Abstract
Cardiopulmonary exercise testing (CPET) plays an important role in the assessment of functional capacity in patients with interstitial lung disease. The aim of this study was to identify CPET measures that might be helpful in predicting the vital capacity and diffusion capacity outcomes of patients with thoracic sarcoidosis. A longitudinal study was conducted on 42 nonsmoking patients with thoracic sarcoidosis (median age = 46.5 years, 22 females). At the first evaluation, spirometry, the measurement of single-breath carbon monoxide diffusing capacity (DLCOsb) and CPET were performed. Five years later, the patients underwent a second evaluation consisting of spirometry and DLCOsb measurement. After 5 years, forced vital capacity (FVC)% and DLCOsb% had decreased significantly [95.5 (82-105) vs 87.5 (58-103) and 93.5 (79-103) vs 84.5 (44-102), respectively; P < 0.0001 for both]. In CPET, the peak oxygen uptake, maximum respiratory rate, breathing reserve, alveolar-arterial oxygen pressure gradient at peak exercise (P(A-a)O2), and Δ SpO2 values showed a strong correlation with the relative differences for FVC% and DLCOsb% (P < 0.0001 for all). P(A-a)O2 ≥22 mmHg and breathing reserve ≤40% were identified as significant independent variables for the decline in pulmonary function. Patients with thoracic sarcoidosis showed a significant reduction in FVC% and DLCOsb% after 5 years of follow-up. These data show that the outcome measures of CPET are predictors of the decline of pulmonary function.
Keywords: Sarcoidosis, Exercise, Respiratory function tests, Respiratory mechanics
Introduction
Sarcoidosis is a multisystem granulomatous disease of unknown origin that occurs in mediastinal and pulmonary sites in 90% of cases. The clinical course varies widely. Parenchymal abnormalities often resolve spontaneously, but progress toward pulmonary fibrosis in 20-25% of cases (1).
Clinicians frequently find it difficult to manage patients with thoracic sarcoidosis due to the significant variability in disease manifestation and multiple, non-specific symptoms. Pulmonary function tests (PFTs) are important for measuring initial lung impairment and providing a baseline to assess improvement or deterioration of lung disease. In clinical practice, the most common parameters that indicate functional impairment are forced vital capacity (FVC) and single-breath diffusion of carbon monoxide across the lung (DLCOsb); these measures are often combined with radiological assessment. However, the management of some patients requires a thoracic computed tomography scan and further physiological measurements, including exercise testing (2). Cardiopulmonary exercise testing (CPET) provides an accurate assessment of functional capacity in patients with interstitial lung disease, allowing the clinician to grade the severity of the disease. Many sarcoidosis patients experience significant symptoms only with exertion and remain asymptomatic at rest (3,4).
The American Thoracic Society (ATS), the European Thoracic Society (ETS), and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) have issued a joint statement (2) of recommendations for monitoring patients with thoracic sarcoidosis. The statement recommends that a chest radiograph and resting PFTs be performed regularly. Although these exams are considered to be sufficient to follow the evolution of the disease, they provide no information on the patients' functional capacity.
Therefore, in the present study, we sought to identify CPET measures that may be helpful in predicting the vital capacity and diffusion capacity outcomes of patients with thoracic sarcoidosis.
Patients and Methods
Patients
This was a longitudinal study involving 57 nonsmoking patients with thoracic sarcoidosis determined by a chest radiograph. The criteria for the diagnosis of sarcoidosis included compatible clinical, radiographic, and laboratory findings; histological evidence of noncaseating granuloma; the absence of mycobacterial infection, and no exposure to aerocontaminants or medication known to cause granulomatous disorders (2). Subjects with a medical history or laboratory findings of concomitant respiratory, cardiac or neuromuscular disease were excluded from the study. Participants were previously informed about the objective of the study and gave written informed consent according to current ethical standards. The study protocol was approved by the Research Ethics Committee of Hospital Universitário Pedro Ernesto, Universidade do Estado do Rio de Janeiro (Process No. 2273-CEP/HUPE).
Study protocol and measurements
The study consisted of two evaluations. At the first evaluation, in addition to chest radiographs, spirometry and the measurement of DLCOsb and CPET were performed on the patients. The severity of dyspnea was estimated using a standard 5-point scale (Medical Research Council): grade 0 = absent; grade 1 = hurrying on hills or after two flights of stairs; grade 2 = hurrying on flat ground or after one flight of stairs; grade 3 = breathlessness on minimal exertion, and grade 4 = breathlessness at rest (5). Five years later, the patients underwent the second evaluation of the study, consisting of another round of PFTs (spirometry and measurement of DLCOsb).
Each pair of chest radiographs was examined by two radiologists, who then reached a consensus about the final classification. The following classification was used: stage 0 = no radiographic abnormalities; stage 1 = bilateral hilar adenopathy without parenchymal abnormalities; stage 2 = bilateral hilar adenopathy with interstitial parenchymal infiltrates; stage 3 = interstitial parenchymal infiltrates without hilar adenopathy, and stage 4 = cicatricial changes (6).
PFTs were completed using the Collins Plus Pulmonary Function Testing Systems (Warren E. Collins, USA) following the ATS standards for both the procedure and the interpretation (7). Maximum voluntary ventilation (MVV) was performed by coaching the patients to hyperventilate as vigorously as possible for 10 s; the goal was a minimum frequency of 80 breaths/min, while the mobilized volume was recorded in liters per minute (8). The results are reported as a percent of the predicted values for the Brazilian population (9,10).
Each subject performed symptom-limited maximal exercise testing using an electronically braked cycle ergometer connected to the Collins Plus Pulmonary Function Testing System. Following the collection of 2 to 5 min of resting data, subjects pedaled for 3 min at 60 rpm without resistance, after which the work rate (WR) was incremented by 10 to 25 W each minute. The WR increment for each ramped exercise test was individualized based on each patient's pretest activity level; the objective was to achieve 8 to 12 min of progressive exercise before stopping (11). Heart rate (HR) was monitored continuously using a 12-lead electrocardiogram monitor (Marquette Electronics, USA), and blood pressure measurements (Hewlett Packard, USA) were obtained every 2 min throughout the exercise and recovery periods. Oxygen saturation (SpO2) was monitored noninvasively with a fiber optic earpiece oxymeter (Nonin Medical, USA). Oxygen desaturation during exercise was defined as a reduction of oxygen saturation greater than 4% from baseline values (12). Oxygen uptake (VO2), carbon dioxide output (VCO2), minute ventilation (VE), and related variables were calculated breath-by-breath. Arterial blood gas and lactate levels were measured (Roche Diagnostics, Brazil) at rest and at the end of the exercise period (peak performance) using samples taken from an indwelling radial arterial line. Breathing reserve was calculated as the difference between the measured resting MVV and the peak VE and is reported as a percentage of MVV [1 - (VE / MVV) × 100]. Heart rate reserve was calculated as the difference between the peak and resting heart rates [(220 - age) - peak HR]. The VO2 at the anaerobic threshold (VO2θL) was estimated using both the modified V-slope and ventilator methods (13). The alveolar-arterial oxygen pressure gradient (P(A-a)O2) was calculated using the measured respiratory exchange ratio (VCO2/VO2 or RER) values. The values were compared to those predicted by Neder et al. (14) for the adult Brazilian population.
Data analysis
Data were analyzed using the SAS 6.11 software (SAS Inc., USA). Data are reported as the median and interquartile range values or frequencies (percentage). The Kolmogorov-Smirnov test was used to check the homogeneity of the sample. Peak VO2 was compared to various parameters using nonparametric testing, including the Mann-Whitney test, and Kruskal-Wallis analysis of variance followed by Dunn's multiple comparisons post-test. The relative variation of FVC and DLCOsb over the 5-year period was evaluated using the Wilcoxon signed-rank test. Correlations between the relative variation for the FVC and DLCOsb over a 5-year period and the outcome measures of the CPET were studied using Spearman's rank correlation, with the exception of the correlation between breathing reserve and relative variations of FVC (logarithmic equation).
The clinical potential of the decline in pulmonary function to detect the potential factors related to outcome measures of the CPET was evaluated by means of receiver operating characteristic (ROC) analyses. Logistic regression was used for joint analysis to determine the factors that were independently related to the decreased pulmonary function. Only those variables found to be significant were retained in the model. Differences were considered to be significant when P < 0.05.
Results
Fifty-seven outpatients were evaluated. Fifteen were excluded due to a history of smoking (10), concomitant respiratory disease (2) and cardiac (2) or neuromuscular disease (1). Of the 42 patients studied, 22 (52.4%) were females. Median age was 46.5 (39-53) years. When white subjects were considered separately from non-white subjects (Mulatto or Black), only 11 (26.2%) described themselves as white. The time since the diagnosis of sarcoidosis in the 42 outpatients ranged from 1 to 360 months. Of the 42 subjects, 32 (76.2%) had criteria for chronic disease (presence of the disease for 2 or more years) (2). Thirty-three patients (78.6%) were receiving systemic therapy (corticosteroids alone or in combination with an immunosuppressive drug), which reflects the policy of the tertiary care clinic. However, no subject presented evidence of steroid toxicity.
Data from the pulmonary function studies and the CPET results at the first evaluation are summarized in Table 1. Thirty-seven of the 42 patients (88.1%) failed to reach at least 80% of their predicted peak VO2. Arterial oxygen desaturation occurred with exercise in 20 patients (47.6%), while a breathing reserve of less than 25% was observed in 17 patients (40.5%). Fifteen patients (35.7%) had a P(A-a) O2 (mmHg) value greater than 35 mmHg.
Table 1. Pulmonary function parameters and outcome measures of the cardiopulmonary exercise testing.
| Variables | Median (interquartile range) |
|---|---|
| Pulmonary function parameters | |
| FVC (% predicted) | 95.5 (82-105) |
| FEV1 (% predicted) | 90 (73-101) |
| FEV1/FVC (%) | 78.5 (74-84) |
| DLco (% predicted) | 93.5 (79-103) |
| Cardiopulmonary exercise testing results | |
| Peak VO2 (% predicted) | 56.5 (33-65) |
| VO2θL (%) | 41.5 (30-55) |
| RER max | 1.24 (1.11-1.33) |
| O2 pulse max (% predicted) | 61.3 (51.3-81.1) |
| HRR (beats/min) | 47 (34-54) |
| BR max (breaths/min) | 41 (34-56) |
| Breathing reserve (%) | 45.6 (13.9-62.6) |
| P(A-a)O2 (mmHg) | 18.6 (15-36) |
| Δ SpO2 (%) | 2.5 (1-7) |
| Δ blood lactate (mM) | 1.86 (1.13-3.21) |
Data are reported as median (interquartile range) for 42 patients. FVC = forced vital capacity; FEV1 = forced expiratory volume in one second; DLco = carbon monoxide diffusing capacity; peak VO2 = peak oxygen uptake; VO2θL = % peak VO2 at the estimated lactate threshold; RER max = maximum respiratory exchange ratio (VCO2/VO2); O2 pulse max = maximum oxygen pulse (VO2/heart rate); HRR = heart rate reserve; BR max = maximum respiratory rate; P(A-a)O2 = alveolar-arterial oxygen pressure gradient at peak exercise; Δ SpO2 = difference between peak and resting oxygen saturation; Δ blood lactate = difference between peak and resting blood lactate.
The characteristics of the patients, along with the peak VO2 value for the different patient groups, are listed in Table 2. Females reached a lower peak VO2 than males, but the difference was not statistically significant. While patients diagnosed with higher chest roentgenogram stage generally displayed a lower peak VO2, the association between the radiographic stage and peak VO2 was not statistically significant. Patients receiving therapy tended to achieve a lower peak VO2, but the difference was not statistically significant.
Table 2. Characteristics of the patients studied as well as the differences in peak oxygen uptake.
| Characteristics | N (%) | Peak VO2 (% predicted) |
|---|---|---|
| Gender | ||
| Male | 20 (47.6) | 59 (36-67.5) |
| Female | 22 (52.4) | 44 (31-62) |
| Degree of dyspnea | ||
| 0 | 14 (33.3) | 59 (38-65) |
| 1-2 | 24 (57.2) | 55 (31-63) |
| 3-4 | 4 (9.5) | 41 (29-58.5) |
| Receiving systemic therapy | ||
| Yes | 33 (78.6) | 48.5 (29-64) |
| No | 9 (21.4) | 56.5 (33-65) |
| Chest roentgenogram stage | ||
| 1 | 5 (11.9) | 60 (60-61) |
| 2 | 9 (21.4) | 51 (35-62) |
| 3 | 23 (54.8) | 62 (32-69) |
| 4 | 5 (11.9) | 33 (26-37) |
Data are reported as number (%) or median (interquartile range) for 42 patients. Peak VO2 = peak oxygen uptake. There were no statistically significant differences when comparing the patients' characteristics and peak oxygen uptake (Mann-Whitney test for gender and receiving systemic therapy, and Kruskal-Wallis analysis for degree of dyspnea and chest roentgenogram stage).
Five years after the first evaluation, FVC% and DLCOsb% were significantly decreased [95.5 (82-105) vs 87.5 (58-103) and 93.5 (79-103) vs 84.5 (44-102), respectively; P < 0.0001]. The relative variation of FVC and DLCOsb between the first and the second assessment was -5.1% (-23.1-0%) and -2.5% (-44.4-0.93%), respectively. The median interval between measurements was 61.5 months (range: 55-67 months). In this second evaluation, 26 patients (61.9%) were under systemic therapy (treatment decisions were taken according by the treating physician). The relative variations of FVC and DLCOsb did not differ significantly between subjects receiving therapy or not at the time of follow-up evaluation.
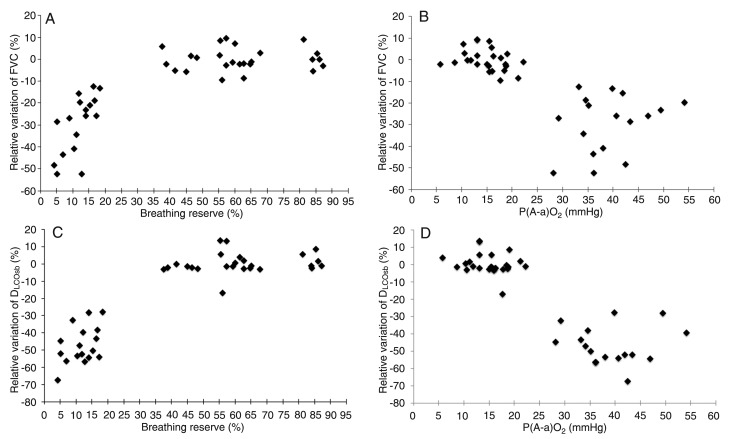
We compared the relative variations of the pulmonary function tests with the CPET measures. The results of univariate analysis are given in Table 3 and Figure 1. Among the variables studied, peak VO2, maximum respiratory rate (BR max), breathing reserve, P(A-a)O2 max, and Δ SpO2 values showed a better correlation.
Table 3. Univariate analysis comparing the relative variations of pulmonary function tests and cardiopulmonary exercise testing measures.
| Variables | Relative variations of FVC (% of predicted) | Relative variations of DLCOsb (% of predicted) | ||
|---|---|---|---|---|
| Correlation coefficient | P | Correlation coefficient | P | |
| Peak VO2 (% predicted) | 0.764 | <0.0001 | 0.803 | <0.0001 |
| VO2θL (%) | -0.159 | 0.32 | -0.143 | 0.37 |
| RER max | -0.238 | 0.13 | -0.221 | 0.16 |
| O2 pulse max (% predicted) | -0.046 | 0.77 | -0.038 | 0.81 |
| HRR (beats/min) | -0.246 | 0.12 | -0.225 | 0.15 |
| BR max (breaths/min) | -0.813 | <0.0001 | -0.685 | <0.0001 |
| Breathing reserve (%) | 0.887 | <0.0001 | 0.795 | <0.0001 |
| P(A-a)O2 (mmHg) | -0.781 | <0.0001 | -0.765 | <0.0001 |
| Δ SpO2 (%) | -0.752 | <0.0001 | -0.707 | <0.0001 |
| Δ blood lactate (mM) | 0.002 | 0.99 | -0.071 | 0.66 |
FVC = forced vital capacity; DLCOsb = single-breath diffusion of carbon monoxide across the lung; peak VO2 = peak oxygen up-take; VO2θL = % peak VO2 at the estimated lactate threshold; RER max = maximum respiratory exchange ratio (VCO2/VO2); O2 pulse max = maximum oxygen pulse (VO2/heart rate); HRR = heart rate reserve; BR max = maximum respiratory rate; P(A-a)O2 = alveolar-arterial oxygen pressure gradient at peak exercise; Δ SpO2 = difference between peak and resting oxygen saturation; Δ blood lactate = difference between peak and resting blood lactate. Correlation coefficient was determined by Spearman's rank correlation for all variables, except for the correlation between breathing reserve and relative variations of FVC (logarithmic equation).
Figure 1. Relationship of relative variation of forced vital capacity (FVC) over a 5-year period with the breathing reserve (r2 = 0.887; P < 0.0001) (Panel A) and the alveolar-arterial oxygen pressure gradient [P(A-a)O2] at peak exercise (ρ = -0.781; P < 0.0001) (Panel B) obtained by cardiopulmonary exercise testing. Relationship of relative variation of single-breath diffusion of carbon monoxide across the lung (DLCOsb) over a 5-year period with the breathing reserve (ρ = 0.795; P < 0.0001) (Panel C) and the P(A-a)O2 at peak exercise (ρ = -0.765; P < 0.0001) (Panel D) obtained by cardiopulmonary exercise testing. Correlation was determined by univariate analysis using Spearman's rank correlation (Panels B, C and D), with the exception of the correlation between breathing reserve and relative variations of FVC (logarithmic equation; Panel A).
A significant decline in pulmonary function (a decrease of ≥10% from the initial FVC values or a decrease of ≥15% from the values initially measured in the DLCOsb) was observed in 18 outpatients; a decrease in both FVC and DLCOsb was observed in 17 patients. According to this criterion, the values for the area under the ROC curve identified the following optimal cut-off points: peak VO2 ≤50% of the predicted value; BR max ≥40 breaths/min; breathing reserve ≤40%; P(A-a)O2 ≥22 mmHg, and Δ SpO2 ≥4%. Table 4 shows the baseline CPET results for 42 patients according to the decline in pulmonary function after a 5-year follow-up.
Table 4. Results of cardiopulmonary exercise testing according to the decline in pulmonary function after a 5-year follow-up in thoracic sarcoidosis patients.
| Significant variables* | Decline in pulmonary function+ | |||||
|---|---|---|---|---|---|---|
| Present (N = 18) | Absent (N = 24) | RR | 95%CI | |||
| N | % | N | % | |||
| Peak VO2 ≤50% of predicted | 17 | 94.4 | 2 | 8.3 | 20.6 | 3.01-140.8 |
| BR max ≥40 breaths/min | 16 | 88.9 | 6 | 25.0 | 7.27 | 1.91-27.8 |
| Breathing reserve ≤40% | 17 | 94.4 | 2 | 8.3 | 20.6 | 3.01-140.8 |
| P(A-a)O2 ≥22 mmHg | 17 | 94.4 | 1 | 4.2 | 22.7 | 3.32-154.9 |
| Δ SpO2 ≥4% | 17 | 94.4 | 3 | 12.5 | 18.7 | 2.73-128.0 |
RR = relative risk; 95%CI = 95% confidence interval; peak VO2 = peak oxygen uptake; BR max = maximum respiratory rate; P(A-a)O2 = alveolar-arterial oxygen pressure gradient at peak exercise; Δ SpO2 = difference between peak and resting oxygen saturation. *Significant variables resulting from Spearman's rank correlation between the relative variations of pulmonary function tests and cardiopulmonary exercise testing measures (optimal cut-off points of the area under the ROC curve). +Decrease >10% in relation to the initial values of forced vital capacity or single-breath diffusion of carbon monoxide across the lung.
We also investigated whether outcome measures of the CPET would demonstrate an independent role in predicting the decline of pulmonary function. All variables were assessed in a forward stepwise logistic regression model. In the model, a P(A-a)O2 ≥22 mmHg and a breathing reserve ≤40% were identified as significant and independent variables (Table 5).
Table 5. Forward stepwise regression analysis: relationship between the decline in pulmonary function and study variables.
| Outcome variable | Independent variables | Unstandardized coefficient | P | RR | 95%CI | |
|---|---|---|---|---|---|---|
| B | SE | |||||
| Decline in pulmonary function* | P(A-a)O2 ≥22 mmHg | 4.25 | 1.60 | 0.001 | 70.0 | 3.03-161.3 |
| Breathing reserve ≤40% | 3.03 | 1.63 | 0.014 | 20.8 | 0.85-507.6 | |
SE = standard error; RR = relative risk; 95%CI = 95% confidence interval; P(A-a)O2 = alveolar-arterial oxygen pressure gradient at peak exercise. *Decrease of >10% from the values initially measured in the forced vital capacity or in the single-breath diffusion of carbon monoxide across the lung.
Discussion
This study evaluated the role of CPET as a predictor of the decline in pulmonary function after a 5-year follow-up in a sample of 42 nonsmoking patients with sarcoidosis. To our knowledge, no study has previously conducted a similar evaluation in this patient subset.
A 2-year follow-up period has been considered a standard time to determine the outcome of sarcoidosis (15,16). However, in order to define the clinical phenotypes of the disease, WASOG developed a recent task force using a 5-year follow-up period (17). Compared to the 2-year follow-up outcome, nearly one-quarter of the patients had a different clinical outcome after 5 years. Thus, the 5-year follow-up period is an important methodological characteristic of our study.
The main findings of this research were as follows. First, we observed that FVC and DLCOsb significantly decreased after a 5-year follow-up in patients with thoracic sarcoidosis (P < 0.0001 for both). Second, a spectrum of ventilatory and pulmonary gas exchange abnormalities obtained in the CPET was associated with the reduction in FVC and DLCOsb five years later. In addition, we determined the cut-off of the CPET measures responsible for a significant decline in pulmonary function and demonstrated that P(A-a)O2 and breathing reserve were independent predictors of functional impairment.
Winterbauer and Hutchinson (18) reported a reduction in FVC and DLCOsb in more than two thirds of patients (discordant changes occurred in fewer than 5% of patients). However, in contrast to these authors, we found a higher relative variation of DLCOsb when considering the two study time points. In sarcoidosis, the reduction of DLCOsb may reflect impairments of the gas exchange area, barrier thickness, or ventilation-perfusion-diffusion mismatching of the lung (19). Interestingly, DLCOsb has also been found to be fairly correlated with gas exchange abnormalities during exercise and in particular to be the best predictive and sensitive index of a fall in PaO2 (8).
Exercise testing is a remarkably robust and versatile tool that provides valuable diagnostic and prognostic information regarding patients with pulmonary diseases (20). A low peak VO2 is usually the starting point in the evaluation of reduced exercise capacity. In our study, approximately 90% of patients failed to reach at least 80% of peak VO2 (% predicted) at the initial evaluation. Interestingly, peak VO2 showed a significant correlation with the relative variations of both FVC and DLco (P < 0.0001). However, changes in VO2 can be due to multiple factors, including gas exchange across the lung, the oxygen content of blood, oxygen delivery to tissues, and the oxygen uptake in the tissues (13).
Patients with significant interstitial lung disease often display low breathing reserve and arterial desaturation during exercise. In the present study, these two variables correlated with the decline in pulmonary function at the 5-year follow-up. Because the majority of patients in this sample (66.7%) had radiographic stages 3 and 4 at the initial evaluation may reflect a limitation by ventilatory mechanisms (21). Moreover, ventilatory abnormalities during CPET have been reported in up to 47% of sarcoidosis patients (11,22). The ventilatory stress (demand/capacity) for a given work rate is much greater in patients with interstitial lung disease than in normal subjects. Additionally, it is possible that the exercise limitation in these patients is due to the marked arterial oxygen desaturation, which usually develops during exercise. Arterial hypoxemia can impair exercise tolerance because of inadequate oxygen delivery to working muscles (23).
The persistence of abnormal pulmonary gas exchange is related to a poor prognosis and is an important criterion indicating long-term steroid therapy (2). Eklund et al. (24) showed that diffusion limitation contributed up to 50% of the P(A-a)O2 widening during exercise in sarcoidosis patients; these investigators found only a moderate degree of ventilation-perfusion mismatch. In the present study, we found a significant correlation between P(A-a)O2 and the relative variations of both FVC and DLco (P < 0.0001). At rest, the apical regions of the lung contribute relatively little to ventilation and gas exchange; however, during exercise, apical pulmonary circulation is normally recruited and contributes significantly to the capacity of the lung to increase the rate of gas exchange. Because the lesions of sarcoidosis favor the upper lobes of the lungs, lung function measured during exercise may have a higher sensitivity for detecting the presence and progression of parenchymal disease (25).
Using logistic regression analysis of the independent factors affecting the decline of pulmonary function at the 5-year follow-up, the only predictive factors were P(A-a)O2 ≥22 mmHg and breathing reserve ≤40%, in that order. In fact, the P(A-a)O2 value during exercise has been found to be the best pulmonary gas exchange impairment index in sarcoidosis patients, showing associations with both DLco and FVC (8,12). Kollert et al. (26) performed a retrospective study to determine the extent to which the gas exchange measurement during exercise reflects disease activity and the clinical course of sarcoidosis. They showed that the P(A-a)O2 during exercise represents disease activity and its extent and is associated with a prolonged need for immunosuppressive treatment during follow-up care in patients with pulmonary sarcoidosis. In interstitial lung disease, a smaller pulmonary vascular bed reserve is available for recruitment, limiting the increase in lung surface area for gas exchange during exercise. Hence, both mixed venous oxygen tension and arterial oxygen tension drop with the progressively increasing workload and increased drop of peripheral oxygen uptake (8). Regarding the breathing reserve, patients with more advanced stages of interstitial lung disease often display higher levels of minute ventilation than those observed in normal subjects during exercise. These higher levels are largely due to the increased dead space ventilation; however, other contributing mechanisms responsible for this heightened ventilatory response may include inputs from mechanoreceptors and chemoreceptors and mediators of inflammation (3).
The present investigation has some limitations. First, there was, by definition, a selection bias because the study was performed at a tertiary hospital to which patients with severe complaints and advance stages of sarcoidosis are more likely to be referred. In fact, 32 patients (76.2%) had criteria for the definition of chronic disease. Second, in 17 of 42 patients (40.1%), the VO2θL was low, and the O2 pulse max was abnormal, suggesting cardiocirculatory dysfunction (12). Although some patients may have had cardiac sarcoidosis or pulmonary hypertension, we did not directly determine cardiac circulatory status; thus, this can be considered a limitation of the study. A new CPET in the second evaluation could also have helped to define the cases difficult to interpret. Finally, the conclusion was based on a small number of patients, resulting in high values for relative risk and longer intervals for the 95% confidence interval in the logistic regression model. Carefully controlled prospective studies conducted on a larger sample are required to confirm these preliminary results and to validate our prediction model. In future studies, a number of potential prognostic variables, including clinical and radiological findings, may also be tested along with the CPET variables.
Patients with thoracic sarcoidosis showed significant reductions in FVC and DLCOsb at the 5-year follow-up. Additionally, the outcome measures of the CPET (P(A-a) O2 and breathing reserve) were predictors of a decline in pulmonary function. Determining the CPET measures may be helpful in predicting the outcomes of patients with thoracic sarcoidosis.
References
- 1.Abehsera M, Valeyre D, Grenier P, Jaillet H, Battesti JP, Brauner MW. Sarcoidosis with pulmonary fibrosis: CT patterns and correlation with pulmonary function. AJR Am J Roentgenol. 2000;174:1751–1757. doi: 10.2214/ajr.174.6.1741751. [DOI] [PubMed] [Google Scholar]
- 2.Statement on sarcoidosis. Joint Statement of the American Thoracic Society (ATS), the European Respiratory Society (ERS) and the World Association of Sarcoidosis and Other Granulomatous Disorders (WASOG) adopted by the ATS Board of Directors and by the ERS Executive Committee, February 1999. Am J Respir Crit Care Med. 1999;160:736–755. doi: 10.1164/ajrccm.160.2.ats4-99. [DOI] [PubMed] [Google Scholar]
- 3.Marciniuk DD, Gallagher CG. Clinical exercise testing in interstitial lung disease. Clin Chest Med. 1994;15:287–303. [PubMed] [Google Scholar]
- 4.Weisman IM, Zeballos RJ. Clinical exercise testing. Clin Chest Med. 2001;22:679–701, viii. doi: 10.1016/s0272-5231(05)70060-5. [DOI] [PubMed] [Google Scholar]
- 5.Xaubet A, Rodriguez-Roisin R, Bombi JA, Marin A, Roca J, Augusti-Vidal A. Correlation of bronchoalveolar lavage and clinical and functional findings in asbestosis. Am Rev Respir Dis. 1986;133:848–854. [PubMed] [Google Scholar]
- 6.Scadding JG. Prognosis of intrathoracic sarcoidosis in England. A review of 136 cases after five years' observation. Br Med J. 1961;2:1165–1172. doi: 10.1136/bmj.2.5261.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Standardization of Spirometry American Thoracic Society. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 8.Medinger AE, Khouri S, Rohatgi PK. Sarcoidosis: the value of exercise testing. Chest. 2001;120:93–101. doi: 10.1378/chest.120.1.93. [DOI] [PubMed] [Google Scholar]
- 9.Pereira CA, Sato T, Rodrigues SC. New reference values for forced spirometry in white adults in Brazil. J Bras Pneumol. 2007;33:397–406. doi: 10.1590/s1806-37132007000400008. [DOI] [PubMed] [Google Scholar]
- 10.Neder JA, Andreoni S, Peres C, Nery LE. Reference values for lung function tests. III. Carbon monoxide diffusing capacity (transfer factor) Braz J Med Biol Res. 1999;32:729–737. doi: 10.1590/s0100-879x1999000600008. [DOI] [PubMed] [Google Scholar]
- 11.Sietsema KE, Kraft M, Ginzton L, Sharma OP. Abnormal oxygen uptake responses to exercise in patients with mild pulmonary sarcoidosis. Chest. 1992;102:838–845. doi: 10.1378/chest.102.3.838. [DOI] [PubMed] [Google Scholar]
- 12.Gibbons WJ, Levy RD, Nava S, Malcolm I, Marin JM, Tardif C, et al. Subclinical cardiac dysfunction in sarcoidosis. Chest. 1991;100:44–50. doi: 10.1378/chest.100.1.44. [DOI] [PubMed] [Google Scholar]
- 13.Barros WG, Neder JA, Pereira CA, Nery LE. Clinical, radiographic and functional predictors of pulmonary gas exchange impairment at moderate exercise in patients with sarcoidosis. Respiration. 2004;71:367–373. doi: 10.1159/000079641. [DOI] [PubMed] [Google Scholar]
- 14.Neder JA, Nery LE, Castelo A, Andreoni S, Lerario MC, Sachs A, et al. Prediction of metabolic and cardiopulmonary responses to maximum cycle ergometry: a randomised study. Eur Respir J. 1999;14:1304–1313. doi: 10.1183/09031936.99.14613049. [DOI] [PubMed] [Google Scholar]
- 15.Baughman RP, Shipley R, Eisentrout CE. Predictive value of gallium scan, angiotensin-converting enzyme level, and bronchoalveolar lavage in two-year follow-up of pulmonary sarcoidosis. Lung. 1987;165:371–377. doi: 10.1007/BF02714452. [DOI] [PubMed] [Google Scholar]
- 16.Judson MA, Baughman RP, Thompson BW, Teirstein AS, Terrin ML, Rossman MD, et al. Two year prognosis of sarcoidosis: the ACCESS experience. Sarcoidosis Vasc Diffuse Lung Dis. 2003;20:204–211. [PubMed] [Google Scholar]
- 17.Baughman RP, Nagai S, Balter M, Costabel U, Drent M, du Bois R, et al. Defining the clinical outcome status (COS) in sarcoidosis: results of WASOG Task Force. Sarcoidosis Vasc Diffuse Lung Dis. 2011;28:56–64. [PubMed] [Google Scholar]
- 18.Winterbauer RH, Hutchinson JF. Use of pulmonary function tests in the management of sarcoidosis. Chest. 1980;78:640–647. doi: 10.1378/chest.78.4.640. [DOI] [PubMed] [Google Scholar]
- 19.Lamberto C, Nunes H, Le Toumelin P, Duperron F, Valeyre D, Clerici C. Membrane and capillary blood components of diffusion capacity of the lung for carbon monoxide in pulmonary sarcoidosis: relation to exercise gas exchange. Chest. 2004;125:2061–2068. doi: 10.1378/chest.125.6.2061. [DOI] [PubMed] [Google Scholar]
- 20.Arena R, Sietsema KE. Cardiopulmonary exercise testing in the clinical evaluation of patients with heart and lung disease. Circulation. 2011;123:668–680. doi: 10.1161/CIRCULATIONAHA.109.914788. [DOI] [PubMed] [Google Scholar]
- 21.Baughman RP, Teirstein AS, Judson MA, Rossman MD, Yeager H, Jr, Bresnitz EA, et al. Clinical characteristics of patients in a case control study of sarcoidosis. Am J Respir Crit Care Med. 2001;164:1885–1889. doi: 10.1164/ajrccm.164.10.2104046. [DOI] [PubMed] [Google Scholar]
- 22.Miller A, Brown LK, Sloane MF, Bhuptani A, Teirstein AS. Cardiorespiratory responses to incremental exercise in sarcoidosis patients with normal spirometry. Chest. 1995;107:323–329. doi: 10.1378/chest.107.2.323. [DOI] [PubMed] [Google Scholar]
- 23.Marciniuk DD, Watts RE, Gallagher CG. Dead space loading and exercise limitation in patients with interstitial lung disease. Chest. 1994;105:183–189. doi: 10.1378/chest.105.1.183. [DOI] [PubMed] [Google Scholar]
- 24.Eklund A, Broman L, Broman M, Holmgren A. V/Q and alveolar gas exchange in pulmonary sarcoidosis. Eur Respir J. 1989;2:135–144. [PubMed] [Google Scholar]
- 25.Arcasoy SM, Christie JD, Pochettino A, Rosengard BR, Blumenthal NP, Bavaria JE, et al. Characteristics and outcomes of patients with sarcoidosis listed for lung transplantation. Chest. 2001;120:873–880. doi: 10.1378/chest.120.3.873. [DOI] [PubMed] [Google Scholar]
- 26.Kollert F, Geck B, Suchy R, Jorres RA, Arzt M, Heidinger D, et al. The impact of gas exchange measurement during exercise in pulmonary sarcoidosis. Respir Med. 2011;105:122–129. doi: 10.1016/j.rmed.2010.09.007. [DOI] [PubMed] [Google Scholar]