Abstract
Apatone™, a combination of menadione (2-methyl-1,4-naphthoquinone, VK3) and ascorbic acid (vitamin C, VC) is a new strategy for cancer treatment. Part of its effect on tumor cells is related to the cellular pro-oxidative imbalance provoked by the generation of hydrogen peroxide (H2O2) through naphthoquinone redox cycling. In this study, we attempted to find new naphthoquinone derivatives that would increase the efficiency of H2O2 production, thereby potentially increasing its efficacy for cancer treatment. The presence of an electron-withdrawing group in the naphthoquinone moiety had a direct effect on the efficiency of H2O2 production. The compound 2-bromo-1,4-naphthoquinone (BrQ), in which the bromine atom substituted the methyl group in VK3, was approximately 10- and 19-fold more efficient than VK3 in terms of oxygen consumption and H2O2 production, respectively. The ratio [H2O2]produced / [naphthoquinone]consumed was 68 ± 11 and 5.8 ± 0.2 (µM/µM) for BrQ and VK3, respectively, indicating a higher efficacy of BrQ as a catalyst for the autoxidation of ascorbic acid. Both VK3 and BrQ reacted with glutathione (GSH), but BrQ was the more effective substrate. Part of GSH was incorporated into the naphthoquinone, producing a nucleophilic substitution product (Q-SG). The depletion of BrQ by GSH did not prevent its redox capacity since Q-SG was also able to catalyze the production of reactive oxygen species. VK3/VC has already been submitted to clinical trials for the treatment of prostate cancer and has demonstrated promising results. However, replacement of VK3 with BrQ will open new lines of investigation regarding this approach to cancer treatment.
Keywords: Cancer; Apatone™; Ascorbic acid; Hydrogen peroxide; 2-Bromo-1,4-naphthoquinone
Introduction
The combination of ascorbic acid (vitamin C, VC) and menadione (2-methyl-1,4-naphthoquinone, vitamin K3, VK3) has been widely explored in several studies in vitro (1-5) and in vivo (5-8) as a new therapy against cancer in which the use of both vitamins demonstrated a synergistic action compared to the administration of either vitamin alone (1,9). The United States Patent and Trademark Office (USPTO) approved a patent and assigned the trademark name Apatone™ (Indian Creek Medical Technologies) with serial number 78475364 to this compound. Apatone™ is characterized by the administration of a combination of VC and VK3 to target and kill cancer cells. Apatone™ was used in end-stage prostate cancer patients who were not responding to conventional therapy. The treatment was considered safe and effective since, of the 15 patients who continued Apatone™ for more than 12 weeks, only 1 died after 14 months of treatment (10).
The antitumor activity of the VC/VK3 combination has been associated with the generation of reactive oxygen species (ROS), particularly hydrogen peroxide (H2O2) (3). This reaction occurs when VK3 is non-enzymatically reduced by ascorbate to form the VK3 equivalent semiquinone free radical and dehydroascorbate. The transient semiquinone free radical is reoxidized to its quinone form by molecular oxygen, thus generating ROS such as superoxide radical anion (O2−•), H2O2 and hydroxyl radicals (•OH) (3). One characteristic of tumor cells seems to be directly related to their susceptibility to VC/VK3 therapy. This includes the reduced level of ROS detoxifying enzymes such as catalase, superoxide dismutase (SOD) and glutathione (GSH) peroxidase (11,12), which elicits a redox imbalance. In this regard, the VC/VK3 combination provokes an additional oxidative stress characterized by decreased intracellular thiol levels, increased intracellular Ca2+ levels and lipid peroxidation in cells already deficient in their intrinsic antioxidant protection (13). The VC/VK3 combination also inhibits the glycolytic pathway (14), which is the primary mechanism responsible for respiration in cancer cells, known as “The Warburg Effect” (15). Additionally, VC accumulates in tumor cells through isoforms of the glucose transporter (GLUT) family, i.e, GLUT1, 3 and 4 (16,17). An important aspect of the signaling pathway involved in the cytotoxicity of VC/VK3 is that, besides necrosis and apoptosis, the antitumor activity of this vitamin combination has been attributed to a new type of cell death first observed in 1993 (18) and named autoschizis in 1998 (19). In contrast to apoptosis, the activation of caspase 3 is not verified in autoschizis (20). Autoschizis is also characterized by the excision of cytoplasmic fragments (7,9,21).
Since a pro-oxidative imbalance in tumor cells provoked by incubation with VC/VK3 seems to underlie the pharmacological mechanism for cancer treatment with this drug combination, we studied and compared the efficiency of different VK3-related compounds, searching for higher efficacy in the production of H2O2 when these molecules catalyze the autoxidation of ascorbic acid.
Material and Methods
Chemicals
Ascorbic acid, VK3, 2-bromo-1,4-naphthoquinone (BrQ), 2-methoxy-1,4-naphthoquinone (MQ), SOD, catalase, GSH, oxidized glutathione (GSSG), N-ethylmaleimide (NEM), cytochrome C, ortho-phthalaldehyde (OPA), and reduced nicotinamide adenine dinucleotide (NADH) were purchased from Sigma-Aldrich Chemical Co. (USA). H2O2 was prepared by diluting a 30% stock solution and calculating the concentration from the absorption of the solution at 240 nm (λ = 43.6 M−1 cm−1). All reagents used for solutions, buffers, and mobile phases were of analytical grade.
Measurement of oxygen consumption
The consumption of dissolved molecular oxygen during the redox cycling reactions was monitored using a YSI 5300A Oxygen Monitor (USA). Unless otherwise stated, the reaction mixture contained 500 µM ascorbic acid and 10 µM naphthoquinones (BrQ, VK3, or MQ) in 10 mM phosphate-buffered saline (PBS), pH 7.4, at 37°C. Before the beginning of the reaction, the opened reaction cuvette containing PBS and naphtoquinones was equilibrated to reach air saturation at 37°C. Then, the electrode was inserted and the reaction triggered by adding ascorbic acid.
Measurement of H2O2 production
The production of H2O2 during the redox cycling reaction was monitored using an amperometric detector and a biosensor specific for H2O2 (TRB 4100, World Precision Instruments, USA). Initially, the instrument was calibrated using H2O2 standards ranging from 10 to 200 µM and an analytical curve was constructed to convert ΔpA to the H2O2 concentration. Unless otherwise stated, the reaction mixture contained 500 µM ascorbic acid and 10 µM naphthoquinones (BrQ, VK3 or MQ) in 10 mM PBS, pH 7.4, at 37°C.
Measurement of naphthoquinone consumption
For the measurement of naphthoquinone consumption during the redox cycling, the reaction mixtures consisting of 10 µM naphthoquinones (BrQ or VK3) and 500 µM ascorbic acid in 10 mM PBS, pH 7.4, at 37°C were incubated for 15 min and the remaining concentration was measured by HPLC coupled to a diode array detector set at 265 nm (Jasco, USA). The analyses were conducted isocratically on a Luna C18 reversed-phase column (250 × 4.6 mm, 5 µm) with a mobile phase consisting of 0.1% formic acid:methanol (40:60, v/v). The flow rate was 1.0 mL/min.
Measurement of GSH consumption and GSSG production
The effect of GSH on the redox cycling reaction was studied in terms of the production of ROS, depletion of GSH and production of GSSG using OPA as a fluorescence derivatization reagent and HPLC analysis, as previously described (22) and as explained below.
GSH assay. After incubation of the reaction mixture consisting of naphthoquinones (BrQ or VK3) and GSH in the presence or absence of ascorbic acid, a 50-µL aliquot was removed and added to 500 µL 0.1% EDTA in 0.1 M Na2HPO4, pH 8.0. A 300-µL amount of 0.1% EDTA in 0.1 M Na2HPO4 and 20 µL 0.1% OPA in methanol were added to a 20-µL aliquot of this mixture. This final mixture was incubated at 25°C for 15 min in the absence of light and injected (20 µL) into the HPLC system.
GSSG assay. Next, 200 µL of the same reaction mixture as used for analysis of GSH was incubated at 25°C with 200 µL NEM for 25 min in the absence of light to interact with the remaining GSH present in the sample. A 750-µL amount of 0.1 M NaOH was added to this mixture and an aliquot of 20 µL was removed and added to 300 µL 0.1 M NaOH and 20 µL 0.1% OPA in methanol. This final mixture was incubated at 25°C for 15 min in the absence of light and injected (20 µL) into the HPLC system.
HPLC method. The analyses were carried out on a Luna C18 reverse-phase column (250 × 4.6 mm, 5 µm) using an HPLC-fluorescence detection system (Jasco). The mobile phase consisted of 15% methanol in 25 mM NaH2PO4 (v/v), pH 6.0. The flow rate was maintained at 1.0 mL/min. The detection of the fluorescent derivative was observed at 350 nm excitation and 420 nm emission. Standard curves were constructed to calculate the consumption of GSH and formation of GSSG (data not shown).
Identification of the GSH-conjugated product
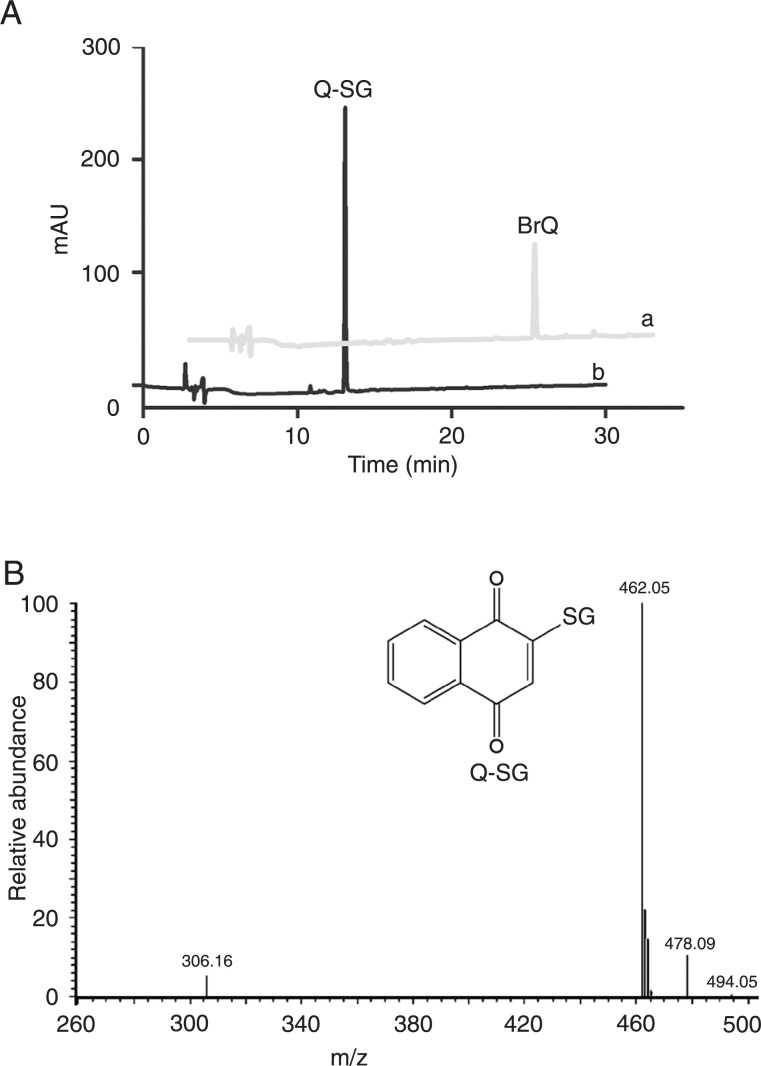
The identification of the product obtained when BrQ was incubated with GSH (100 µM) was performed by direct injection of the previously HPLC-purified product into the mass spectrometer operating in negative ion mode (LCQ Fleet Ion Trap Mass spectrometer, Thermo Scientific, USA). The negative molecular ion peak [M - 1] = 462.05 corresponded to the product that was generated by the substitution of the bromine atom at position 2 of the naphthoquinone moiety with GSH, leading to Q-SG (MW 463.09). An additional confirmation of this product was the fragment observed at 306.16, which corresponded to GS-. The reaction conditions were 10 µM BrQ and 100 µM GSH for 3 min in PBS at 37°C.
Analysis of superoxide production
The generation of a superoxide anion during the redox cycling was measured by adding 100 µM cytochrome c to the reaction mixture containing 10 µM naphthoquinones and 50 µM VC in PBS, pH 7.4. The blank for absorbance measurements consisted of cytochrome c and BrQ in PBS. An HP8452 diode array spectrophotometer (Agilent, USA) was used to measure absorbance. The reaction was initiated by adding VC and then scanned at 30-s intervals. A sharp peak at 550 nm is characteristic of reduced cytochrome c generated by a superoxide anion.
Measurement of the effect of NADH on ascorbic acid consumption
The effect of NADH on the redox cycling reaction was studied by measuring the rate of consumption of ascorbic acid in the presence or absence of this reduced coenzyme. The reaction mixture contained 100 µM NADH, 10 µM naphthoquinones (BrQ or VK3) and 50 µM VC in PBS, pH 7.4. The blank for absorbance measurements contained PBS and naphthoquinone. An HP8452 diode array spectrophotometer (Agilent) was used to measure the variation in absorbance. The reaction was initiated by adding VC and then scanned at 30-s intervals. The band centered at 270 nm was chosen to monitor ascorbic acid consumption and the band centered at 340 nm was followed to monitor the oxidation of NADH.
Results
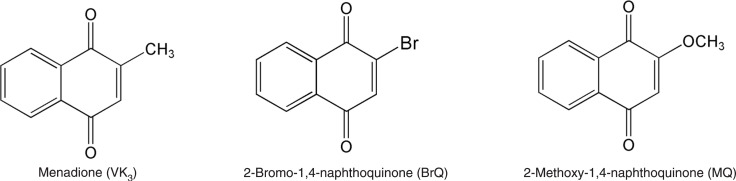
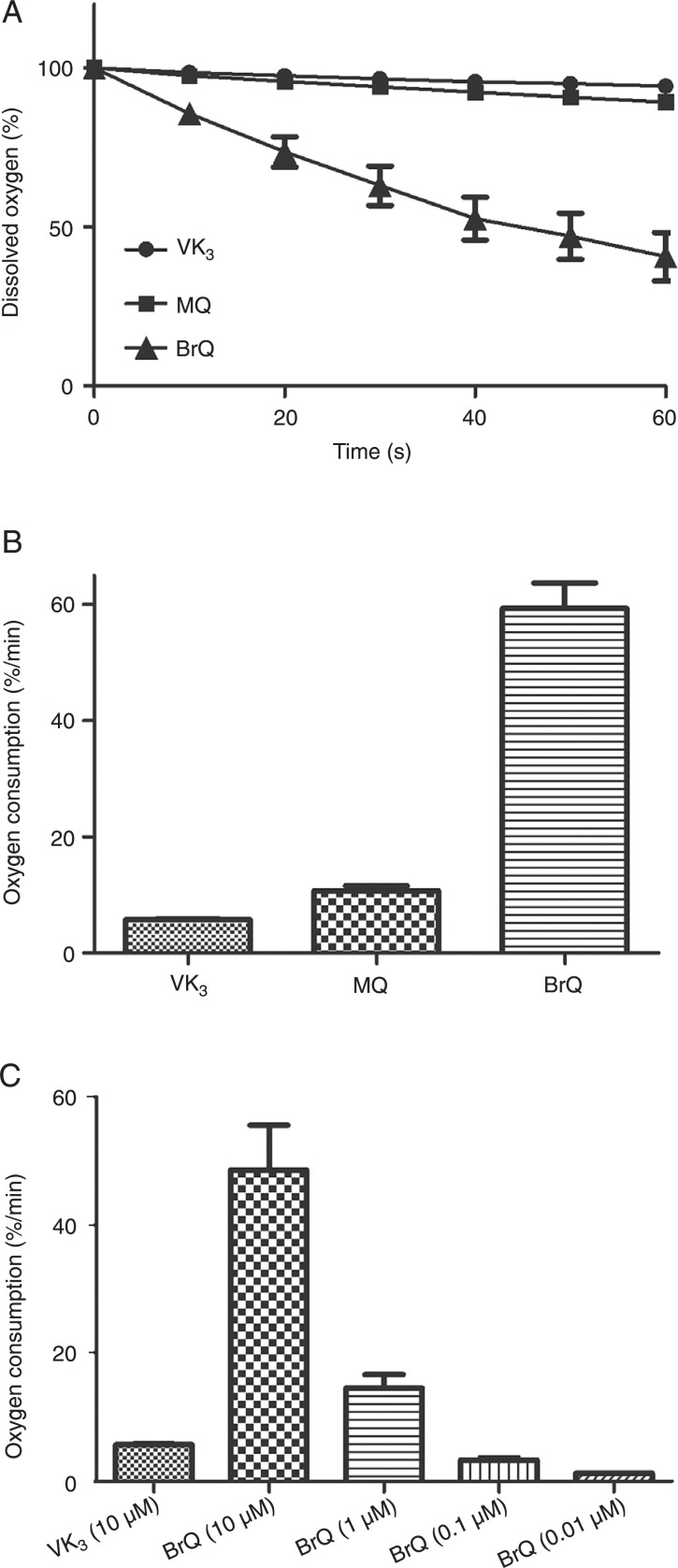
We studied the capacity of naphthoquinone derivatives in terms of their efficacy as generators of H2O2 when combined with ascorbic acid. The naphthoquinone moiety is an important pharmacophoric element for cytotoxic activity (23) and the reduction potential of the aromatic compounds is sensitive to the presence of substituent groups in the ring (24). Thus, we compared the redox properties of menadione with BrQ and MQ (Figure 1). These compounds were chosen because bromine is an electron-withdrawing atom and, conversely, methoxy is an electron-donating group stronger than the methyl group present in the parent molecule (24). Hence, we were able to analyze both electronic effects on the redox cycling of the naphthoquinone moiety driven by ascorbic acid. The concentration of ascorbic acid (500 µM) and naphthoquinones (10 µM) were chosen to confirm the catalytic effect of the latter compound and are of the same proportions of compounds used in Apatone™ (10). Figure 2A shows that the presence of an electron-withdrawing group in the naphthoquinone moiety provoked a significant increase in the rate of oxygen consumption compared to menadione or to the methoxy derivative. As observed, approximately 75% of the dissolved molecular oxygen was depleted within 1 min of reaction using BrQ, compared to only 5% using VK3 or 7% MQ. In these experiments, the reaction medium was previously equilibrated at 37°C. Hence, the relative concentration of 100% is equivalent to approximately 200 µM of dissolved oxygen in PBS. Therefore, considering that only 10 µM BrQ was added to the reaction mixture, this is evidence of its efficient recycling during the reaction course. Figure 2B depicts the comparison the relative initial rate of oxygen depletion measured in the linear phase of the reaction. Considering that the higher efficiency of BrQ as a redox cycling agent could offer a therapeutic advantage since it could be applied in a lower-dose regimen, we tried to find the lowest concentration of BrQ that was as efficient as VK3. Figure 2C shows that using about one-hundredth the concentration of BrQ, the rate of oxygen depletion was still comparable to that obtained with VK3.
Figure 1. Molecular structures of the naphthoquinones.
Figure 2. Consumption of dissolved molecular oxygen. A, Kinetic profiles of oxygen depletion. B, Rate of depletion (% oxygen/min). The reaction mixtures consisted of naphthoquinones (10 µM VK3, MQ or BrQ) and vitamin C (500 µM) in PBS, pH 7.4, at 37°C. C, Effect of the concentration of BrQ on oxygen depletion. The results are representative of at least three different experiments. VK3 = 2-methyl-1,4-naphthoquinone; MQ = 2-methoxy-1,4-naphthoquinone; BrQ = 2-bromo-1,4-naphthoquinone.
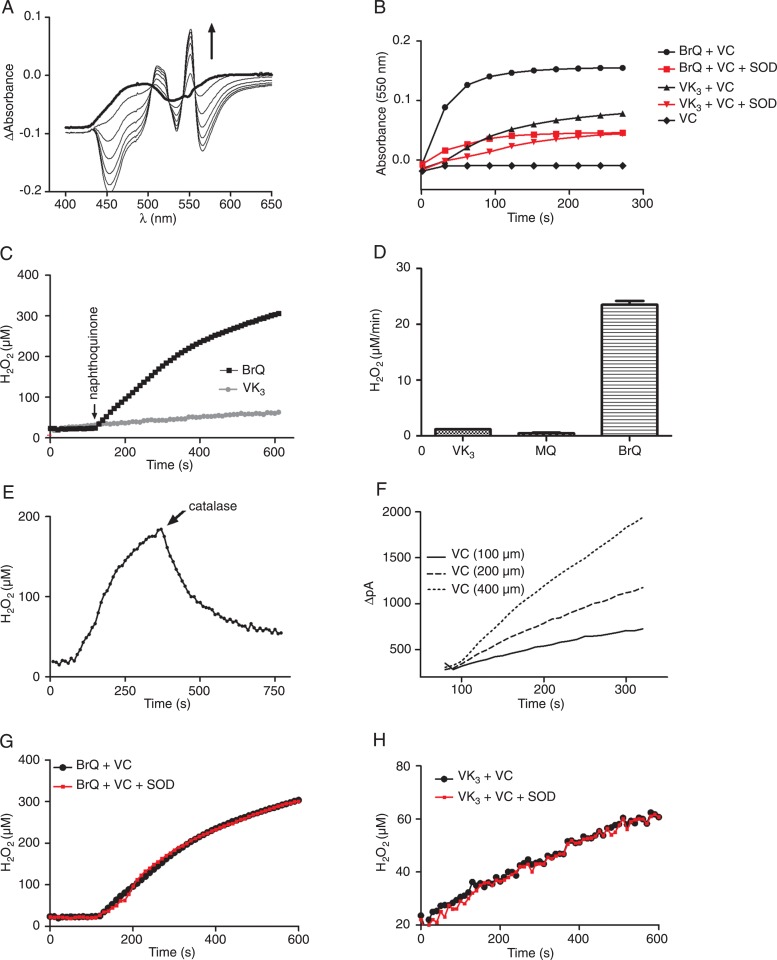
The superoxide anion generated by the redox cycling of the naphthoquinone was transient, being promptly dismuted to H2O2. The evidence of a peak at 550 nm when cytochrome c was added to the reaction medium was a confirmation of the production of superoxide anion (Figure 3A and B). Here we followed the production of H2O2 using a specific electrode and an amperometric detector, which was previously calibrated with standard solutions of H2O2. The results displayed in Figure 3C and D confirmed the higher catalytic effect of BrQ compared to VK3 and MQ. The addition of catalase during the course of the reaction confirmed the formation of H2O2 (Figure 3E).
Figure 3. A, Detection of the formation of transient superoxide anion during the redox cycling reaction (VC/BrQ) by the reduction of cytochrome c (100 µM). Arrow shows the direction of absorbance increase as the reaction proceeds. B, Effect of SOD (3 mg/mL) on the production of transient superoxide anion. C, Kinetic profile of H2O2 production during the redox cycling reactions. D, Rate of H2O2 production. E, Evidence of the formation of H2O2 (VC/BrQ, catalase 3 mg/mL). F, Effect of ascorbic acid on the rate of H2O2 production in the reaction with BrQ. G, Effect of SOD (3 mg/mL) on the H2O2 production (VC/BrQ). H, Effect of SOD on H2O2 production (VC/VK3). The reaction mixtures consisted of naphthoquinones (10 µM) and VC (500 µM) in PBS, pH 7.4, at 37°C. The results are representative of at least three different experiments. VC = vitamin C; BrQ = bromoquinone; SOD = superoxide dismutase; VK3 = 2-methyl-1,4-naphthoquinone; MQ = 2-methoxy-1,4-naphthoquinone.
We used the rate of H2O2 production as an experimental approach to study the effect of the reactants in the redox cycling reaction. As depicted in Figure 3F, the rate of H2O2 production was dependent on the concentration of ascorbic acid. On the other hand, the addition of SOD, which increased the rate of H2O2 production by catalyzing the dismutation of the superoxide anion, did not provoke any alteration (Figure 3G and H). Taken together, these experiments demonstrated that the rate-determining step in the redox cycling reaction is the interaction between ascorbic acid and BrQ.
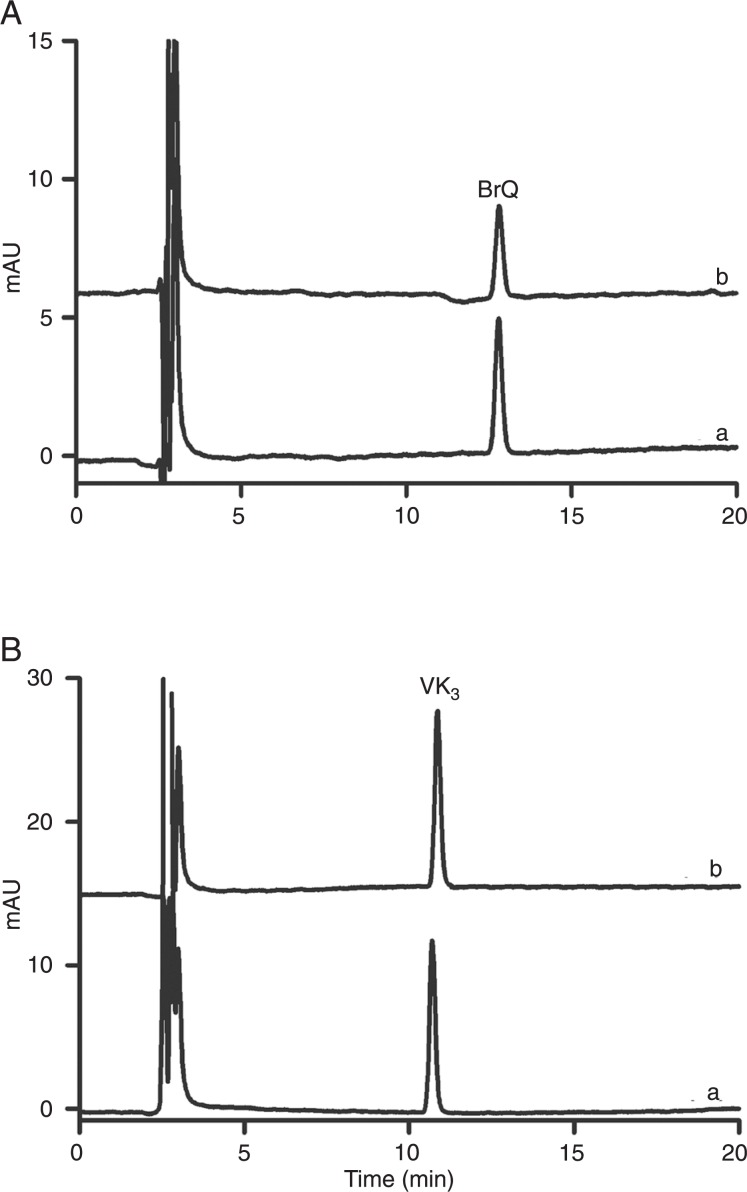
From another point of view, the combination of ascorbic acid and naphthoquinone is a way to exacerbate the autoxidation of ascorbic acid. In other words, naphthoquinone acts as a catalyst for these reactions (3). However, part of the naphthoquinone can be transformed to other products, which, consequently, decreases its capacity for ROS production. For this reason, we measured the consumption of naphthoquinones during the reaction course. We noted that VK3 was consumed less than BrQ (Figure 4). However, considering the production of H2O2 within the same time interval, the ratio [H2O2] µM / minproduced / [naphthoquinone]. µM / minconsumed was 68 ± 11 and 5.8 ± 0.2 for BrQ and VK3, respectively, which revealed the higher efficacy of BrQ as a catalyst for the autoxidation of ascorbic acid.
Figure 4. Consumption of the naphthoquinones during the redox cycling reaction. Aa, Pure BrQ (10 µM); Ab, VC/BrQ reaction. Ba, Pure VK3 (10 µM); Bb, VK3/VC reaction. The reaction mixtures consisted of naphthoquinones (10 µM) and VC (500 µM) in PBS at 37°C and the remaining concentration of naphthoquinones measured after 15 min. BrQ = bromoquinone; VC = vitamin C; VK3 = 2-methyl-1,4-naphthoquinone.
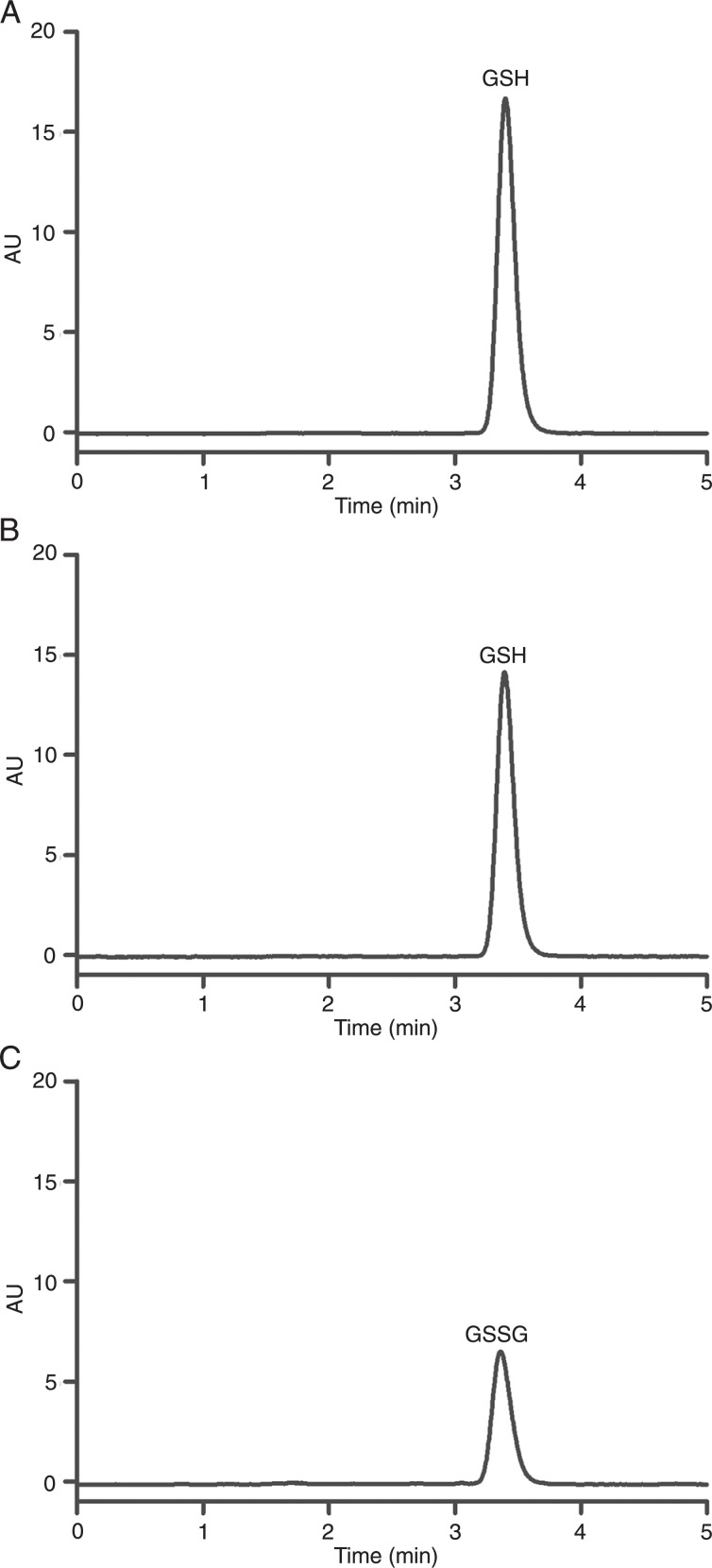
In addition to acting as a catalyst for the generation of H2O2, naphthoquinone may have other effects in the cellular medium. First, as an electrophilic molecule, naphthoquinone is able to react with the intracellular antioxidant and nucleophilic GSH. The consequence of this reaction is the depletion of the intracellular antioxidant reserve (25). Here, we found that both VK3 and BrQ reacted with GSH, but BrQ was the more effective substrate. Part of the GSH was incorporated into the naphthoquinone, producing a nucleophilic substitution product (Q-SG; Figure 5). Another part of the GSH was oxidized to GSSG (Figure 6).
Figure 5. Reaction between BrQ and GSH. Aa, Pure BrQ (10 µM); Ab, consumption of BrQ and production of Q-SG. B, Mass spectrum of Q-SG. The reaction conditions were BrQ (10 µM) and GSH (100 µM) for 3 min in PBS at 37°C. BrQ = bromoquinone; GSH = glutathione; Q-SG = nucleophilic substitution product.
Figure 6. Consumption of GSH and production of GSSG. A, Pure GSH (100 µM); B, remaining concentration of GSH after the redox cycling reaction; C, production of GSSG after the redox cycling reaction. The reaction conditions were bromoquinone (10 µM) and GSH (100 µM) in PBS, pH 7.4, at 37°C. After 15 min, aliquots of the reaction mixtures were removed and submitted to derivatization and analysis. GSH = glutathione; GSSG = oxidized glutathione.
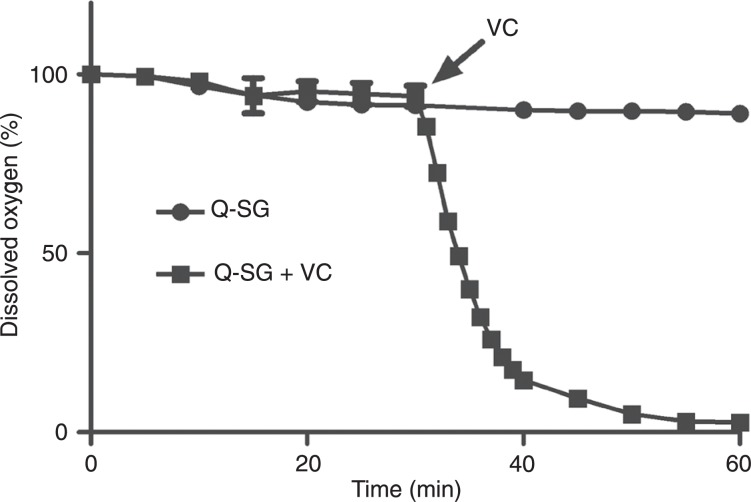
The depletion of GSH took place in the absence and, without a significant difference, in the presence of ascorbic acid, but it did not avoid the redox cycling reaction. Considering that the consumption of BrQ by GSH could hamper its efficacy in the generation of ROS, we tested the capacity of the substitution product Q-SG as a catalyst for the production of ROS in the presence of ascorbic acid. In this case, BrQ and GSH were incubated for 25 min to allow time for the total conversion to Q-SG. Ascorbic acid was then added and oxygen was measured. The results depicted in Figure 7 show that the redox cycling reaction was also effective using Q-SG.
Figure 7. Consumption of dissolved molecular oxygen by Q-SG. BrQ and GSH were previously incubated for 30 min to guarantee the total conversion to Q-SG. The reaction conditions were BrQ (10 µM) and GSH (100 µM) in PBS, pH 7.4, at 37°C. Ascorbic acid (500 µM; VC) was added at the indicated time. Q-SG = nucleophilic substitution product; BrQ = bromoquinone; GSH = glutathione.
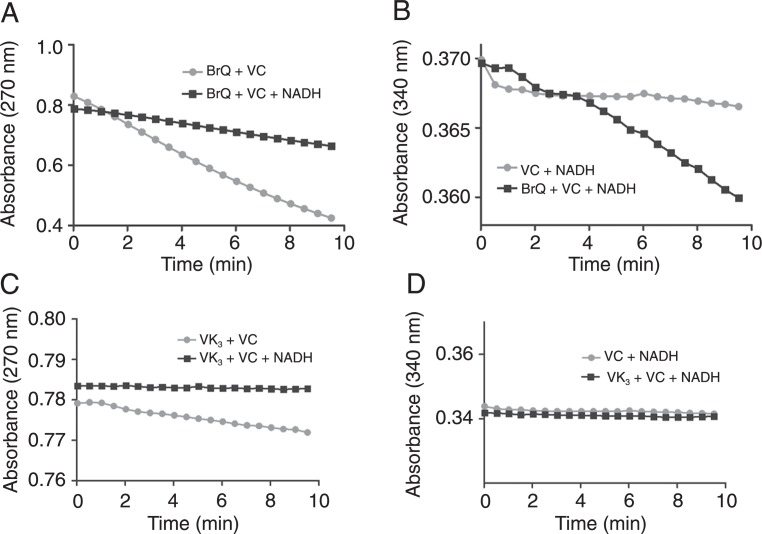
We also monitored the consumption of ascorbic acid and the effect of the addition of the coenzyme NADH. As depicted in Figure 8, the addition of NADH to the reaction mixture consisting of BrQ and VC promoted its oxidation and inhibited the depletion of ascorbic acid. Again, BrQ was significantly more effective than VK3, which had a small effect on ascorbic acid depletion and no effect on NADH oxidation.
Figure 8. Oxidation of NADH during the redox cycling reaction. A,C, Oxidation of ascorbic acid monitored by its absorbance at 270 nm. B,D, Oxidation of NADH monitored by its absorbance at 340 nm. The reaction mixtures consisted of BrQ or VK3 (10 µM) and VC (50 µM) in PBS at 37°C in the presence or absence of NADH (100 µM). NADH = nicotinamide adenine dinucleotide; BrQ = bromoquinone; VK3 = 2-methyl-1,4-naphthoquinone; VC = vitamin C.
Discussion
ROS, particularly H2O2, have dose-dependent, but somehow opposite effects on cancer cells. For instance, H2O2 at low concentration acts as a second messenger in the pathways that lead to increased cell proliferation through activation of important receptor tyrosine kinases, cytokines and transcription factors such as activator protein 1, hypoxia-inducible factor-1, nuclear factor-κB (26-28), and p53 (29). Other events related to H2O2 signaling involve the induction of the expression of proto-oncogenes such as c-fos, c-jun and c-myc, thus contributing to the expression of genes related to proteins of the cell cycle that promote persistent cell proliferation, apoptosis inhibition and metastases (26). On the other hand, the increased concentration of ROS at the cellular level is considered to be an effective way to promote the death of cancer cells (30,31). For instance, a high concentration of H2O2 induces cell cycle arrest and DNA alterations, including DNA damage, mutations and genetic instability, which trigger alternative signaling pathways and changes in cellular metabolism leading to cell death (31). Considering this last characteristic, several therapeutic approaches to cancer are based on or linked to the exacerbation of the generation of ROS in tumors. Obviously, this is the case with the use of VK3/VC in several in vitro (1-5,9,14,18-21,32) and in vivo (6-8,10,14) models. It is important to note that, when used alone, both vitamins present cytotoxic effects through mechanisms that include the generation of H2O2 (2), but the combined administration of VC/VK3 shows synergistic and selective cytotoxic activity at doses as much as 50 times lower than either vitamin alone (1). It is well established that, in the VC/VK3 combination, the latter vitamin works as a catalyst for the autoxidation of ascorbate, increasing the intracellular concentration of H2O2, inducing the G1/S and G2/M cell cycle, blocking the cell cycle, and decreasing DNA synthesis, among other cellular events, where the exacerbated production of H2O2 is of pivotal importance (2,32).
Considering these findings, the present study was performed to search for new and more efficient drug combinations that could enhance the production of ROS. The VC/BrQ pair presented here was more effective than the well-established VC/VK3 combination in terms of the production of ROS, particularly H2O2. This augmented potency is related to the electron-withdrawing feature of the bromine atom compared to the methyl group present in VK3. Indeed, the presence of electron-withdrawing groups at aromatic rings increase their redox potential and, consequently, their pro-oxidant capacity (33). This property was observed here, since BrQ was more effective in the oxidation of ascorbic acid and GSH and the production of a superoxide anion and H2O2. These results are consistent with the increased redox cycling of quinones of higher redox potential (4).
The success of the VC/VK3 combination is also related to the fact that tumor cells have an increased capacity to accumulate VC compared to normal cells (16). Indeed, a characteristic of tumor cells is the uptake of dehydroascorbate through one or more glucose transporter isoforms, including GLUT1, GLUT3 or GLUT4 (34). This oxidized form of ascorbate is produced in the pro-oxidant microenvironments around tumors. Once inside these cells, dehydroascorbate is reduced back to ascorbate by NADH-dependent semidehydroxyascorbate reductase or glutathione-dependent dehydroxyascorbate reductase (35), which prevents its reverse transport and, consequently, results in the accumulation of up to 100-fold higher concentrations of VC compared to the extracellular milieu (17,34). We propose that this property of the VC/VK3 combination can also be improved by using BrQ instead of VK3, since the oxidation of ascorbate to dehydroascorbate was more effective using BrQ.
Another important difference between normal and tumor cells is the increased use of the glycolytic pathway by the latter (15). For example, the use of Apatone™ provokes an 80% decrease in the respiration of tumor cells due to a 30% inhibition in the activity of glyceraldehyde-3-phosphate dehydrogenase and 100% depletion of cellular NAD+ (36). The high level of dehydroascorbate has also been proposed to be involved in the inhibition of other enzymes in this metabolic pathway, including hexokinase and glucose-6-phosphate dehydrogenase (37). In addition to the high levels of ROS produced by BrQ/VC, we demonstrated that this combination, but not VK3/VC, can chemically deplete the coenzyme NADH, which could have direct implications for the glycolytic pathway.
The VC/VK3 combination also decreases the intracellular GSH level and initiates membrane lipid peroxidation. The oxidation of GSH has been correlated with the release of mitochondrial Ca2+, thus activating endonucleases, lipases and proteases leading to signaling pathways related to cell death (13). This is another feature that could be improved using BrQ/VC. First, this drug combination was more effective in the depletion of GSH, leading to the production of its oxidized form, GSSG. Second, BrQ also reacted directly with GSH, leading to a compound in which the bromine was replaced with GSH. This molecule, which we called Q-SG, did not lose its catalytic effect on ascorbic acid autoxidation, which enhanced its efficacy, since, in addition to the depletion of GSH, the products of the reaction were still able to generate ROS.
In conclusion, we performed a complete chemical study of the redox cycling reaction using BrQ/VC (Figure 9). Our results show that the substitution of VK3 by BrQ significantly increases the generation of a pro-oxidative state compared to VK3. As we have demonstrated, the concentration of BrQ could be reduced by a factor of 100 and still maintain an effect similar to that of VK3. This property could lead to a lower-dose regimen for cancer treatment. We propose that this combination should be applied in in vitro and in vivo studies that use Apatone™.
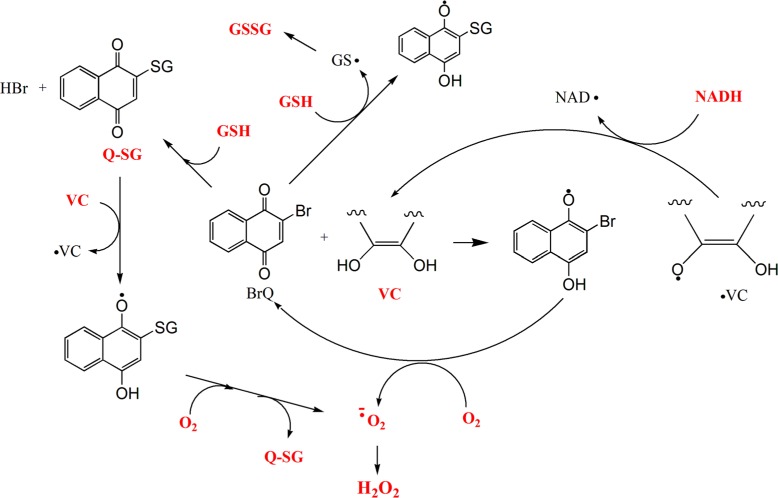
Figure 9. Pathways proposed for the BrQ/VC redox cycling reaction in the presence or absence of GSH or NADH. The compounds that were measured by its consumption or production are marked in red. BrQ = bromoquinone; VC = vitamin C (simplified structure of ascorbic acid); GSH = glutathione; NADH = nicotinamide adenine dinucleotide; Q-SG = substitution product of the reaction between BrQ and GSH.
Acknowledgments
Research supported by FAPESP, CAPES, and CNPq.
References
- 1.Noto V, Taper HS, Jiang YH, Janssens J, Bonte J, De Loecker W. Effects of sodium ascorbate (vitamin C) and 2-methyl-1,4-naphthoquinone (vitamin K3) treatment on human tumor cell growth in vitro. I. Synergism of combined vitamin C and K3 action. Cancer. 1989;63:901–906. doi: 10.1002/1097-0142(19890301)63:5<901::aid-cncr2820630518>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 2.Venugopal M, Jamison JM, Gilloteaux J, Koch JA, Summers M, Hoke J, et al. Synergistic antitumour activity of vitamins C and K3 against human prostate carcinoma cell lines. Cell Biol Int. 1996;20:787–797. doi: 10.1006/cbir.1996.0102. [DOI] [PubMed] [Google Scholar]
- 3.Verrax J, Cadrobbi J, Delvaux M, Jamison JM, Gilloteaux J, Summers JL, et al. The association of vitamins C and K3 kills cancer cells mainly by autoschizis, a novel form of cell death. Basis for their potential use as coadjuvants in anticancer therapy. Eur J Med Chem. 2003;38:451–457. doi: 10.1016/s0223-5234(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 4.Verrax J, Delvaux M, Beghein N, Taper H, Gallez B, Buc Calderon P. Enhancement of quinone redox cycling by ascorbate induces a caspase-3 independent cell death in human leukaemia cells. An in vitro comparative study. Free Radic Res. 2005;39:649–657. doi: 10.1080/10715760500097906. [DOI] [PubMed] [Google Scholar]
- 5.Verrax J, Stockis J, Tison A, Taper HS, Calderon PB. Oxidative stress by ascorbate/menadione association kills K562 human chronic myelogenous leukaemia cells and inhibits its tumour growth in nude mice. Biochem Pharmacol. 2006;72:671–680. doi: 10.1016/j.bcp.2006.05.025. [DOI] [PubMed] [Google Scholar]
- 6.Taper HS, Roberfroid M. Non-toxic sensitization of cancer chemotherapy by combined vitamin C and K3 pretreatment in a mouse tumor resistant to oncovin. Anticancer Res. 1992;12:1651–1654. [PubMed] [Google Scholar]
- 7.Taper HS, Jamison JM, Gilloteaux J, Gwin CA, Gordon T, Summers JL. In vivo reactivation of DNases in implanted human prostate tumors after administration of a vitamin C/K(3) combination. J Histochem Cytochem. 2001;49:109–120. doi: 10.1177/002215540104900111. [DOI] [PubMed] [Google Scholar]
- 8.Taper HS, Jamison JM, Gilloteaux J, Summers JL, Calderon PB. Inhibition of the development of metastases by dietary vitamin C:K3 combination. Life Sci. 2004;75:955–967. doi: 10.1016/j.lfs.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Gilloteaux J, Jamison JM, Neal DR, Summers JL. Cell death by autoschizis in TRAMP prostate carcinoma cells as a result of treatment by ascorbate: menadione combination. Ultrastruct Pathol. 2005;29:221–235. doi: 10.1080/01913120590951239. [DOI] [PubMed] [Google Scholar]
- 10.Tareen B, Summers JL, Jamison JM, Neal DR, McGuire K, Gerson L, et al. A 12 week, open label, phase I/IIa study using apatone for the treatment of prostate cancer patients who have failed standard therapy. Int J Med Sci. 2008;5:62–67. doi: 10.7150/ijms.5.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, Oberley LW, Elwell JH, Sierra-Rivera E. Antioxidant enzyme activities in normal and transformed mouse liver cells. Int J Cancer. 1989;44:1028–1033. doi: 10.1002/ijc.2910440615. [DOI] [PubMed] [Google Scholar]
- 12.Oberley TD, Oberley LW. Antioxidant enzyme levels in cancer. Histol Histopathol. 1997;12:525–535. [PubMed] [Google Scholar]
- 13.Di Monte D, Bellomo G, Thor H, Nicotera P, Orrenius S. Menadione-induced cytotoxicity is associated with protein thiol oxidation and alteration in intracellular Ca2+ homeostasis. Arch Biochem Biophys. 1984;235:343–350. doi: 10.1016/0003-9861(84)90207-8. [DOI] [PubMed] [Google Scholar]
- 14.Jamison MJ, Gilloteaux J. The in vitro and in vivo antitumor activity of vitamin C:K3 combination against. In: Lucas JN, editor. Trends in prostate cancer research. New York: Hauppauge, Nova Science Publishers, Inc.; 2005. pp. 129–236. [Google Scholar]
- 15.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 16.Langemann H, Torhorst J, Kabiersch A, Krenger W, Honegger CG. Quantitative determination of water- and lipid-soluble antioxidants in neoplastic and non-neoplastic human breast tissue. Int J Cancer. 1989;43:1169–1173. doi: 10.1002/ijc.2910430634. [DOI] [PubMed] [Google Scholar]
- 17.Agus DB, Vera JC, Golde DW. Stromal cell oxidation: a mechanism by which tumors obtain vitamin C. Cancer Res. 1999;59:4555–4558. [PubMed] [Google Scholar]
- 18.Gilloteaux J, Jamison JM, Venugopal M, Giammar D, Summers JL. Scanning electron microscopy and transmission electron microscopy aspects of synergistic antitumor activity of vitamin C - vitamin K3 combinations against human prostatic carcinoma cells. Scanning Microsc. 1995;9:159–173. [PubMed] [Google Scholar]
- 19.Gilloteaux J, Jamison JM, Arnold D, Ervin E, Eckroat L, Docherty JJ, et al. Cancer cell necrosis by autoschizis: synergism of antitumor activity of vitamin C: vitamin K3 on human bladder carcinoma T24 cells. Scanning. 1998;20:564–575. doi: 10.1002/sca.4950200805. [DOI] [PubMed] [Google Scholar]
- 20.Verrax J, Cadrobbi J, Marques C, Taper H, Habraken Y, Piette J, et al. Ascorbate potentiates the cytotoxicity of menadione leading to an oxidative stress that kills cancer cells by a non-apoptotic caspase-3 independent form of cell death. Apoptosis. 2004;9:223–233. doi: 10.1023/B:APPT.0000018804.26026.1a. [DOI] [PubMed] [Google Scholar]
- 21.Gilloteaux J, Jamison JM, Lorimer HE, Jarjoura D, Taper HS, Calderon PB, et al. Autoschizis: a new form of cell death for human ovarian carcinoma cells following ascorbate:menadione treatment. Nuclear and DNA degradation. Tissue Cell. 2004;36:197–209. doi: 10.1016/j.tice.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Kand'ar R, Zakova P, Lotkova H, Kucera O, Cervinkova Z. Determination of reduced and oxidized glutathione in biological samples using liquid chromatography with fluorimetric detection. J Pharm Biomed Anal. 2007;43:1382–1387. doi: 10.1016/j.jpba.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 23.Perez-Sacau E, Diaz-Penate RG, Estevez-Braun A, Ravelo AG, Garcia-Castellano JM, Pardo L, et al. Synthesis and pharmacophore modeling of naphthoquinone derivatives with cytotoxic activity in human promyelocytic leukemia HL-60 cell line. J Med Chem. 2007;50:696–706. doi: 10.1021/jm060849b. [DOI] [PubMed] [Google Scholar]
- 24.March J. Advanced organic chemistry. New York: John Wiley & Sons; 1982. [Google Scholar]
- 25.Ross D, Thor H, Orrenius S, Moldeus P. Interaction of menadione (2-methyl-1,4-naphthoquinone) with glutathione. Chem Biol Interact. 1985;55:177–184. doi: 10.1016/s0009-2797(85)80126-5. [DOI] [PubMed] [Google Scholar]
- 26.Toyokuni S, Okamoto K, Yodoi J, Hiai H. Persistent oxidative stress in cancer. FEBS Lett. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 27.Hirota K, Matsui M, Iwata S, Nishiyama A, Mori K, Yodoi J. AP-1 transcriptional activity is regulated by a direct association between thioredoxin and Ref-1. Proc Natl Acad Sci U S A. 1997;94:3633–3638. doi: 10.1073/pnas.94.8.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerald D, Berra E, Frapart YM, Chan DA, Giaccia AJ, Mansuy D, et al. JunD reduces tumor angiogenesis by protecting cells from oxidative stress. Cell. 2004;118:781–794. doi: 10.1016/j.cell.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 29.Bensaad K, Vousden KH. Savior and slayer: the two faces of p53. Nat Med. 2005;11:1278–1279. doi: 10.1038/nm1205-1278. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Lazaro M. Dual role of hydrogen peroxide in cancer: possible relevance to cancer chemoprevention and therapy. Cancer Lett. 2007;252:1–8. doi: 10.1016/j.canlet.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 31.Lopez-Lazaro M. A new view of carcinogenesis and an alternative approach to cancer therapy. Mol Med. 2010;16:144–153. doi: 10.2119/molmed.2009.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamison JM, Gilloteaux J, Nassiri MR, Venugopal M, Neal DR, Summers JL. Cell cycle arrest and autoschizis in a human bladder carcinoma cell line following Vitamin C and Vitamin K3 treatment. Biochem Pharmacol. 2004;67:337–351. doi: 10.1016/j.bcp.2003.08.040. [DOI] [PubMed] [Google Scholar]
- 33.Kanegae MP, da Fonseca LM, Brunetti IL, Silva SO, Ximenes VF. The reactivity of ortho-methoxy-substituted catechol radicals with sulfhydryl groups: contribution for the comprehension of the mechanism of inhibition of NADPH oxidase by apocynin. Biochem Pharmacol. 2007;74:457–464. doi: 10.1016/j.bcp.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 34.Liang WJ, Johnson D, Jarvis SM. Vitamin C transport systems of mammalian cells. Mol Membr Biol. 2001;18:87–95. doi: 10.1080/09687680110033774. [DOI] [PubMed] [Google Scholar]
- 35.De Laurenzi V, Melino G, Savini I, Annicchiarico-Petruzzelli M, Finazzi-Agro A, Avigliano L. Cell death by oxidative stress and ascorbic acid regeneration in human neuroectodermal cell lines. Eur J Cancer. 1995;31A:463–466. doi: 10.1016/0959-8049(95)00059-r. [DOI] [PubMed] [Google Scholar]
- 36.Gilloteaux J, Jamison JM, Neal DR, Loukas M, Doberzstyn T, Summers JL. Cell damage and death by autoschizis in human bladder (RT4) carcinoma cells resulting from treatment with ascorbate and menadione. Ultrastruct Pathol. 2010;34:140–160. doi: 10.3109/01913121003662304. [DOI] [PubMed] [Google Scholar]
- 37.Fiorani M, De Sanctis R, Scarlatti F, Vallorani L, De Bellis R, Serafini G, et al. Dehydroascorbic acid irreversibly inhibits hexokinase activity. Mol Cell Biochem. 2000;209:145–153. doi: 10.1023/a:1007168032289. [DOI] [PubMed] [Google Scholar]