be as easily addressed in a transient model, where the initiating agent or event is artificial and apparent; however, we can expect that this model will provide many answers and lead to better, more representative, and workable models of AIED.
Footnotes
See the related article beginning on page 1210.
Nonstandard abbreviations used: antigen (Ag); autoimmune inner ear disease (AIED); autoimmune sensorineural hearing loss (ASNHL); inner ear (IE); sensorineural hearing loss (SNHL).
Conflict of interest: The author has declared that no conflict of interest exists.
References
- 1.Harris, J.P., Moscicki, R.A., and Hughes, G.B. 1997. Immunologic disorders of the inner ear. In Clinical otology. G.B. Hughes and M.L. Pensak, editors. Thieme Medical Publishers. New York, New York, USA. 381–391.
- 2.Keithley EM, Chen M-C, Linthicum F. Clinical diagnoses associated with histologic findings of fibrotic tissue and new bone in the inner ear. Laryngoscope. 1998;108:87–91. doi: 10.1097/00005537-199801000-00016. [DOI] [PubMed] [Google Scholar]
- 3.Hughes GB, Moscicki R, Barna BP, San Martin JE. Laboratory diagnosis of immune inner ear disease. Am. J. Otol. 1994;15:198–202. [PubMed] [Google Scholar]
- 4.García Berrocal JR, Ramírez-Camacho R, Arellano B, Vargas JA. Validity of the Western blot immunoassay for heat shock protein-70 in associated and isolated immunorelated inner ear disease. Laryngoscope. 2002;112:304–309. doi: 10.1097/00005537-200202000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Cao MY, Deggouj N, Gersdorff M, Tomasi J-P. Guinea pig inner ear antigens: extraction and application to the study of human autoimmune inner ear disease. Laryngoscope. 1996;106:207–212. doi: 10.1097/00005537-199602000-00019. [DOI] [PubMed] [Google Scholar]
- 6.Harris JP, Woolf NK, Ryan AF. A re-examination of experimental type II collagen autoimmunity: middle and inner ear morphology and function. Ann. Otol. Rhinol. Laryngol. 1986;95:176–180. doi: 10.1177/000348948609500214. [DOI] [PubMed] [Google Scholar]
- 7.Billings PB, Shin S-O, Harris JP. Assessing the role of anti-hsp70 in cochlear function. Hear. Res. 1998;126:210–212. doi: 10.1016/s0378-5955(98)00172-5. [DOI] [PubMed] [Google Scholar]
- 8.Boulassel MR, et al. No evidence of auditory dysfunction in guinea pigs immunized with myelin P0 protein. Hear. Res. 2001;152:10–16. doi: 10.1016/s0378-5955(00)00212-4. [DOI] [PubMed] [Google Scholar]
- 9.Harris JP. Experimental autoimmune sensorineural hearing loss. Laryngoscope. 1987;97:63–76. doi: 10.1288/00005537-198701000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Ikezono T, et al. Passive transfer of experimental labyrinthitis. Audiol. Neurootol. 2000;5:292–299. doi: 10.1159/000013893. [DOI] [PubMed] [Google Scholar]
- 11.Bouman H, et al. Experimental autoimmune inner ear disease: an electrocochleographic and histophysiologic study. Ann. Otol. Rhinol. Laryngol. 2000;109:457–466. doi: 10.1177/000348940010900504. [DOI] [PubMed] [Google Scholar]
- 12.Tomiyama S. Experimental autoimmune labyrinthitis: assessment of molecular size of autoantigens in fractions of inner ear proteins eluted on the Mini Whole Gel Eluter. Acta Otolaryngol. 2002;122:692–697. [PubMed] [Google Scholar]
- 13.Gloddek B, Gloddek J, Arnold W. A rat T-cell line that mediates autoimmune disease of the inner ear in the Lewis rat. ORL J. Otorhinolaryngol. Relat. Spec. 1999;61:181–187. doi: 10.1159/000027668. [DOI] [PubMed] [Google Scholar]
- 14.Nair TS, et al. Monoclonal antibody induced hearing loss. Hear. Res. 1995;83:101–113. doi: 10.1016/0378-5955(94)00194-u. [DOI] [PubMed] [Google Scholar]
- 15.Nair, T.S., et al. 2002. Evidence that the inner ear supporting cell antigen (IESCA) is CTL2, a member of the choline transporter-like family. Assoc. Res. Otolaryngol. 256 (Abstr.). http://www.aro.org/archives/2002/2002258.html.
- 16.Solares CA, et al. Murine autoimmune hearing loss mediated by CD4+ T cells specific for inner ear peptides. J. Clin. Invest. 2004;113:1210–1217. doi:10.1172/JCI200418195. doi: 10.1172/JCI18195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jane-wit D, et al. A novel class II-binding motif selects peptides that mediate organ-specific autoimmune disease in SWXJ, SJL/J, and SWR/J mice. J. Immunol. 2002;169:6507–6514. doi: 10.4049/jimmunol.169.11.6507. [DOI] [PubMed] [Google Scholar]
- 18.Boulassel M-R, Tomasi J-P, Deggouj N, Gersdorff M. COCH5B2 is a target antigen of anti-inner ear antibodies in autoimmune inner ear diseases. Otol. Neurotol. 2001;22:614–618. doi: 10.1097/00129492-200109000-00009. [DOI] [PubMed] [Google Scholar]