Abstract
Agmatine, an endogenous polyamine and putative neuromodulator, is known to have neuroprotective effects on various neurons in the central nervous system. We determined whether or not topically administered agmatine could reduce ischemic retinal injury. Transient ocular ischemia was achieved by intraluminal occlusion of the middle cerebral artery of ddY mice (30-35 g) for 2 h, which is known to also induce occlusion of the ophthalmic artery. In the agmatine group (N = 6), a 1.0 mM agmatine-containing ophthalmic solution was administered four times daily for 2 weeks before occlusion. In the control group (N = 6), a 0.1% hyaluronic acid ophthalmic solution was instilled at the same times. At 22 h after reperfusion, the eyeballs were enucleated and the retinal sections were stained by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL). Transient ocular ischemia induced apoptosis of retinal cells in the entire retinal layer, and topically administered agmatine can significantly reduce this ischemic retinal injury. The proportion of apoptotic cells was definitely decreased (P < 0.001; Kruskal-Wallis test). Overall, we determined that topical agmatine application effectively decreases retinal damage in an in vivo ocular ischemic injury model. This implies that agmatine is a good candidate as a direct neuroprotective agent for eyes with ocular ischemic diseases.
Keywords: Agmatine, Apoptosis, Ischemia, Neuroprotection, Retina
Introduction
Agmatine is an endogenous aminoguanidine compound formed by the decarboxylation of L-arginine, and has been reported to be a putative neuromodulator in neurons of the central nervous system (CNS) (1,2). Although agmatine was initially described as a ligand for imidazoline receptors (3), it is now recognized to act as an agonist on imidazoline I and α2-adrenergic receptors, as an antagonist on N-methyl-D-aspartic acid (NMDA) receptors, and as an inhibitor of neuronal/inducible nitric oxide synthases (NOSs) (1,2).
A large body of experimental evidence has demonstrated the neuroprotective effects of agmatine on various noxious neuronal injuries (4-6). Given the suggestions that agmatine can reduce neuronal loss after cerebral/spinal cord ischemia (5,6), we confirmed its protective effects on hypoxia-induced apoptosis of RGC-5 cells in vitro (7). Although we also revealed that topically administered agmatine rescues retinal ganglion cells (RGCs) in the eyes of chronic ocular hypertensive rats (8), the in vivo data providing evidence for the neuroprotective effects of agmatine are not yet sufficient.
Theoretically, agmatine is a good neuroprotective drug candidate because of its high binding affinity for α2-adrenergic and NMDA receptors. Although various neuroprotective agents, including α2-adrenergic receptor agonists and NMDA receptor antagonists, have been widely studied, a novel candidate drug is not yet available (9). If we can prove the neuroprotective effects of agmatine and identify its mechanisms of action, agmatine might provide new therapeutic strategies as a neuroprotective drug.
In the present report, we determined whether or not topically administered agmatine could reduce retinal injury induced by transient ocular ischemia using intraluminal occlusion of the ophthalmic artery.
Material and Methods
Animals and topical agmatine administration
A total of 12 adult male ddY mice (7 weeks old, 30 to 35 g) were used for this study. The animals were maintained under controlled conditions with a 12:12-h light/dark cycle and standard food and water provided ad libitum. They were treated in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research after obtaining permission of the Institutional Animal Care and Use Committee. Every effort was made to minimize the number of animals sacrificed and their suffering throughout our experiments.
An agmatine-containing ophthalmic solution (1.0 mM; Sigma-Aldrich, USA) was formulated as previously described (8). Mice in the agmatine group (N = 6) received agmatine eye drops in both eyes four times daily for 2 consecutive weeks. Mice in the control group (N = 6) received a 0.1% hyaluronic acid ophthalmic solution (Santen Pharmaceutical Co., Japan) in both eyes at the same times.
Transient ocular ischemia caused by ophthalmic artery occlusion
Intraluminal occlusion of the middle cerebral artery (MCA) was induced as previously described (10,11) using a silicone rubber-coated monofilament (Doccol Co., USA). Briefly, after the mice were anesthetized with an intraperitoneal injection of a mixture of zolazepam/tiletamine (80 mg/kg; Zoletil 50®, Virbac, France) and xylazine (20 mg/ kg; Rompun®, Bayer HealthCare, Germany), the filament was introduced into the left internal carotid artery through an arteriotomy in the left common carotid artery. The filament was advanced up to the origin of the anterior cerebral artery and caused occlusion of the ophthalmic artery as well as the MCA (12).
After 2 h of occlusion, the animals were re-anesthetized and the filament was withdrawn to permit restoration of blood flow. After an additional 22 h, the mice were perfused with 4% paraformaldehyde and their eyeballs were enucleated.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)
The enucleated eyeballs were immersed overnight in 4% paraformaldehyde at 4°C and embedded in paraffin; 4-µm sections were cut parallel to the maximum circumference of the eyeball through the optic disc. Apoptotic cells were identified using TUNEL (DeadEnd™ Fluorometric TUNEL System; Promega, USA), and fluorescein isothyocyanate (FITC)-12-dUTP-labeled fragmented DNA was directly visualized using a fluorescence microscope. Nuclear counterstaining was performed using 4′,6-diamidino-2-phenylindole (DAPI). Four sections were selected from each eyeball and areas within a field between 375 and 625 µM from the optic disc were evaluated.
Results
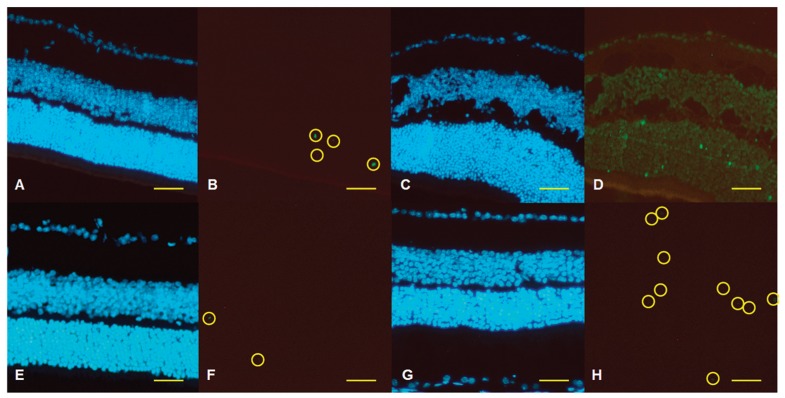
Representative retinal sections of the TUNEL are shown in Figure 1. Mice in the control group were treated with a 0.1% hyaluronic acid ophthalmic solution (vehicle) in both eyes four times daily for 2 consecutive weeks before occlusion of their left MCA. Their right eyes were defined as the ‘no treatment control’ group (Figure 1A,B) and their left eyes were defined as the ‘ocular ischemic injury only’ group (Figure 1C,D). Mice in the agmatine group received agmatine eye drops in both eyes four times daily for 2 consecutive weeks before occlusion of their left ophthalmic artery. Their right eyes were defined as the ‘agmatine treatment only’ group (Figure 1E,F) and their left eyes were defined as the ‘ocular ischemic injury after agmatine treatment’ group (Figure 1G,H).
Figure 1. Representative retinal sections of terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL), in which the fluorescein isothyocyanate (FITC)-12-dUTP-labeled fragmented DNA and nuclear counterstaining were performed using 4′,6-diamidino-2-phenylindole (DAPI). A, B, No treatment control; C, D, ocular ischemic injury only; E, F, agmatine treatment only; G, H, ocular ischemic injury after agmatine treatment. A, C, E, G, Blue fluorescence indicates DAPI; B, D, F, H, green fluorescence indicates FITC. The TUNEL-positive cells are emphasized as yellow circles in Panels B, F, and H, but all cells are TUNEL-positive in Panel D. Scale bar = 100 µM.
In the retinas of the control group, a few TUNEL-positive apoptotic cells were noticed in the outer nuclear layer (Figure 1B). Transient ocular ischemia definitely induced extensive retinal damage (Figure 1D); every single cell in the whole retinal layer was TUNEL-positive and large vacuolations were found in the inner nuclear and plexiform layers. However, topically administered agmatine dramatically reduced the proportion of apoptotic cells (P < 0.001; Kruskal-Wallis test) and improved the retinal integrity (Figure 1H).
Discussion
In this investigation, we demonstrated that topically administered agmatine effectively rescues retinal cells from ischemic damage. Although many researchers have used an acute ischemia-reperfusion injury model caused by a transient elevation in intraocular pressure (13-15), this animal model has the mixed pathophysiology of pressure-dependent and pressure-independent mechanisms. Retrobulbar/intravitreal injection of endothelin-1 has also been reported to completely obstruct retinal vessels (16,17); however, this is a very expensive method and it is hard to control the duration of occlusion, especially in small rodents (18). Intraluminal MCA occlusion also causes transient/permanent occlusion of the ophthalmic artery (12). While intraluminal MCA occlusion requires a skilled technician, it can precisely control the time of occlusion. Thus, transient intraluminal occlusion of the MCA can serve as a useful experimental model that mimics ocular ischemiareperfusion injury.
Agmatine is a putative neuromodulator in CNS neurons (1,2) and is known to act as an agonist on imidazoline I and α2-adrenergic receptors, as an antagonist on NMDA receptors, and as an inhibitor of neuronal/inducible NOSs (1,2). Recently, agmatine has received considerable attention due to its neuroprotective effects on various neuronal injuries (4-6). Regarding ocular tissue, we demonstrated that agmatine protects damaged hypoxic RGC-5 cells (7) and that topically administered agmatine rescues RGCs in chronic ocular hypertensive rat eyes (8). In the present report, we investigated whether topically administered agmatine could reduce ischemic retinal injury. In a previous investigation, high concentrations of agmatine (0.1 M) were presumed to have caused corneal toxicity, while 1.0 mM agmatine exhibited good ocular hypotensive effects (8). As such, in this study we also used 1.0 mM agmatine eye drops. In addition, since we have some evidence of the neuroprotective effects of agmatine pretreatment (19,20), we decided to start agmatine treatment before inducing ischemic injury.
Our results confirm that topically administered agmatine can significantly reduce ischemia-reperfusion retinal injury in a mouse model of transient ocular ischemia. Although the precise mechanisms of agmatine in this retinal protective process have not been well established, agmatine is a good potential candidate as a direct neuroprotective agent for eyes with ocular ischemic diseases, including glaucoma.
Acknowledgments
Research supported by a Faculty Research Grant from the Yonsei University College of Medicine, Seoul, Republic of Korea (#6-2010-0046).
References
- 1.Reis DJ, Regunathan S. Is agmatine a novel neurotransmitter in brain? Trends Pharmacol Sci. 2000;21:187–193. doi: 10.1016/s0165-6147(00)01460-7. [DOI] [PubMed] [Google Scholar]
- 2.Halaris A, Plietz J. Agmatine: metabolic pathway and spectrum of activity in brain. CNS Drugs. 2007;21:885–900. doi: 10.2165/00023210-200721110-00002. [DOI] [PubMed] [Google Scholar]
- 3.Li G, Regunathan S, Barrow CJ, Eshraghi J, Cooper R, Reis DJ. Agmatine: an endogenous clonidine-displacing substance in the brain. Science. 1994;263:966–969. doi: 10.1126/science.7906055. [DOI] [PubMed] [Google Scholar]
- 4.Gilad GM, Salame K, Rabey JM, Gilad VH. Agmatine treatment is neuroprotective in rodent brain injury models. Life Sci. 1996;58:L–6. doi: 10.1016/0024-3205(95)02274-0. [DOI] [PubMed] [Google Scholar]
- 5.Gilad GM, Gilad VH. Accelerated functional recovery and neuroprotection by agmatine after spinal cord ischemia in rats. Neurosci Lett. 2000;296:97–100. doi: 10.1016/s0304-3940(00)01625-6. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, Yenari MA, Giffard RG, Cho SW, Park KA, Lee JE. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp Neurol. 2004;189:122–130. doi: 10.1016/j.expneurol.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 7.Hong S, Lee JE, Kim CY, Seong GJ. Agmatine protects retinal ganglion cells from hypoxia-induced apoptosis in transformed rat retinal ganglion cell line. BMC Neurosci. 2007;8:81. doi: 10.1186/1471-2202-8-81. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Hong S, Kim CY, Lee WS, Shim J, Yeom HY, Seong GJ. Ocular hypotensive effects of topically administered agmatine in a chronic ocular hypertensive rat model. Exp Eye Res. 2010;90:97–103. doi: 10.1016/j.exer.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 9.Cheung W, Guo L, Cordeiro MF. Neuroprotection in glaucoma: drug-based approaches. Optom Vis Sci. 2008;85:406–416. doi: 10.1097/OPX.0b013e31817841e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hara H, Huang PL, Panahian N, Fishman MC, Moskowitz MA. Reduced brain edema and infarction volume in mice lacking the neuronal isoform of nitric oxide synthase after transient MCA occlusion. J Cereb Blood Flow Metab. 1996;16:605–611. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Hyakkoku K, Hamanaka J, Tsuruma K, Shimazawa M, Hara H. Proteomic approach with LCMS-IT-TOF identified an increase of Rab33B after transient focal cerebral ischemia in mice. Exp Transl Stroke Med. 2010;2:20. doi: 10.1186/2040-7378-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steele EC, Jr, Guo Q, Namura S. Filamentous middle cerebral artery occlusion causes ischemic damage to the retina in mice. Stroke. 2008;39:2099–2104. doi: 10.1161/STROKEAHA.107.504357. [DOI] [PubMed] [Google Scholar]
- 13.Ju WK, Lindsey JD, Angert M, Patel A, Weinreb RN. Glutamate receptor activation triggers OPA1 release and induces apoptotic cell death in ischemic rat retina. Mol Vis. 2008;14:2629–2638. [PMC free article] [PubMed] [Google Scholar]
- 14.Russo R, Cavaliere F, Berliocchi L, Nucci C, Gliozzi M, Mazzei C, et al. Modulation of pro-survival and deathassociated pathways under retinal ischemia/reperfusion: effects of NMDA receptor blockade. J Neurochem. 2008;107:1347–1357. doi: 10.1111/j.1471-4159.2008.05694.x. [DOI] [PubMed] [Google Scholar]
- 15.Sakamoto K, Ohki K, Saito M, Nakahara T, Ishii K. Histological protection by donepezil against neurodegeneration induced by ischemia-reperfusion in the rat retina. J Pharmacol Sci. 2010;112:327–335. doi: 10.1254/jphs.09302fp. [DOI] [PubMed] [Google Scholar]
- 16.Takei K, Sato T, Nonoyama T, Miyauchi T, Goto K, Hommura S. A new model of transient complete obstruction of retinal vessels induced by endothelin-1 injection into the posterior vitreous body in rabbits. Graefes Arch Clin Exp Ophthalmol. 1993;231:476–481. doi: 10.1007/BF02044235. [DOI] [PubMed] [Google Scholar]
- 17.Brooks DE, Kallberg ME, Cannon RL, Komaromy AM, Ollivier FJ, Malakhova OE, et al. Functional and structural analysis of the visual system in the rhesus monkey model of optic nerve head ischemia. Invest Ophthalmol Vis Sci. 2004;45:1830–1840. doi: 10.1167/iovs.03-0950. [DOI] [PubMed] [Google Scholar]
- 18.Masuzawa K, Jesmin S, Maeda S, Kaji Y, Oshika T, Zaedi S, et al. A model of retinal ischemia-reperfusion injury in rats by subconjunctival injection of endothelin-1. Exp Biol Med. 2006;231:1085–1089. [PubMed] [Google Scholar]
- 19.Iizuka Y, Hong S, Kim CY, Kim SK, Seong GJ. Agmatine pretreatment protects retinal ganglion cells (RGC-5 cell line) from oxidative stress in vitro. Biocell. 2008;32:245–250. [PubMed] [Google Scholar]
- 20.Iizuka Y, Hong S, Kim CY, Yang WI, Lee JE, Seong GJ. Protective mechanism of agmatine pretreatment on RGC-5 cells injured by oxidative stress. Braz J Med Biol Res. 2010;43:356–358. doi: 10.1590/S0100-879X2010007500018. [DOI] [PubMed] [Google Scholar]