Abstract
The hypothalamus is a forebrain structure critically involved in the organization of defensive responses to aversive stimuli. Gamma-aminobutyric acid (GABA)ergic dysfunction in dorsomedial and posterior hypothalamic nuclei is implicated in the origin of panic-like defensive behavior, as well as in pain modulation. The present study was conducted to test the difference between these two hypothalamic nuclei regarding defensive and antinociceptive mechanisms. Thus, the GABAA antagonist bicuculline (40 ng/0.2 µL) or saline (0.9% NaCl) was microinjected into the dorsomedial or posterior hypothalamus in independent groups. Innate fear-induced responses characterized by defensive attention, defensive immobility and elaborate escape behavior were evoked by hypothalamic blockade of GABAA receptors. Fear-induced defensive behavior organized by the posterior hypothalamus was more intense than that organized by dorsomedial hypothalamic nuclei. Escape behavior elicited by GABAA receptor blockade in both the dorsomedial and posterior hypothalamus was followed by an increase in nociceptive threshold. Interestingly, there was no difference in the intensity or in the duration of fear-induced antinociception shown by each hypothalamic division presently investigated. The present study showed that GABAergic dysfunction in nuclei of both the dorsomedial and posterior hypothalamus elicit panic attack-like defensive responses followed by fear-induced antinociception, although the innate fear-induced behavior originates differently in the posterior hypothalamus in comparison to the activity of medial hypothalamic subdivisions.
Keywords: Panic-like behavior, Fear-induced antinociception, Dorsomedial hypothalamus, Posterior hypothalamus
Introduction
The medial hypothalamus is thought to be part of a neurobiological substrate controlling defensive behavior (1,2). It has been suggested that some hypothalamic nuclei such as the anterior hypothalamic nucleus, the dorsomedial division of the ventromedial nucleus and the dorsal premammillary nucleus are part of a circuit proposed as a medial hypothalamic zone (MHZ) defensive system, since there is an increase in Fos immunoreactivity in these structures during innate defensive responses elicited during predatory threatening (3). There is also evidence that the MHZ defensive system is connected to other hypothalamic nuclei, including the lateral preoptic area, the dorsomedial rostral perifornical region, as well as the dorsomedial (DMH) and posterior (PH) hypothalamic nuclei (3) that justify more profound investigation of the specific role of these nuclei in panic attack-related behavior.
In humans, stimulation of the ventromedial hypothalamus (4) and PH (5) evokes panic attacks such as shortness of breath and increased arterial pressure and heart rate, although the possibility of activation of both excitatory neurons and fibers of passage connecting these hypothalamic nuclei to other structures of the brain aversion system, such as the periaqueductal gray matter, cannot be ruled out.
Panic is a severe anxiety disorder characterized by spontaneous panic attacks, accompanied by multiple physiological symptoms (6). In rats, acute gamma-aminobutyric acid (GABAA) receptor blockade in the DMH (7,8) and PH (9) causes panic-like behavior. In addition, chronic inhibition of GABA synthesis in the DMH of rats provokes anxiety and panic-like attacks (7).
Moreover, oriented escape attempts (oriented jumps toward the upper levels of the open-field test arena) were also observed during GABAergic dysfunction in the DMH caused by intradiencephalic microinjections of bicuculline (2,10). Thus, the blockade of the GABAA receptor in the DMH can be used to simulate some behavioral responses commonly reported by human patients during panic attacks.
It was recently reported that panic-like behavior elicited by GABAergic receptor antagonism in the DMH is followed by fear-induced antinociception (2). Innate fear-related oscillations of nociceptive thresholds were also observed after electrical and chemical stimulation of other encephalic structures, for example those situated in the dorsal midbrain (11-15).
Activation of antinociceptive process-related neurons has been proposed to be part of defensive reactions modulated by fear (2,14), engaging the animal in fear-induced defensive behavior instead of pain-related recuperative responses.
Given the importance of the DMH and PH in antinociceptive processes associated with the defensive response or panic-like behavior, the aim of the present study was to investigate the characteristics of defensive responses elicited by GABAA receptor blockade in different divisions of the hypothalamus, including medial and posterior nuclei, and to determine if these reactions are followed by oscillation of the nociceptive threshold.
Material and Methods
Animals
Male Wistar rats (Rattus norvegicus, Rodentia, Muridae) weighing 220-260 g (N = 8 per group) from the Animal Facility of the School of Medicine of Ribeirão Preto, University of São Paulo (FMRP-USP), were studied. They were housed 4 to a cage and kept in the experimental room for at least 48 h prior to the experiments, with free access to water and food, on a 12:12-h light/dark cycle (lights on at 7:00 am) at 22-23°C. The enclosure was kept under a light/dark cycle of 12/12 h (lights on from 7:00 am to 7:00 pm) and at a constant room temperature of 25° ± 1°C (40-70% humidity). All experiments were performed in accordance with the recommendation of the Ethics Commission of Animal Experimentation of FMRP-USP (protocol No. 130/2008), which are consistent with the ethical principles in animal research adopted by the Brazilian Society of Laboratory Animal Sciences (SBCAL), and approved by the Ethics Committee of Animal Research (CETEA) on 12/15/2008.
Drugs
Bicuculline methiodide (40 ng/0.2 µL; Sigma, USA) was dissolved in saline (0.9% NaCl) shortly before use. Saline also served as vehicle control.
Surgical procedure
Animals were anesthetized with 92 mg/kg ketamine (Ketamina Agener®, União Química Farmacêutica Nacional, Brazil; 0.2 mL 10% solution) and 10 mg/kg xylazine (0.1 mL; Dopaser®, Hertape Calier, Brazil) and fixed in a stereotaxic frame (David Kopf, USA). A stainless steel guide cannula (OD 0.6 mm, ID 0.4 mm) was implanted in the diencephalon aiming at the DMH and PH. The upper incisor bar was set at 3.3 mm below the interaural line, such that the skull was horizontal between bregma and lambda. The unilateral guide cannula was vertically introduced in 84 animals, using the following coordinates, with bregma serving as the reference: anteroposterior, -2.80 mm; mediolateral, 0.5 mm, and dorsoventral, 7.8 mm to DMH, and anteroposterior, -3.72 mm; mediolateral, -0.4 mm, and dorsoventral, -7.4 mm to the PH, in an independent group of rodents. The guide cannula was fixed to the skull with acrylic resin and two stainless steel screws. At the end of surgery, each guide cannula was sealed with a stainless steel wire to protect it from obstruction.
Antinociceptive procedure
Independent groups of rats (N = 8) had their nociception thresholds compared using the tail-flick test. Each animal was placed in a restraining apparatus (Insight, Brazil) with acrylic walls, and its tail was placed on a heating sensor (tail-flick Analgesia Instrument; Insight). The progressive heat elevation was automatically interrupted when the animal removed its tail from the apparatus. The current raised the temperature of the coil (Ni/Cr alloy; 26.04 cm in length × 0.02 cm in diameter) at the rate of 9°C/s starting at room temperature (approximately 20°C). Small current intensity adjustments were performed, if necessary, at the beginning of the experiment (baseline records), in order to obtain three consecutive tail-flick latencies (TFL) between 2.5 and 3.5 s. If the animal did not remove its tail from the heater within 6 s, the apparatus was turned off in order to prevent damage to the skin. Three baseline measurements of control TFL were made at 5-min intervals. TFL were also measured for 60 min immediately after the induction of escape behavior.
Experimental procedure
The nociceptive threshold of the animals (N = 8) was measured for baseline records. The animal was submitted to surgery for implantation of the DMH or PH nuclei. Five days after surgery, the rats were gently wrapped in a cloth and hand held in order to be randomly microinjected with either bicuculline methiodide (40 ng/0.2 µL) or saline (0.2 µL) in the DMH or PH. The following responses were subsequently recorded: exploratory behavior, expressed by the number of crossings (four paws in a given division of the open-field floor after crossing the limit between each division); frequency and duration of rearing (upright posture); behavioral defensive reactions expressed by the frequency and duration of defensive attention (alertness, a response operationally defined as the interruption of ongoing behavior as if the rodents oriented themselves toward the stimulus, evoking attentive posture, with small head movements, rearing and smelling the surrounding air); the frequency and duration of defensive immobility (“freezing”, operationally defined as immobility with two or more of the following autonomic reactions: defecation, urination, piloerection, and exophthalmos); frequency and duration of defensive backward movements (rapid defensive backward movement), and frequency and duration of induced forward escape behavior (running intercalated with exploratory responses and jumps oriented to the upper level of the open-field test arena). The frequency and duration of grooming (self-cleaning repertory of sequential movements from the head to the hind paws) were also recorded. All of these behavioral responses were recorded immediately after microinjection of bicuculline into the DMH or PH. Each animal received a maximum of one diencephalic treatment with the GABAergic antagonist or its vehicle. The nociceptive responses (TFLs) were measured 15 min before the diencephalic administration of bicuculline or its vehicle and immediately after the escape behavior elicited by microinjection of bicuculline into the hypothalamus, or 10 min after pretreatment of the hypothalamus with saline, and subsequently over a period of 1 h at 10-min interval, after this procedure.
Histology
Upon completion of the experiments, the animals were anesthetized with 92 mg/kg ketamine and 10 mg/kg xylazine and perfused through the left ventricle. The blood was washed out with Tyrode's buffer (40 mL at 4°C) followed by 200 mL ice-cold 4% (w/v) paraformaldehyde (LabSynth, Brazil) in 0.1 M sodium phosphate buffer (LabSynth), pH 7.3, for 15 min at a pressure of 50 mmHg. The brains were quickly removed and soaked for 4 h in fresh fixative at 4°C. After fixation, the brains were sectioned, and the diencephalon was rinsed in 10 and 20% sucrose dissolved in 0.1 M sodium phosphate buffer, pH 7.3, at 4°C for at least 12 h in each solution. Tissue pieces were immersed in 2-methylbutane (Sigma), frozen on dry ice (30 min), embedded in Tissue Tek® (Sakura, The Netherlands) and cut with a cryostat (Leica CM 1950, Germany). Slices were then mounted on glass slides coated with chrome alum gelatin to prevent detachment and stained in a robotized autostainer (CV5030 Leica Autostainer XL) with hematoxylin-eosin in order to localize the positions of the guide cannula tips according to the Paxinos and Watson atlas (16) under a photomicroscope (AxioImager Z1, Zeiss, Germany). Data from rats with the guide cannula tips located outside the DMH or PH were not included in the statistical analysis.
Statistical analysis
Data from independent groups of animals submitted to GABAA receptor blockade in the DMH or PH were submitted to ANOVA followed by the Newman-Keuls post hoc test. Data from experiments carried out to determine the oscillation of the nociceptive thresholds after fear-induced responses of exploratory behavior were submitted to repeated-measure ANOVA, followed by the Duncan post hoc test. All data are reported as means ± SEM for N = 8 rats. P < 0.05 was considered to be statistically significant.
Results
Panic-like defensive behavior
Decreased GABAergic neurotransmission in both the DMH and PH nuclei was followed by panic-like defensive behaviors characterized by defensive alertness, defensive immobility, defensive backward rapid movement, and forward escape reactions. These reactions were accompanied by intense exploratory behavior in the open field test during the hypothalamic GABAA receptor blockade.
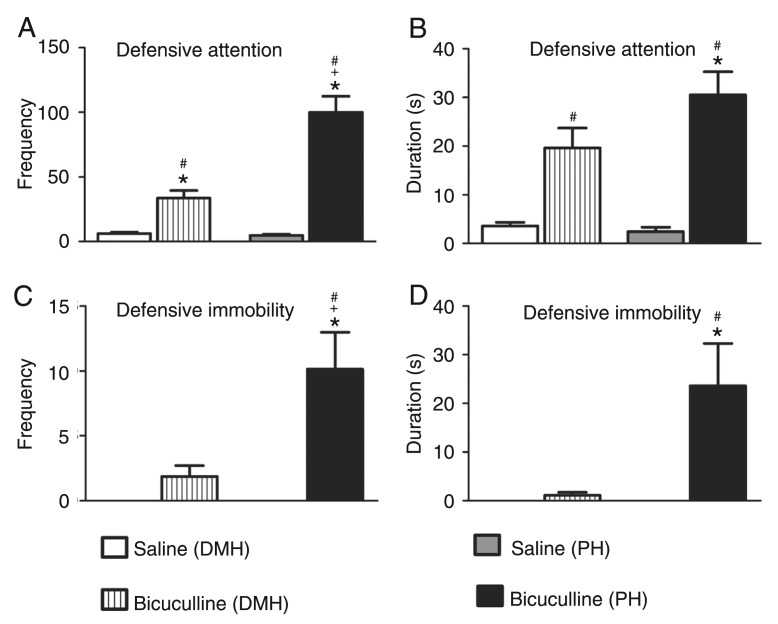
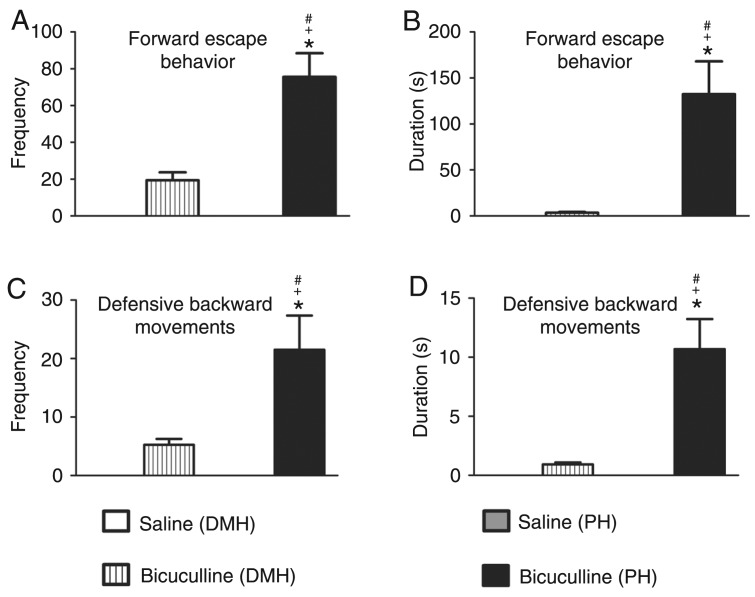
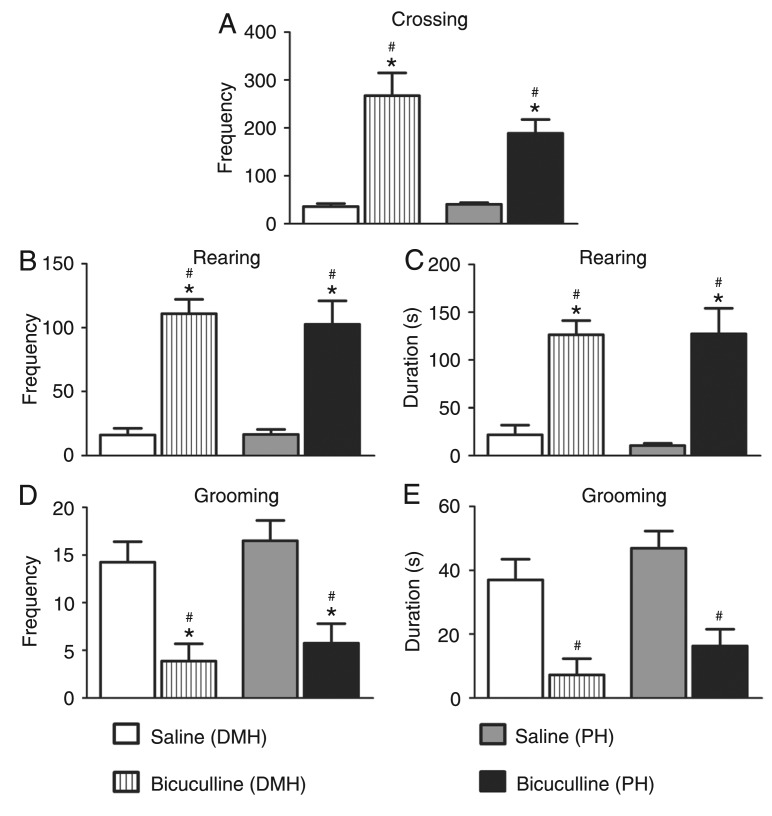
Microinjections of bicuculline into the DMH caused a significant increase in the frequency [F(3,28) = 40.62; P < 0.05; Figure 1A] and duration [F(3,28) = 17.94; P < 0.01; Figure 1B] of defensive alertness and in the frequency [F(3,28) = 10.59; P < 0.001; Figure 1C] and duration [F(3,28) = 7.083; P < 0.001; Figure 1D] of defensive immobility. Blockade of the GABAA receptor in the DMH did not cause a significant increase in the frequency (Figure 2A) and duration (Figure 2B) of forward escape behavior. A non-significant increase in the frequency (Figure 2C) and duration (Figure 2D) of defensive backward movement was also observed. These panic-like responses were accompanied by exploratory behavior characterized by a significant increase in crossing episodes [F(3,28) = 16.76; P < 0.001; Figure 3A] in the open-field test.
Figure 1. Effect of central administration of saline (0.9% NaCl; N = 8) or 40 ng/0.2 µL bicuculline methiodide (N = 8) into the dorsomedial (DMH) and posterior (PH) hypothalamic nuclei on the frequency (A and C) and duration (B and D) of defensive attention (alertness; A and B), defensive immobility (freezing; C and D). The columns indicate the mean and the bars represent the standard error of the mean. *P < 0.05 compared to the saline (DMH)-treated group, +P < 0.001 compared to the bicuculline (DMH)-treated group, #P < 0.01 compared to the saline (PH)-treated group (one-way ANOVA, followed by the Newman-Keuls post hoc test).
Figure 2. Effect of central administration of saline (0.9% NaCl; N = 8) or 40 ng/0.2 µL bicuculline methiodide (N = 8) into the dorsomedial (DMH) and posterior (PH) hypothalamic nuclei on the frequency (A and C) and duration (B and D) of forward escape behavior (A and B), and defensive backward movements (C and D). The columns indicate the mean and the bars represent the standard error of the mean. *P < 0.05 compared to the saline (DMH)-treated group, +P < 0.001 compared to the bicuculline (DMH)-treated group, #P < 0.001 compared to the saline (PH)-treated group (one-way ANOVA, followed by the Newman-Keuls post hoc test).
Figure 3. Effect of central administration of saline (0.9% NaCl; to N = 8) or 40 ng/0.2 µL bicuculline methiodide (N = 8) into the dorsomedial (DMH) and posterior (PH) hypothalamic nuclei on the exploratory behavior expressed by crossings (A), the frequency (B) and duration (C) of rearing, and the frequency (D) and duration (E) of grooming behavior. The columns indicate the mean and the bars represent the standard error of the mean. *P < 0.01 compared to the saline (DMH)-treated group, #P < 0.01 compared to the saline (PH)-treated group (oneway ANOVA, followed by the Newman-Keuls post hoc test).
A clear-cut increase in the frequency [F(3,28) = 21.21; P < 0.001; Figure 3B] and duration [F(3,28) = 15,65; P < 0.001; Figure 3C] of rearing behavior and a significant decrease in the frequency [F(3,28) = 9.204; P < 0.01; Figure 3D], but not in the duration [F(3,28) = 10.71; P < 0.001; Figure 3E] of grooming were also observed in the animals microinjected intra-DMH with the GABAA antagonist compared to the control group.
Microinjections of bicuculline into the PH resulted in an increase in the frequency [F(3,28) = 40.62; P < 0.001; Figure 1A] and duration [F(3,28) = 17.94; P < 0.001; Figure 1B] of defensive alertness and an increase in the frequency [F(3,28) = 10.59; P < 0.001; Figure 1C] and duration [F(3,28) = 7.083; P < 0.001; Figure 1D] of defensive immobility compared to control. A significant increase in the frequency [F(3,28) = 27.28; P < 0.001; Figure 2A] and duration [F(3,28) = 13.74; P < 0.001; Figure 2B] of forward escape behavior was also observed. Blockade of the GABAA receptor in the PH also caused a significant increase in the frequency [F(3,28) = 11.81; P < 0.001; Figure 2C] and duration [F(3,28) = 16.72; P < 0.001; Figure 2D] of defensive backward movement.
These panic-like responses were also accompanied by exploratory behavior characterized by a significant increase of crossing [F(3,28) = 16.76; P < 0.001; Figure 3A] compared to control. We also observed an increase in the frequency [F(3,28) = 21.21; P < 0.001; Figure 3B] and duration [F(3,28) = 15.65; P < 0.001; Figure 3C] of rearing behavior. There was also a significant decrease in the frequency [F(3,28) = 9.204; P < 0.01; Figure 3D] and duration [F(3,28) = 10.71; P < 0.001; Figure 3E] of grooming compared to control.
Microinjection of bicuculline into the PH caused a significant increase in the frequency (P < 0.001) of defensive attention, frequency (P < 0.001) of defensive immobility, frequency (P < 0.001) and duration (P < 0.001) of forward escape behavior and defensive backward movements compared to the effect of GABAA receptor blockade in the DMH (Figure 1A and C; 2A and B; 2C and D).
Fear-induced antinociception
The panic-like escape behavior organized by both DMH and PH nuclei was followed by significant fear-induced antinociception compared to the control group. Repeated-measures ANOVA revealed a significant effect of treatment [F(3,28) = 12.41; P < 0.001], time [F(9,20) = 10.881; P < 0.001] and a treatment versus time interaction [F(27,56) = 3.433; P < 0.001]. The Duncan post hoc test revealed that significant antinociception occurred immediately after the fear-induced escape behavior up to 20 min after this defensive behavioral response [F(3,28) ranging from 5.51 to 39.01; P < 0.001; Figure 4].
Figure 4. Effect of microinjections of saline (0.9% NaCl, 0.2 µL; N = 8) or 40 ng/0.2 µL bicuculline methiodide (N = 8) into the dorsomedial (DMH) and posterior (PH) hypothalamic nuclei on nociceptive thresholds demonstrated by the tail-flick test (TFT). Innate fear-induced antinociception was studied after panic-like escape behavior elicited by GABAA receptor blockade in the hypothalamus. Data are reported as means ± SEM. *P < 0.05 compared to the saline (DMH)-treated group; #P < 0.05 compared to the saline (PH)-treated group (repeated-measures ANOVA followed by the Duncan post hoc test). The arrow in A indicates the time of microinjection of either saline or bicuculline into hypothalamic nuclei. BL1-3 = baseline tail-flick latencies.
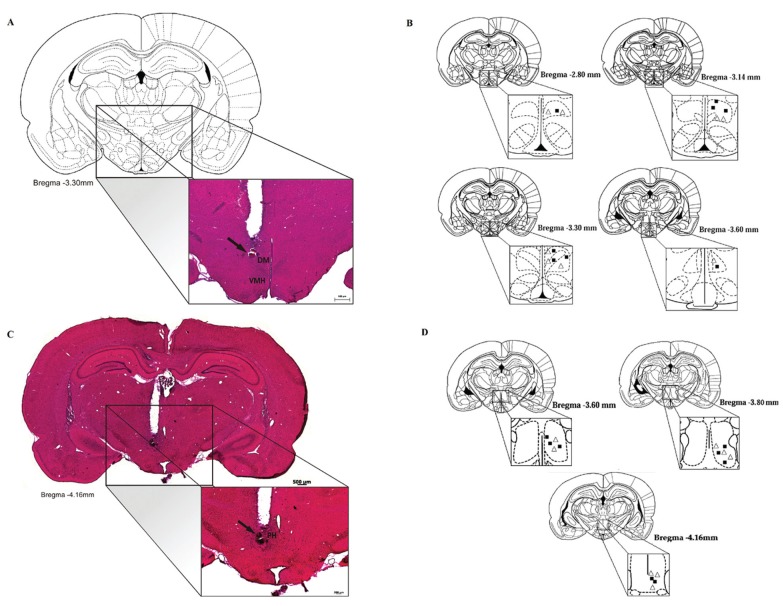
Schematic drawings and representative photomicrographs of coronal sections of R. norvegicus brain showing the sites of central administration of bicuculline methiodide or saline are presented in Figure 5.
Figure 5. A, Schematic drawing of brain structures depicted in illustrations from Paxinos and Watson's (16) atlas (reproduction authorized by Elsevier) at bregma -3.30 mm, at the level of the dorsomedial hypothalamus (DMH; original photomicrograph amplified at the bottom). Scale bar: 500 µm. C, Original photomicrograph of a diencephalic coronal section at bregma -4.16, showing a representative site (arrow) of central microinjection of 40 ng/0.2 µL bicuculline methiodide into the posterior hypothalamus (PH). Scale bar: 200 µm, stained with hematoxylin and eosin. B and D, Schematic coronal sections of the Rattus norvegicus brain, showing the sites of central administration of saline (open triangles) or 40 ng/0.2 µL bicuculline methiodide (filled squares) in the DMH (B) or PH (D).
Discussion
Panic-like defensive behavior
The present data clearly show that a reduction of tonic GABAergic inhibition of hypothalamic neurons by local administration of bicuculline, a selective GABAA receptor antagonist, microinjected into the DMH and PH evoked defensive behavioral responses such as defensive attention, defensive immobility and a flight response interspersed with exploratory behavior. These findings are consistent with a previous report showing that panic-like defensive behavior can be elicited by chemical stimulation of the dorsomedial part of the ventromedial hypothalamic nucleus and dorsomedial hypothalamic nucleus (2). It has been suggested that the GABAA receptor-mediated neurotransmission is involved in the anxiety and panic-like defensive behavior organized by the DMH (17). Indeed, several lines of evidence suggest that the DMH plays an important role in the generation of behavioral and autonomic responses to aversive stimuli (8,18). Similarly, a GABAA antagonist microinjected into the PH has also been reported to evoke defensive behavior (9).
The defensive behavior observed in the present study is consistent with studies using electrical and chemical simulation of other prosencephalic and mesencephalic structures (2,14,19-21). However, the stimulation of hypothalamic nuclei evokes defensive reactions characterized by low intensity and much more oriented and elaborate defensive motor reactions compared to those elicited from mesencephalic structures (2,10). Another outstanding defensive reaction elaborated by hypothalamic and mesencephalic structures concerns defensive immobility or “freezing”. Recently, “freezing” has been described as a defensive response due to potential or distal aversive stimuli, when the animals need to acquire environmental information (22). GABAergic dysfunction and chemical stimulation of the dorsolateral column of the periaqueductal gray matter has been widely reported to produce freezing behavior (23,24). Despite previous reports suggesting that defensive immobility is more commonly related to activation of neurons situated in the mesencephalic tectum during the organization of defensive behavior (23,25), defensive immobility was also reported as an aversive stimulus-induced defensive reaction elaborated by medial hypothalamus neurons (2). It is interesting to note that a “freezing” defensive response was also observed in the present study after GABAergic system dysfunction in both DMH and PH.
Interestingly, the present results suggest that DMH GABAergic dysfunction evokes more attenuated defensive responses than PH. Both DMH and PH were reported to elaborate the same defensive behavioral pattern of responses (9,17), although the present study provides the first evidence of critical differences between the defensive behavior organized by each hypothalamic division presently studied. Moreover, the evidence suggests that the DMH, but not the PH, is activated in situations of high concentrations of carbon dioxide (hypercarbic gas exposure), which increase anxiety-like behavior in rats and humans and induce panic attacks in the most patients with a diagnosis of panic disorder. In addition, the perifornical nucleus and the paraventricular hypothalamic nucleus are reported to be activated under the same condition, i.e., hypercarbic gas exposure (26). Since the dorsal premammillary hypothalamic nucleus is relatively close to the PH, we also must consider its possible involvement in the origin of panic attack-like behaviors. As a matter of fact, other hypothalamic nuclei might also be involved in the organization of defensive behavior and in fear-induced antinociception elicited by threatening situations.
In addition, innate defensive responses elicited in the presence of a natural predator increased Fos protein levels in the medial zone of the hypothalamus (anterior hypothalamic nucleus, dorsomedial division of the ventromedial nucleus, and dorsal pre-mammillary hypothalamic nuclei) (27). Thus, it has been proposed that these structures are part of a so-called medial hypothalamic defensive system (3,27). All of these data suggest that different hypothalamic nuclei might be recruited in different aversive environments; consequently, dysfunction of the hypothalamic system may be implicated at least in part in the triggering of panic disorder in humans.
In view of this possibility, the precise function of each hypothalamic nucleus and its anatomic divisions should be clarified to provide a better knowledge of the neural circuitry involved in the physiologic panic-like behavior and also in panic syndrome.
Fear-induced antinociception
The present results have also shown an important role of the DMH and PH in pain modulation. Besides panic-like defensive behavior, the present findings provide evidence for a significant fear-induced antinociception after GABAA receptor antagonism in the DMH and PH. It is known that in situations of proximal or imminent danger, such as the presence of a predator, the encephalic aversion system recruits a series of brainstem structures that comprise the pain endogenous inhibitory system (28,29). This allows the organism to engage in fight or flight responses without the risk of affecting its performance by the pain caused by a given corporal injury (14,30). Other studies have provided additional evidence that some hypothalamic nuclei such as the lateral hypothalamus and paraventricular hypothalamic nucleus (31,32) are involved in pain modulation. However, there is little evidence demonstrating the involvement of dorsomedial and posterior hypothalamic nuclei in fearinduced antinociception.
Although the present results showed an increase in the defensive behavior triggered by the PH, the fear-induced antinociception was not different in duration and intensity from that triggered by DMH neurons. Interestingly, PH has been the target of an invasive approach (deep brain stimulation) aiming at the treatment of chronic pain. A case report about the first patient submitted to this new treatment, consisting of deep brain stimulation focused on the posterior hypothalamic area, was recently published (5). The purpose of this invasive approach is to treat cases with chronic cluster headache, which are refractory to pharmacological therapy. However, the side effects of this particular approach include panic attacks (33,34), and this reinforces the involvement of hypothalamic nuclei in the organization of fear-induced responses in human.
It is known that innate fear-induced behavioral responses, such as defensive immobility and escape behavior (24,25), as well as fear-induced antinociception (15,25) are commonly related to the neuronal activity of the periaqueductal gray matter. In view of these findings, it is possible that both DMH and PH recruit the dorsal columns of the periaqueductal gray matter for the organization of at least part of the antinociception induced by panic-like responses. In fact, there is evidence that descending PH projections, particularly those to the periaqueductal gray matter, are involved in PH modulation of complex behaviors (35), and neuroimaging approaches have shown that traumatic nociceptive pain activates neurons in both the hypothalamus and periaqueductal gray matter (36).
In conclusion, GABAergic neurons can exert tonic control on dorsomedial and posterior hypothalamic nuclei involved in the generation and expression of panic-like response and fear-induced antinociception. In contrast, DMH and PH give origin to defensive behaviors of distinct expression and intensity. More detailed studies are still necessary to understand the exact role of each hypothalamic nucleus in the organization of panic-like defensive behavior and how these neural substrates are involved in the modulation of pain in dangerous situations.
Acknowledgments
The authors are grateful to D.H. Elias-Filho for expert technical assistance. We thank Mr. Farhad Ullah for English review. Research supported by CNPq (#470119/2004-7), FAEPA (#1291/97, #355/2000, #68/2001, and #15/2003) and FAPESP (#03/07202-6, #03/01768-8, #03/01794-9, and #07/01174-1). A.F. Biagioni and J.A. Silva were supported by FAPESP (#2010/15140-4) and CNPq (#142844/2011-0) fellowships, respectively. N.C. Coimbra is the recipient of a research fellowship (level 1A) from CNPq (#301905/2010-0). D.H. Elias Filho received a technician scholarship from FAPESP (TT-2, #02/01497-1) and was the recipient of scholarships sponsored by CNPq (#501858/2005-9, #500896/2008-9, and #505461/2010-2).
References
- 1.Graeff FG. Minor tranquilizers and brain defense systems. Braz J Med Biol Res. 1981;14:239–265. [PubMed] [Google Scholar]
- 2.Freitas RL, Uribe-Mariño A, Castiblanco-Urbina MA, Elias-Filho DH, Coimbra NC. GABAA receptor blockade in dorsomedial and ventromedial nuclei of the hypothalamus evokes panic-like elaborated defensive behaviour followed by innate fear-induced antinociception. Brain Res. 2009;1305:118–131. doi: 10.1016/j.brainres.2009.09.096. [DOI] [PubMed] [Google Scholar]
- 3.Canteras NS. The medial hypothalamic defensive system: hodological organization and functional implications. Pharmacol Biochem Behav. 2002;71:481–491. doi: 10.1016/s0091-3057(01)00685-2. [DOI] [PubMed] [Google Scholar]
- 4.Wilent WB, Oh MY, Buetefisch CM, Bailes JE, Cantella D, Angle C, et al. Induction of panic attack by stimulation of the ventromedial hypothalamus. J Neurosurg. 2010;112:1295–1298. doi: 10.3171/2009.9.JNS09577. [DOI] [PubMed] [Google Scholar]
- 5.Rasche D, Foethke D, Gliemroth J, Tronnier VM. [Deep brain stimulation in the posterior hypothalamus for chronic cluster headache. Case report and review of the literature] Schmerz. 2006;20:439–444. doi: 10.1007/s00482-005-0462-3. [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association . Diagnostic and statisti-calmanual of mental disorders. 4th edn. Washington: American Psychiatric Association; 1994. [Google Scholar]
- 7.Shekhar A. GABA receptors in the region of the dorsomedial hypothalamus of rats regulate anxiety in the elevated plusmaze test. I. Behavioral measures. Brain Res. 1993;627:9–16. doi: 10.1016/0006-8993(93)90742-6. [DOI] [PubMed] [Google Scholar]
- 8.Nascimento JO, Zangrossi H, Jr, Viana MB. Effects of reversible inactivation of the dorsomedial hypothalamus on panicand anxiety-related responses in rats. Braz J Med Biol Res. 2010;43:869–873. doi: 10.1590/s0100-879x2010007500075. [DOI] [PubMed] [Google Scholar]
- 9.Shekhar A, Hingtgen JN, DiMicco JA. GABA receptors in the posterior hypothalamus regulate experimental anxiety in rats. Brain Res. 1990;512:81–88. doi: 10.1016/0006-8993(90)91173-e. [DOI] [PubMed] [Google Scholar]
- 10.Brandão ML, Di Scala GM, Bouchet J, Schmitt P. Escape behavior produced by the blockade of glutamic acid decarboxylase (GAD) in mesencephalic central gray or medial hypothalamus. Pharmacol Biochem Behav. 1984;24:497–501. doi: 10.1016/0091-3057(86)90547-2. [DOI] [PubMed] [Google Scholar]
- 11.Reynolds DV. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science. 1969;164:444–445. doi: 10.1126/science.164.3878.444. [DOI] [PubMed] [Google Scholar]
- 12.Fardin V, Oliveras JL, Besson JM. A reinvestigation of the analgesic effects induced by stimulation of the periaqueductal gray matter in the rat. I. The production of behavioral side effects together with analgesia. Brain Res. 1984;306:105–123. doi: 10.1016/0006-8993(84)90360-3. [DOI] [PubMed] [Google Scholar]
- 13.Coimbra NC, Osaki MY, Eichenberger GCD, Ciscato JG, Jr, Jucá CEB, Biojone CR. Effects of opioid receptor blockade on defensive behavior elicited by electrical stimulation of the aversive substrates of the inferior colliculus in Rattus norvegicus (Rodentia, Muridae) Psychopharmacology. 2000;152:422–430. doi: 10.1007/s002130000544. [DOI] [PubMed] [Google Scholar]
- 14.Coimbra NC, de Oliveira R, Freitas RL, Ribeiro SJ, Borelli KG, Pacagnella RC, et al. Neuroanatomical approaches of the tectum-reticular pathways and immunohistochemical evidence for serotonin-positive perikarya on neuronal substrates of the superior colliculus and periaqueductal gray matter involved in the elaboration of the defensive behavior and fear-induced analgesia. Exp Neurol. 2006;197:93–112. doi: 10.1016/j.expneurol.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 15.Coimbra NC, Brandão ML. Effects of 5-HT2 receptors blockade on fear-induced analgesia elicited by electrical stimulation of the deep layers of the superior colliculus and dorsal periaqueductal gray. Behav Brain Res. 1997;87:97–103. doi: 10.1016/s0166-4328(96)02267-x. [DOI] [PubMed] [Google Scholar]
- 16.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. San Diego: Elsevier Academic Press; 1997. [Google Scholar]
- 17.Shekhar A, Keim SR, Simon JR, McBride WJ. Physiological arousal elicited by sodium lactate infusion in rats with dorsomedial hypothalamic GABA dysfunction. Pharmacol Biochem Behav. 1996;55:249–256. doi: 10.1016/s0091-3057(96)00077-9. [DOI] [PubMed] [Google Scholar]
- 18.Milani H, Graeff FG. GABA-benzodiazepine modulation of aversion in the medial hypothalamus of the rat. Pharmacol Biochem Behav. 1987;28:21–27. doi: 10.1016/0091-3057(87)90005-0. [DOI] [PubMed] [Google Scholar]
- 19.Graeff FG, Brandão ML, Audi EA, Milani H. Role of GABA in the anti-aversive action of anxiolytics. Adv Biochem Psychopharmacol. 1986;42:79–86. [PubMed] [Google Scholar]
- 20.Eichenberger GCD, Ribeiro SJ, Osaki MY, Maruoka RY, Resende GCC, Castellan-Baldan L, et al. Neuroanatomical and psychopharmacological evidence for interaction between opioid and GABAergic neural pathways in the modulation of fear and defense elicited by electrical and chemical stimulation of the deep layers of the superior colliculus and dorsal periaqueductal gray matter. Neuropharmacology. 2002;42:48–59. doi: 10.1016/s0028-3908(01)00155-1. [DOI] [PubMed] [Google Scholar]
- 21.Brandão ML, Borelli KG, Nobre MJ, Santos JM, Albrechet-Souza L, Oliveira AR, et al. GABAergic regulation of the neural organization of fear in the midbrain tectum. Neurosci Biobehav Rev. 2005;29:1299–1311. doi: 10.1016/j.neubiorev.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Ferreira-Netto C, Borelli KG, Brandão ML. Distinct Fos expression in the brain following freezing behavior elicited by stimulation with NMDA of the ventral or dorsal inferior colliculus. Exp Neurol. 2007;204:693–704. doi: 10.1016/j.expneurol.2006.12.023. [DOI] [PubMed] [Google Scholar]
- 23.Brandão ML, de Aguiar JC, Graeff FG. GABA mediation of the anti-aversive action of minor tranquilizers. Pharmacol Biochem Behav. 1982;16:397–402. doi: 10.1016/0091-3057(82)90441-5. [DOI] [PubMed] [Google Scholar]
- 24.Vianna DM, Landeira-Fernandez J, Brandão ML. Dorsolateral and ventral regions of the periaqueductal gray matter are involved in distinct types of fear. Neurosci Biobehav Rev. 2001;25:711–719. doi: 10.1016/s0149-7634(01)00052-5. [DOI] [PubMed] [Google Scholar]
- 25.Coimbra NC, Tomaz C, Brandão ML. Evidence for the involvement of serotonin in the antinociception induced by electrical or chemical stimulation of the mesencephalic tectum. Behav Brain Res. 1992;50:77–83. doi: 10.1016/s0166-4328(05)80289-x. [DOI] [PubMed] [Google Scholar]
- 26.Johnson PL, Fitz SD, Hollis JH, Moratalla R, Lightman SL, Shekhar A, et al. Induction of c-Fos in ‘panic/defence’-related brain circuits following brief hypercarbic gas exposure. J Psychopharmacol. 2011;25:26–36. doi: 10.1177/0269881109353464. [DOI] [PubMed] [Google Scholar]
- 27.Canteras NS, Chiavegatto S, Ribeiro do Vale LE, Swanson LW. Severe reduction of rat defensive behavior to a predator by discrete hypothalamic chemical lesions. Brain Res Bull. 1997;44:297–305. doi: 10.1016/s0361-9230(97)00141-x. [DOI] [PubMed] [Google Scholar]
- 28.Fields HL, Basbaum AI, Clanton CH, Anderson SD. Nucleus raphe magnus inhibition of spinal cord dorsal horn neurons. Brain Res. 1977;126:441–453. doi: 10.1016/0006-8993(77)90596-0. [DOI] [PubMed] [Google Scholar]
- 29.Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Annu Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- 30.Brandão ML, Rees H, Witt S, Roberts MH. Central antiaver-sive and antinociceptive effects of anterior pretectal nucleus stimulation: attenuation of autonomic and aversive effects of medial hypothalamic stimulation. Brain Res. 1991;542:266–272. doi: 10.1016/0006-8993(91)91577-n. [DOI] [PubMed] [Google Scholar]
- 31.Holden JE, Farah EN, Jeong Y. Stimulation of the lateral hypothalamus produces antinociception mediated by 5-HT1A, 5-HT1B and 5-HT3 receptors in the rat spinal cord dorsal horn. Neuroscience. 2005;135:1255–1268. doi: 10.1016/j.neuroscience.2005.07.023. [DOI] [PubMed] [Google Scholar]
- 32.Condes-Lara M, Rojas-Piloni G, Martinez-Lorenzana G, Lopez-Hidalgo M, Rodriguez-Jimenez J. Hypothalamospinal oxytocinergic antinociception is mediated by GABAergic and opiate neurons that reduce A-delta and C fiber primary afferent excitation of spinal cord cells. Brain Res. 2009;1247:38–49. doi: 10.1016/j.brainres.2008.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Bussone G, Franzini A, Proietti CA, Mea E, Curone M, Tullo V, et al. Deep brain stimulation in craniofacial pain: seven years' experience. Neurol Sci. 2007;28(Suppl 2):S146–S149. doi: 10.1007/s10072-007-0768-2. [DOI] [PubMed] [Google Scholar]
- 34.Rasche D, Klase D, Tronnier VM. Neuromodulation in cluster headache. Clinical follow-up after deep brain stimulation in the posterior hypothalamus for chronic cluster headache, case report - Part II. Schmerz. 2008;22(Suppl 1):37–40. doi: 10.1007/s00482-007-0611-y. [DOI] [PubMed] [Google Scholar]
- 35.Vertes RP, Crane AM. Descending projections of the posterior nucleus of the hypothalamus: Phaseolus vulgaris leucoagglutinin analysis in the rat. J Comp Neurol. 1996;374:607–631. doi: 10.1002/(SICI)1096-9861(19961028)374:4<607::AID-CNE9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 36.Hsieh JC, Stahle-Backdahl M, Hagermark O, Stone-Elander S, Rosenquist G, Ingvar M. Traumatic nociceptive pain activates the hypothalamus and the periaqueductal gray: a positron emission tomography study. Pain. 1996;64:303–314. doi: 10.1016/0304-3959(95)00129-8. [DOI] [PubMed] [Google Scholar]