Abstract
The most disabling aspect of human peripheral nerve injuries, the majority of which affect the upper limbs, is the loss of skilled hand movements. Activity-induced morphological and electrophysiological remodeling of the neuromuscular junction has been shown to influence nerve repair and functional recovery. In the current study, we determined the effects of two different treatments on the functional and morphological recovery after median and ulnar nerve injury. Adult Wistar male rats weighing 280 to 330 g at the time of surgery (N = 8-10 animals/group) were submitted to nerve crush and 1 week later began a 3-week course of motor rehabilitation involving either “skilled” (reaching for small food pellets) or “unskilled” (walking on a motorized treadmill) training. During this period, functional recovery was monitored weekly using staircase and cylinder tests. Histological and morphometric nerve analyses were used to assess nerve regeneration at the end of treatment. The functional evaluation demonstrated benefits of both tasks, but found no difference between them (P > 0.05). The unskilled training, however, induced a greater degree of nerve regeneration as evidenced by histological measurement (P < 0.05). These data provide evidence that both of the forelimb training tasks used in this study can accelerate functional recovery following brachial plexus injury.
Keywords: Peripheral nerve injury, Functional recovery, Median nerve, Ulnar nerve, Nerve morphometry
Introduction
Although the majority of human peripheral nerve injuries affect the upper extremity, the sciatic nerve injury is the most widespread experimental model used for the study of nerve repair (1). Several investigators have recently defended the use of experimental brachial plexus nerve injury in studies of functional recovery (2-4).
The brachial plexus injury model has several advantages. The brachial plexus of rats and humans exhibits various similarities in its components and branches (5,6). Furthermore in the upper extremity of rats, the distance between the nerves and their targets is shorter than in the lower extremity, reducing the time required for studies of functional recovery (2). In general, no autonomy or contractures are observed in upper extremity lesions (4,7,8).
The neuronal response following injury includes the expression of growth factors as well as other secreted molecules that are involved in cell-to-cell communication (9). The mechanisms regulating the expression of the different injury-response factors, such as the neurotrophins, are not completely understood. In the central nervous system, the expression of some members of the neurotrophin family is correlated with the degree of neuronal activity (10-12). In the peripheral nervous system, moderate physical motion can exert positive effects on functional recovery after injury by means of modulation of neurotrophin expression, axonal outgrowth, and nerve maturation to promote recovery of muscle contractile properties (13,14).
There is evidence that motor activity can influence the morphological and electrophysiological remodeling of the neuromuscular junction. Although fully established that adult synapses are largely stable structures, motor nerve endings are continuously changing and being modified in response to functional demands (8,15,16). Adaptations of these synapses can, therefore, influence nerve orientation and repair. Moreover, growth factor secretion is one of the mechanisms underlying nerve regeneration (17). Neurotrophins are unique among neurotrophic factors in their ability to act as guidance molecules for nerve growth. Strategies to enhance functional recovery after neuronal injury may depend on neurotrophin secretion to induce improvement of axonal regeneration and target reinnervation as well as to modulate abnormal plasticity of neuronal circuits (14).
Patients suffering from neuromuscular diseases undergo a great diversity in the physical therapy used for rehabilitation. Despite advances in the knowledge of nerve regeneration, as well as surgical techniques, functional recovery after injury is often unsatisfactory (18). In addition, there is no consensus regarding the type and intensity of the most appropriate physical therapy (19). It is extremely important, therefore, to understand how recovery from nerve injury is influenced by different types of exercise (20).
Rats are adept at reaching for, grasping, and bringing food to their mouths using a single paw (skilled reaching) (1). Since an attempt to identify an appropriate strategy for rehabilitation after peripheral nerve injury has unquestionable clinical relevance, we investigated the effects of two types of physical therapies, skilled and unskilled, upon functional and morphological recovery after a brachial plexus crush lesion in adult rats.
Material and Methods
Animals
All procedures were in accordance with Brazilian Law No. 11.794/08 and decree No. 6.899/09, which regulate the use of animals in scientific research.
Male Wistar rats (N = 42) weighing 280 to 330 g at the time of surgery, housed in groups of four or five in Plexiglas cages under standard conditions (12-h light/dark, 22 ± 2°C) were used. Wistar rats were obtained from the Central Animal House of the Biochemistry Department, at the Institute of Basic Health Sciences from the Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brazil. Water and standard laboratory chow were provided ad libitum except during the behavioral training and testing periods. Animals were weighed weekly. On the day before the beginning of training, animals did not receive food (in order to increase interest in new food). From then on, and after each training session, rats were provided with a measured amount of standard laboratory chow each day (12-15 g) to keep their body weight at ∼80-90% of free-feeding level. The training period was completed two days prior to surgery and animals were then returned to ad libitum feeding.
Experimental design
Three weeks before surgery animals were randomly assigned to one of the five groups described in Table 1.
Table 1. Experimental groups and number of animals used for each analysis.
| Group description | Designation (abbreviation) | Total number of animals | Behavioral evaluation | Morphometric analysis |
|---|---|---|---|---|
| Control animals (intact - unoperated) | Control (C) | 8 | 8 | 4 |
| Animals submitted to all procedures (anesthesia, incision and suture), except median and ulnar nerve crush | Sham (S) | 8 | 8 | 4 |
| Animals submitted to median and ulnar nerve crush and not treated | Crush (CC) | 8 | 8 | 5 |
| Animals submitted to median and ulnar nerve crush and treated with a skilled motor task | Skilled (Sk) | 10 | 10 | 5 |
| Animals submitted to median and ulnar nerve crush and treated with repetitive motor movement (unskilled task) | Unskilled (Usk) | 8 | 8 | 5 |
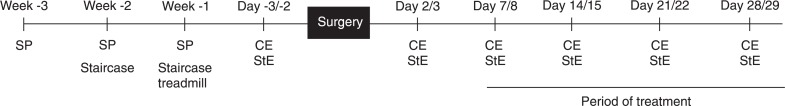
During the 3 weeks (5 days/week) prior to the surgical procedure, animals were habituated to all tasks. The habituation phase consisted of 3 weeks of training in the single pellet task, 2 weeks of training in the staircase test and 1 week of habituation to the electrical treadmill. A time line of experimental events is presented in Figure 1.
Figure 1. Time line of the experimental procedures. SP = Single pellet training; staircase = staircase test training; treadmill = electrical treadmill habituation; CE = cylinder evaluation; StE = staircase test evaluation.
Surgical procedures
For the surgical procedure, all animals, except controls, were deeply anesthetized with a mixture of 90 mg/kg ketamine and 10 mg/kg xylazine administered intraperitoneally. Rats were immobilized on a wooden surface and a horizontal incision was made parallel to the clavicle, from the sternum to the axillary region (the forepaw chosen was that which showed higher performance in the staircase test prior to surgery). The brachial plexus was approached through this opening and the ulnar and median nerves were crushed together (1 cm distal to the sternum) with a 1-mm hemostatic forceps for 30 s (adapted from Refs. 20 and 21). The crush was performed on these two nerves because median and ulnar nerve injury affects the capacity of finger flexion and grasping (2,7,22). This procedure induces axonal interruption while preserving the connective sheaths (axonotmesis) (8).
Rehabilitation protocols
One week following median and ulnar nerve crush, animals received one of the following treatments. Both protocols were performed for 3 weeks, 5 days per week.
Skilled task. This task consisted of reaching for food inside reaching boxes made of clear Plexiglas (20 × 25 × 40 cm high) (1). In the middle of the front wall, a 1.1-cm wide vertical slot allowed animals to reach for food pellets placed on a shelf situated 4 cm above the floor. Two small indentations (0.5 cm in diameter, 0.15 cm deep) on the upper side of this shelf, each aligned with one side of the slit, served to stabilize the food pellets (sweet flavored sucrose spheres: 4.6 mm; 65 mg ± 10%; Brazilian Homeopathic Pharmacopoeia [www.anvisa.gov.br/farmacopeiabrasileira/conteudo/3a_edicao.pdf]). The distance from the indentations to the front wall was 1.5 cm.
Each animal received individual preoperative training. Once animals began to reach, the food pellets were placed in the indentation contralateral to their preferred limb to provide easier access to the food and to prevent simultaneous use of the non-preferred limb. In the preoperative training period, rats were motivated to reach during a 20-min period. The protocol was performed similarly during the postoperative training period. Reaching with the impaired forelimb was effectively enforced by the insertion of an inner chamber wall ipsilateral to this limb, and the placement of pellets, one at a time, in the wall opposite this limb. Since animals needed to cross the midline to reach the pellet, this prevented the use of the unaffected forelimb.
Unskilled task. This task consisted of walking on an adapted motorized rodent treadmill (INBRAMED TK 01, Brazil) for a 20-min (0.03 m/s - 3 initial min; 0.05 m/s - 14 min; 0.03 m/s - last 3 min) period. The grade of the treadmill remained at 0% and no aversive stimuli were used. The low velocity was chosen to avoid possible effects of aerobic treadmill exercise.
Behavioral assessments
Behavioral assessments were performed using a skilled reaching test (staircase test) and an asymmetrical forelimb use test (cylinder test).
Skilled reaching test. Fine motor ability was assessed by means of a staircase test, which provides a sensitive measure of the skilled reaching of both forepaws independently (23). This test has been recently demonstrated to identify the effects of axon misdirection on forelimb function (3). The boxes were made of clear Plexiglas and consisted of a chamber with a central platform. Seven steps were positioned on each side of the platform and three small food pellets were placed on each staircase (Brazilian Homeopathic Pharmacopoeia). Animals were trained for 2 weeks before the surgical procedure (2 trials/day). The rats remained in the staircase chamber for 15 min and the total number of pellets eaten on each side was recorded. Animals were tested on two different days (2 trials/day) before surgery, and the reaching performance was calculated as the average of these trials. Animals were submitted to the staircase test 2 and 7 days after surgery and weekly for the 3-week treatment.
Forelimb asymmetry test. To examine the effects of median and ulnar nerve crush and treatment on spontaneous forelimb use during exploratory activity, animals were placed individually inside a transparent cylinder (20 cm in diameter and 40 cm high) on a glass tabletop and video-recorded from below through an angled mirror for a 4-min test session. The cylindrical shape encouraged rearing and vertical exploration of the walls with the forelimbs. The number of forepaw-wall contacts used for postural support was counted and the percentage asymmetry of single-limb wall contacts [(contralateral / contralateral + ipsilateral) × 100] was calculated (24). A single cylinder test session was performed 2 days prior to surgery, 2 days after surgery, and weekly after the beginning of treatment.
Morphometric analyses
Two days after the end of the treatment period, animals were anesthetized with chloride hydrate (30%, 10 mL/kg, ip) and injected with 1000 IU heparin (Cristália, Brazil). They were then transcardially perfused through the left ventricle using a peristaltic pump (Control Company, Brazil) with 100 mL saline followed by 200 mL of a fixative solution composed of 0.5% glutaraldehyde (Sigma, USA) and 4% paraformaldehyde (Reagen, Brazil) in 0.1 M phosphate buffer (PB), pH 7.4, at room temperature. For nerve regeneration analysis, one short segment (∼3 mm) of the ulnar and median nerves was rapidly excised 5 mm after the crush injury site. The specimens were postfixed by immersion in the same fixative solution for 1 h. They were then postfixed in 1% OsO4 (Sigma) in PB, dehydrated in a graded alcohol series and propylene oxide (Electron Microscopy Sciences, USA), embedded in araldite (Durcupan ACM, Fluka, Switzerland), and polymerized for 48 h at 60°C.
Transverse semithin sections (1 µm) were obtained with an ultramicrotome (MT 6000-XL, RMC, USA) and stained with 1% toluidine blue (Merck, Germany) in 1% sodium tetraborate (Ecibra, Brazil). Images of the distal portions of the nerves were then digitized (initially 100X and further amplified 200X for analysis) using a Nikon Eclipse E-600 microscope (Japan) coupled to a Pro-Series High-Performance CCD camera and Image Pro Plus Software 6.0 (Media Cybernetics, USA). For morphological evaluation, a set of 6 images was obtained from each nerve portion: 3 random images from the periphery and 3 random images from the center of the nerve, in order to obtain a representative area per nerve segment (0.010 mm2; 100% of the area analyzed per segment) (20,25).
The morphometric measurements used to assess the differentiation of regenerating nerves included: 1) myelinated fiber density (number of fibers/mm2); 2) average myelinated fiber area (µm2); 3) average myelinated fiber diameter (µm); 4) average axon diameter (µm) of the myelinated fiber; 5) average myelin sheath thickness (µm); 6) g ratio (the quotient axon diameter/fiber diameter, a measurement of the degree of myelination). Individual myelinated fibers were counted and the myelinated fiber density was determined by examining the ratio of the myelinated fibers/total area analyzed. The average myelin sheath thickness was estimated using the measurement tools of the ImagePro Plus software. The area measurements were estimated with a point-counting technique (20,26) using grids with point density of one point per 1.87 µm2 and the following equation: !!!eq i 0 % MathType!MTEF!2!0!+-% feaagaart1ev2aqatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLn% hiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr% 4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq-Jc9% vqaqpepm0xbba9pwe9Q8fs0-yqaqpepae9pg0FirpepeKkFr0xfr-x% fr-xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGabmyqayaaja% Gaeyypa0Jaeu4OdmLaamiCaiabgwSixpaalyaabaGaamyyaaqaaiaa% dchaaaaaaa!3E7E!$\hat A\equals \Sigma p \cdot {a \mathord{\left/ {\vphantom {a p}} \right. \kern-\nulldelimiterspace} p}$!!!, where !!!eq i 0 % MathType!MTEF!2!0!+-% feaagaart1ev2aqatCvAUfeBSjuyZL2yd9gzLbvyNv2CaerbuLwBLn% hiov2DGi1BTfMBaeXatLxBI9gBaerbd9wDYLwzYbItLDharqqtubsr% 4rNCHbGeaGqiVu0Je9sqqrpepC0xbbL8F4rqqrFfpeea0xe9Lq-Jc9% vqaqpepm0xbba9pwe9Q8fs0-yqaqpepae9pg0FirpepeKkFr0xfr-x% fr-xb9adbaqaaeGaciGaaiaabeqaamaabaabaaGcbaGabmyqayaaja% aaaa!36C4!$\hat A$!!! is the area, Σp the sum of points, and a/p the area/point value (1.87 µm2). To estimate the axon and fiber diameters, the area of each individual fiber was measured and the value obtained was converted to the diameter of a circle having an equivalent area. In each nerve segment, 10.48 µm2 was 100% of the analyzed area.
Statistical analysis
The SPSS® 16.0 (Statistical Package for the Social Sciences, Inc., USA) was used for data analysis. Repeated measures analysis of variance (ANOVA) was used to determine differences between groups in the behavioral evaluation. Morphological measurements were analyzed by one-way ANOVA and the Duncan multiple range test was used when appropriate. Significance was set at P < 0.05 for all analyses and results are reported as means ± SEM.
Results
Staircase test
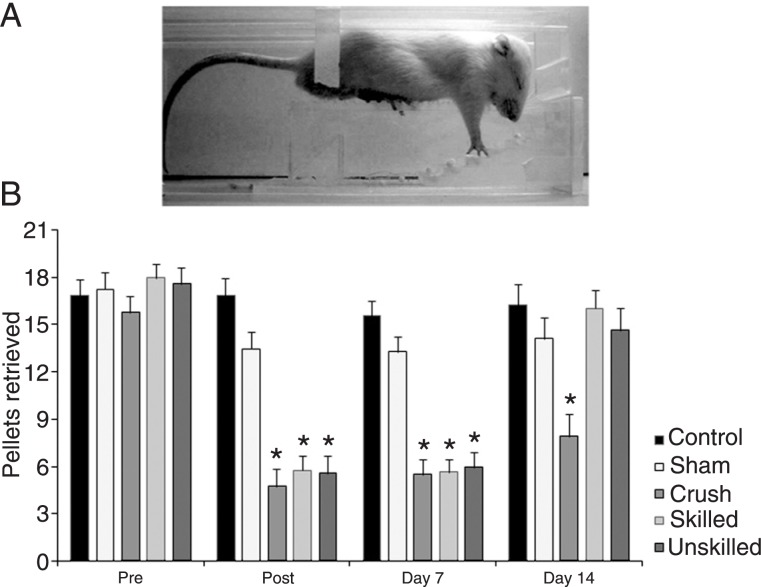
Repeated measures ANOVA of the staircase test showed time (F(1,37) = 12.02, P = 0.01) and group effects (F(4,37) = 7.24, P < 0.01), but no significant interaction between time and group (F(4,37) = 1.71, P = 0.16). As shown in Figure 2, Duncan's post hoc analysis revealed that nerve lesion resulted in a significant decrease in performance of the crushed groups when compared to sham and control groups at post (evaluation performed at 24 h after crush) and day 7 (P < 0.05). At day 14, the crush group (which was not submitted to any exercise protocol) remained impaired (P < 0.05) while no difference between the sham-and control-treated groups were observed. At days 21 and 28, no differences between groups were observed (data not shown).
Figure 2. Staircase test of skilled forelimb reaching. A, Photograph showing the staircase apparatus. The experimental boxes were made of clear Plexiglass with a central raised platform. The narrowness of the compartment was enough to prevent the rat from turning around and to allow unilateral reaches. Pellets were provided into a removable double staircase located at the end of the box. B, Number of pellets retrieved: all damaged groups were significantly impaired compared to control and sham animals at post (24 h) and day 7. At day 14, animals from the crush group remained impaired and animals from both treated groups improved but without a difference between them. Data are reported as means ± SEM. *P < 0.05 compared to the control and sham groups (repeated measures ANOVA followed by the Duncan multiple range test).
Cylinder test
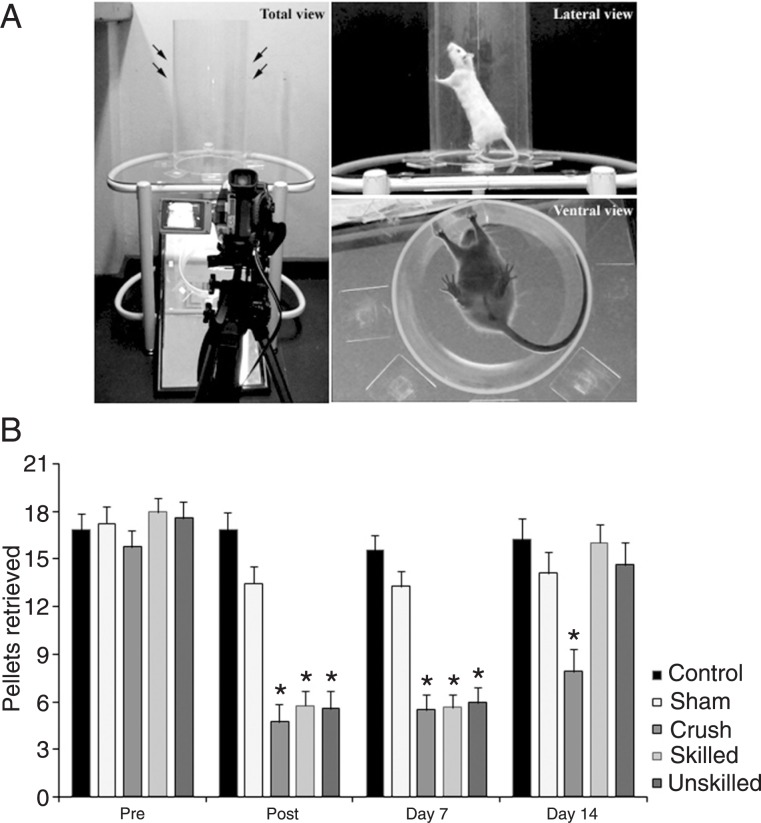
For the forelimb asymmetry task, repeated measures ANOVA showed an effect of time (F(1,37) = 4.21, P < 0.05), group (F(4,37) = 15.50, P < 0.01) and time-group interaction (F(4,37) = 7.61, P < 0.01). As demonstrated in Figure 3, all injured groups were significantly impaired immediately following the median and ulnar nerves crush (P < 0.05) and used the damaged limb for support less frequently than did the sham and control groups. At 14 days post-injury, both treated groups improved to sham and control levels. Untreated animals from the crush group had persistent impairment (P < 0.05) at 14 days, and showed spontaneous recovery at day 21. There were no differences between groups at days 21 and 28 (data not shown).
Figure 3. A, Photograph of the cylinder test apparatus - transparent cylinder (20 cm in diameter and 40 cm high). Cylinder was positioned on a glass tabletop and exploratory movements were video-recorded from below through an angled mirror. B, The forelimb asymmetry task was used to measure the number of contralateral forelimb contacts compared to ipsilateral contacts while the animal reared in a cylinder. All damaged groups showed an increase in contralateral forelimb use after median and ulnar nerve crush at post (24 h) and day 7. At day 14, both treated groups improved, with no difference between treatments. At this time, only the animals from the crush group remained impaired. Data are reported as means ± SEM. *P < 0.05 compared to the control and sham groups (repeated measures ANOVA followed by the Duncan multiple range test).
Histological analysis
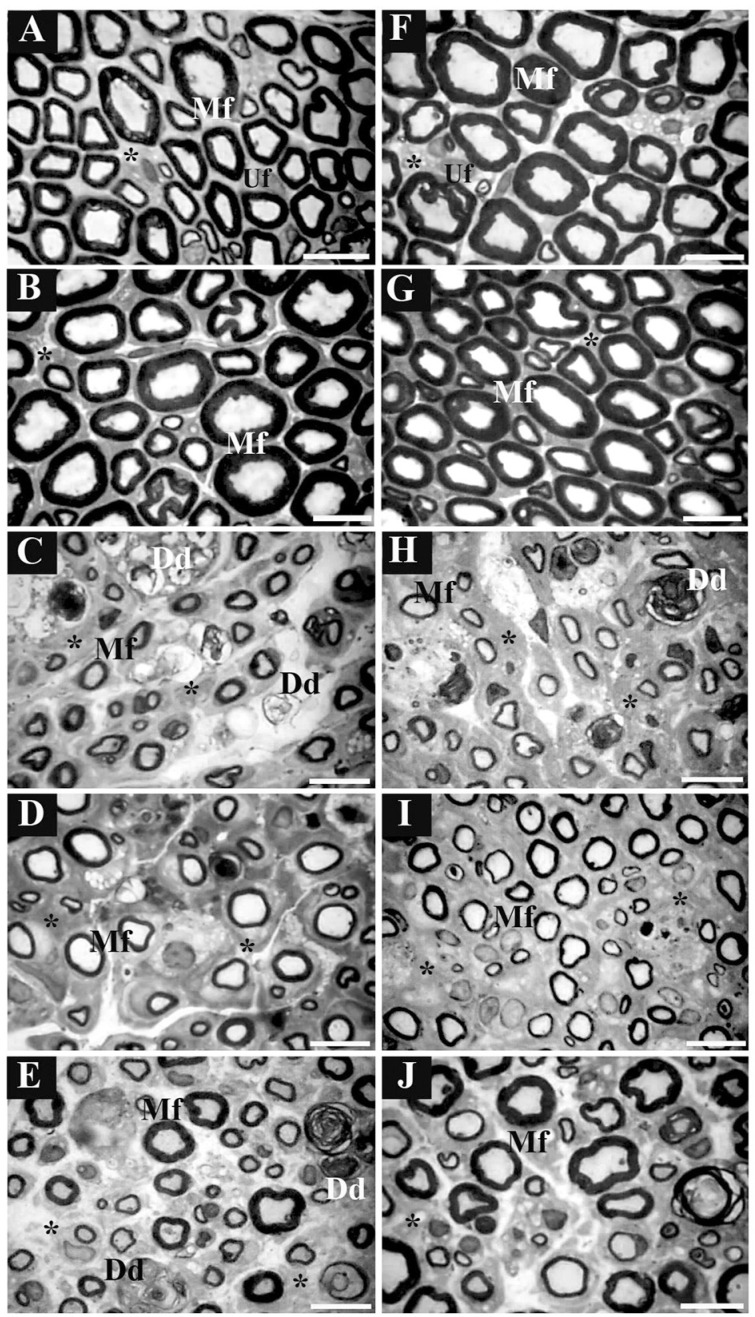
The histological characteristics of the distal portions of the median and ulnar nerves (Figure 4) demonstrated pathological features in the crush group (Figure 4C,H), which were not seen in the control (Figure 4A,F) or sham (Figure 4B,G) groups. These consisted of enlargement of endoneurial connective tissue between the nerve fibers, reduction of myelinated fiber diameter and myelin sheath thickness, and presence of degeneration debris. In injured treated groups (Figure 4D,E,I,J), these pathological features were reduced and less endoneurial connective tissue and tissue debris were observed.
Figure 4. Morphological images of the median and ulnar nerves: digitalized images of transverse semithin sections (1 µm) obtained from regenerating median and ulnar nerves after 3 weeks of skilled and unskilled training. The left columns represent images from distal portions of the median nerve and the right columns represent images from distal portions of the ulnar nerve. Letters indicate distal portions from nerves of A and F, control group; B and G, sham group; C and H, crush group; D and I, skilled training group; E and J, unskilled training group. Mf = myelinated nerve fiber; Uf = unmyelinated nerve fiber; Dd = degeneration debris; asterisks = endoneurial connective tissue. Semithin sections were stained with toluidine blue. Scale bar = 10 µm.
Morphometric analysis of the median nerve
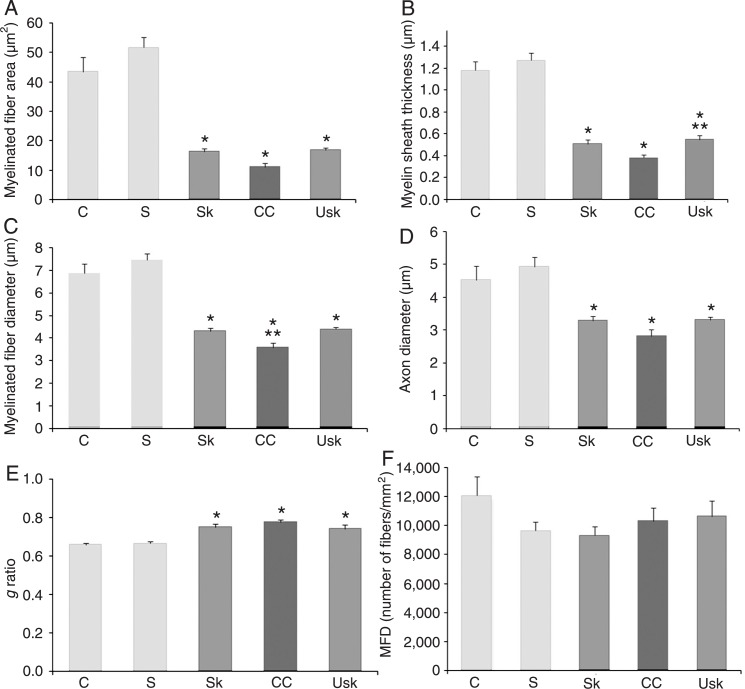
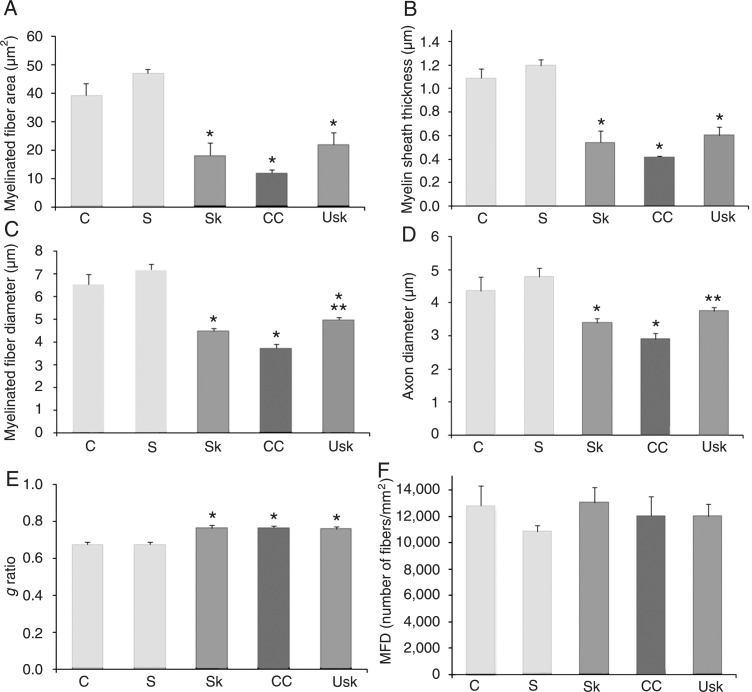
In the median nerve, one-way ANOVA demonstrated an effect of the lesion for myelinated fiber area (F(4,18) = 54.16, P < 0.01); myelin sheath thickness (F(4,18) = 67.06, P < 0.01); myelinated fiber diameter (F(4,18) = 54.60, P < 0.01); axon diameter (F(4,18) = 24.80, P < 0.01), and g ratio (F(4,18) = 11.36, P < 0.01), but not for myelinated fiber density (F(4,18) = 1.23, P = 0.33). After 3 weeks of rehabilitation, Duncan post-hoc analyses demonstrated differences between the injured (crush, skilled task and unskilled) and uninjured groups (sham and control) in myelinated fiber area, axon diameter and g ratio (Figure 5A,D,E, respectively). Differences were found between injured and uninjured groups, as well as between crush and unskilled groups in myelin sheath thickness (Figure 5B). A difference in myelinated fiber diameter (Figure 5C) was demonstrated between injured and uninjured groups and between injured (crush) and injured treated groups (skilled and unskilled task). However, no significant differences were observed between the two types of treatment. In addition, no difference was observed in myelinated fiber density between groups (Figure 5F).
Figure 5. Effects of treatment on the morphometric parameters of regenerating median nerve fibers. Graphics show means ± SEM of A, myelinated fiber area; B, myelin sheath thickness; C, myelinated fiber diameter; D, axon diameter of the myelinated fiber; E, g ratio, and F, myelinated fiber density (MFD). *P < 0.05 compared to the control and sham groups. In B, **P < 0.05 for the difference between the unskilled training and crush group. In C, **P < 0.05 for the difference between the injured treated (skill and unskilled training) groups and the injured group (crush). C = control group; S = sham; Sk = skill training; CC = crush; Usk = unskilled training (one-way ANOVA followed by the Duncan multiple range test).
Morphometric analysis of the ulnar nerve
One-way ANOVA revealed an effect of the lesion for ulnar nerve myelinated fiber area (F(4,18) = 17.17, P < 0.01), myelin sheath thickness (F(4,18) = 19.47, P < 0.01), myelinated fiber diameter (F(4,18) = 15.53, P < 0.01), axon diameter (F(4,18) = 11.15, P < 0.01), and g ratio (F(4,18) = 11.58, P < 0.01), but no significant differences were observed in myelinated fiber density (F(4,18) = 0.48, P = 0.74).
After 3 weeks of rehabilitation, Duncan post hoc analysis revealed differences between the injured (crush, skilled and unskilled task) and uninjured groups (sham and control) in myelinated fiber area, myelin sheath thickness and g ratio (Figure 6A,B, and E, respectively). Differences in myelinated fiber diameter (Figure 6C) were found between the injured and uninjured groups, as well as between the crush and unskilled groups. Differences in axon diameter (Figure 6D) were found between the crush, control and sham groups, although no differences were apparent between the control and unskilled groups, or between the treated groups (skilled and unskilled task). No differences were observed in myelinated fiber density between groups (Figure 6F).
Figure 6. Effects of treatment on the morphometric parameters of regenerating ulnar nerve fibers. Graphics show means ± SEM of A, myelinated fiber area; B, myelin sheath thickness; C, myelinated fiber diameter; D, axon diameter of the myelinated fiber; E, g ratio, and F, myelinated fiber density (MFD). *P < 0.05 compared to the control and sham groups; In C and D, **P < 0.05 for the difference between the unskilled training and crush groups. C = control group; S = sham; Sk = skill training; CC = crush; Usk = unskilled training (one-way ANOVA followed by the Duncan multiple range test).
Discussion
The most disabling aspect of upper extremity peripheral nerve injury in humans is reported to be the loss of skilled hand movements (27). Rats are capable of performing skilled reaching and grasping movements with their forepaws. This ability can be used to model rehabilitative treatment (28) and to assess post-injury performance (1). Previous studies, however, have used neither skill training as treatment for brachial plexus injury, nor tests of skilled forelimb function to assess recovery after peripheral nerve injury and repair. The exception is a study by Galtrey and Fawcett (3).
Although small physiological variations (such as increases and decreases in locomotor activity for a short period) can modify the ultrastructure of the nerve terminal (16,29,30), the differences obtained with different types of physical exercises have not been examined extensively in models of brachial plexus injury.
In the present study, we investigated the hypothesis that skilled and unskilled training, beginning 1 week after the injury, would produce different effects regarding the functional recovery and morphological changes observed in the regenerating nerve. Analysis of functional activity of the forepaws using the grasping strength test revealed that median and ulnar nerve crush led to a marked reduction in grasping on the 5th day after nerve damage, and that this impairment was gradually reduced about 20 days after the lesion (8). We therefore used this chronogram for our experiments.
Our results demonstrate that both types of treatment promoted the normalization of both skilled and gross forelimb motor function after 1 week of treatment, as evaluated by the staircase and cylinder tests (Figures 2 and 3). However, the unskilled training accelerated axonal regeneration as demonstrated by the increased fiber myelination and tissue recovery (Figure 4). Greater myelin thickness and myelinated fiber diameter were found in the median nerve of the animals trained in the unskilled task compared to untreated injured animals (Figure 5). Morphological examination of the ulnar nerve also revealed axons with a more mature appearance in animals of the unskilled training group compared to the crush group (as demonstrated by analysis of myelinated fiber diameter and axon diameter of the myelinated fiber; Figure 6).
As visualized in Figure 6, sham-treated animals displayed a lower density of myelinated fibers compared to control animals. This variability was present both in the ulnar and median nerve evaluations and no statistical difference in this measure was observed between these two groups. These results could possibly be due to unavoidable biological variability.
The major aim of physiotherapy after nervous system injury in humans is to avoid permanent disability as well as to restore the patient's autonomy in activities of daily living. Experimental studies demonstrate that recovery of function does not necessarily correlate with histological and electrophysiological evidence of regeneration (6,31), as confirmed in the present study.
Previous studies have evaluated the effects of treadmill exercise on peripheral nerves following injury (32,33). Regeneration of neural processes is likely to be an activity-dependent process (27). The type of treatment, the intensity and duration of the protocol, and the period during which it is applied after the injury are factors that determine beneficial or detrimental effects on functional recovery (14). In some cases, nerve regeneration is more directly influenced by the quantity, rather than the type, of exercise (34). Sabatier et al. (34) demonstrated that training for longer periods at slower speeds, and training for short amounts of time (as little as 4 min) at higher speeds, increased axon profile lengths 2 weeks after nerve transection.
Physiological activities such as those used in the present study, may induce greater regeneration than more intensive training. Several studies have reported that muscle overwork is harmful to muscle reinnervation, as overexerting muscles might accelerate disease progression (20,33,35,36). However, for the improvement of nerve regeneration, the intervention should be of a high enough intensity to provide a training stimulus (19). This level perhaps was not reached in those animals submitted to skill training in the current study.
Although previous studies have shown that voluntary physical activity can prime enhanced axonal regeneration after subsequent axotomy (17), in our study this bias was avoided by submitting all animals to the same pretreatment protocol.
Fine dexterity is often lost or diminished after nerve injury. Goal-directed activity may promote greater use of the hand and limb (27), and morphological evaluation of animals submitted to unskilled training shows superior nerve regeneration than in those receiving skill training. The discharge of weight on a treadmill (unskilled training) demands more intense and global muscular activity than skilled training, such as a reaching task. The pattern of peripheral regulation of growth factors may differ according to type of training (20). Unskilled training thus may induce a greater release and expression of relevant substances and their respective receptors involved in nerve regeneration, such as brain-derived neurotrophic factor (BDNF), neurotrophins (NT 3, 4, and 5) and specific members of the tyrosine kinase gene family (Trk) (Refs. 13 and 36, for review).
Physical exercise after nerve injuries increases the expression of genes needed for neurite growth. Animals exercised after lesion had higher levels of BDNF, NT3, synapsin I, and GAP43 mRNAs than sedentary animals (17,37). Blocking the Trk neurotrophin receptor activity before exercise inhibits the effects on synapsin I transcription and attenuates the increase in neurite growth, demonstrating that neurotrophin signal transduction pathways play a critical role in exercise inducing nerve growth. Exercise can also influence the injured neurons by stimulating sensory afferents and exerting positive effects on the cell circuitry (17). Even during the denervation period, active exercise stimulates muscle afferents, which may influence the axotomized motoneurons by normally silent spinal synaptic connections (12).
A limitation of our study is that we used a technique that permitted visualization only of the final nerve regeneration. Further studies are necessary to determine the rate of regeneration as well as the latency to target reinnervation in animals submitted to both rehabilitation protocols.
The present study demonstrated that skilled and unskilled forelimb training accelerates the morphological and functional recovery after brachial plexus injury. Morphological analyses revealed that the unskilled training paradigm induced a slight superior degree of tissue recovery. Further studies are needed to verify the utility of the different treatments for promoting neuronal reorganization and functional improvement after peripheral nervous system injury.
Acknowledgments
The authors thank Dr. Dale Corbett and co-workers, especially Kathy McKay and Sue Evans, for methodological assistance. The Write Science Right Company provided English editing of the manuscript. Research supported by CNPq. C.A. Netto is a CNPq investigator and A.S. Pagnussat was the recipient of CNPq postgraduate fellowship.
References
- 1.Whishaw IQ, Pellis SM. The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behav Brain Res. 1990;41:49–59. doi: 10.1016/0166-4328(90)90053-h. [DOI] [PubMed] [Google Scholar]
- 2.Bontioti E, Kanje M, Lundborg G, Dahlin LB. End-to-side nerve repair in the upper extremity of rat. J Peripher Nerv Syst. 2005;10:58–68. doi: 10.1111/j.1085-9489.2005.10109.x. [DOI] [PubMed] [Google Scholar]
- 3.Galtrey CM, Fawcett JW. Characterization of tests of functional recovery after median and ulnar nerve injury and repair in the rat forelimb. J Peripher Nerv Syst. 2007;12:11–27. doi: 10.1111/j.1529-8027.2007.00113.x. [DOI] [PubMed] [Google Scholar]
- 4.Santos AP, Suaid CA, Fazan VP, Barreira AA. Microscopic anatomy of brachial plexus branches in Wistar rats. Anat Rec. 2007;290:477–485. doi: 10.1002/ar.20519. [DOI] [PubMed] [Google Scholar]
- 5.Bertelli JA, Mira JC, Gilbert A, Michot GA, Legagneux J. Anatomical basis of rat brachial plexus reconstruction. Surg Radiol Anat. 1992;14:85–86. doi: 10.1007/BF01628049. [DOI] [PubMed] [Google Scholar]
- 6.Nichols CM, Myckatyn TM, Rickman SR, Fox IK, Hadlock T, Mackinnon SE. Choosing the correct functional assay: a comprehensive assessment of functional tests in the rat. Behav Brain Res. 2005;163:143–158. doi: 10.1016/j.bbr.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Bertelli JA, Mira JC. Behavioral evaluating methods in the objective clinical assessment of motor function after experimental brachial plexus reconstruction in the rat. J Neurosci Methods. 1993;46:203–208. doi: 10.1016/0165-0270(93)90068-3. [DOI] [PubMed] [Google Scholar]
- 8.Rodrigues-Filho R, Santos AR, Bertelli JA, Calixto JB. Avulsion injury of the rat brachial plexus triggers hyperalgesia and allodynia in the hindpaws: a new model for the study of neuropathic pain. Brain Res. 2003;982:186–194. doi: 10.1016/s0006-8993(03)03007-5. [DOI] [PubMed] [Google Scholar]
- 9.Makwana M, Raivich G. Molecular mechanisms in successful peripheral regeneration. FEBS J. 2005;272:2628–2638. doi: 10.1111/j.1742-4658.2005.04699.x. [DOI] [PubMed] [Google Scholar]
- 10.Adkins DL, Boychuk J, Remple MS, Kleim JA. Motor training induces experience-specific patterns of plasticity across motor cortex and spinal cord. J Appl Physiol. 2006;101:1776–1782. doi: 10.1152/japplphysiol.00515.2006. [DOI] [PubMed] [Google Scholar]
- 11.Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004;1028:92–104. doi: 10.1016/j.brainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 12.Koerber HR, Mirnics K, Lawson JJ. Synaptic plasticity in the adult spinal dorsal horn: the appearance of new functional connections following peripheral nerve regeneration. Exp Neurol. 2006;200:468–479. doi: 10.1016/j.expneurol.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Apfel SC, Wright DE, Wiideman AM, Dormia C, Snider WD, Kessler JA. Nerve growth factor regulates the expression of brain-derived neurotrophic factor mRNA in the peripheral nervous system. Mol Cell Neurosci. 1996;7:134–142. doi: 10.1006/mcne.1996.0010. [DOI] [PubMed] [Google Scholar]
- 14.Udina E, Cobianchi S, Allodi I, Navarro X. Effects of activity-dependent strategies on regeneration and plasticity after peripheral nerve injuries. Ann Anat. 2011;193:347–353. doi: 10.1016/j.aanat.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Deschenes MR, Maresh CM, Crivello JF, Armstrong LE, Kraemer WJ, Covault J. The effects of exercise training of different intensities on neuromuscular junction morphology. J Neurocytol. 1993;22:603–615. doi: 10.1007/BF01181487. [DOI] [PubMed] [Google Scholar]
- 16.Tomas J, Santafe M, Lanuza MA, Fenoll-Brunet MR. Physiological activity-dependent ultrastructural plasticity in normal adult rat neuromuscular junctions. Biol Cell. 1997;89:19–28. doi: 10.1016/s0248-4900(99)80078-1. [DOI] [PubMed] [Google Scholar]
- 17.Molteni R, Zheng JQ, Ying Z, Gomez-Pinilla F, Twiss JL. Voluntary exercise increases axonal regeneration from sensory neurons. Proc Natl Acad Sci U S A. 2004;101:8473–8478. doi: 10.1073/pnas.0401443101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gordon T, Sulaiman O, Boyd JG. Experimental strategies to promote functional recovery after peripheral nerve injuries. J Peripher Nerv Syst. 2003;8:236–250. doi: 10.1111/j.1085-9489.2003.03029.x. [DOI] [PubMed] [Google Scholar]
- 19.Cup EH, Pieterse AJ, Ten Broek-Pastoor JM, Munneke M, van Engelen BG, Hendricks HT, et al. Exercise therapy and other types of physical therapy for patients with neuromuscular diseases: a systematic review. Arch Phys Med Rehabil. 2007;88:1452–1464. doi: 10.1016/j.apmr.2007.07.024. [DOI] [PubMed] [Google Scholar]
- 20.Ilha J, Araujo RT, Malysz T, Hermel EE, Rigon P, Xavier LL, et al. Endurance and resistance exercise training programs elicit specific effects on sciatic nerve regeneration after experimental traumatic lesion in rats. Neurorehabil Neural Repair. 2008;22:355–366. doi: 10.1177/1545968307313502. [DOI] [PubMed] [Google Scholar]
- 21.Bridge PM, Ball DJ, Mackinnon SE, Nakao Y, Brandt K, Hunter DA, et al. Nerve crush injuries - a model for axonotmesis. Exp Neurol. 1994;127:284–290. doi: 10.1006/exnr.1994.1104. [DOI] [PubMed] [Google Scholar]
- 22.Papalia I, Tos P, Scevola A, Raimondo S, Geuna S. The ulnar test: a method for the quantitative functional assessment of posttraumatic ulnar nerve recovery in the rat. J Neurosci Methods. 2006;154:198–203. doi: 10.1016/j.jneumeth.2005.12.012. [DOI] [PubMed] [Google Scholar]
- 23.Pagnussat AS, Michaelsen SM, Achaval M, Netto CA. Skilled forelimb reaching in Wistar rats: evaluation by means of Montoya staircase test. J Neurosci Methods. 2009;177:115–121. doi: 10.1016/j.jneumeth.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Schallert T. Behavioral tests for preclinical intervention assessment. NeuroRx. 2006;3:497–504. doi: 10.1016/j.nurx.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Medinaceli L. Interpreting nerve morphometry data after experimental traumatic lesions. J Neurosci Methods. 1995;58:29–37. doi: 10.1016/0165-0270(94)00156-b. [DOI] [PubMed] [Google Scholar]
- 26.Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- 27.Duff SV. Impact of peripheral nerve injury on sensorimotor control. J Hand Ther. 2005;18:277–291. doi: 10.1197/j.jht.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Ploughman M, Attwood Z, White N, Dore JJ, Corbett D. Endurance exercise facilitates relearning of forelimb motor skill after focal ischemia. Eur J Neurosci. 2007;25:3453–3460. doi: 10.1111/j.1460-9568.2007.05591.x. [DOI] [PubMed] [Google Scholar]
- 29.Ferre J, Brunet R, Santafe M, Mayayo E. Changes in motor nerve terminals during bupivacaine-induced postsynaptic deprivation. J Anat. 1989;162:225–234. [PMC free article] [PubMed] [Google Scholar]
- 30.Tomas J, Batlle J, Fenoll MR, Santafe M, Lanuza MA. Activity-dependent plastic changes in the motor nerve terminals of the adult rat. Biol Cell. 1993;79:133–137. doi: 10.1111/j.1768-322x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- 31.Munro CA, Szalai JP, Mackinnon SE, Midha R. Lack of association between outcome measures of nerve regeneration. Muscle Nerve. 1998;21:1095–1097. doi: 10.1002/(sici)1097-4598(199808)21:8<1095::aid-mus20>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 32.Marqueste T, Alliez JR, Alluin O, Jammes Y, Decherchi P. Neuromuscular rehabilitation by treadmill running or electrical stimulation after peripheral nerve injury and repair. J Appl Physiol. 2004;96:1988–1995. doi: 10.1152/japplphysiol.00775.2003. [DOI] [PubMed] [Google Scholar]
- 33.van Meeteren NL, Brakkee JH, Helders PJ, Gispen WH. The effect of exercise training on functional recovery after sciatic nerve crush in the rat. J Peripher Nerv Syst. 1998;3:277–282. [PubMed] [Google Scholar]
- 34.Sabatier MJ, Redmon N, Schwartz G, English AW. Treadmill training promotes axon regeneration in injured peripheral nerves. Exp Neurol. 2008;211:489–493. doi: 10.1016/j.expneurol.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutmann E, Jakoubek B. Effect of increased motor activity on regeneration of the peripheral nerve in young rats. Physiol Bohemoslov. 1963;12:463–468. [PubMed] [Google Scholar]
- 36.van der Kooi EL, Lindeman E, Riphagen I. Strength training and aerobic exercise training for muscle disease. Cochrane Database Syst Rev. 2005:CD003907. doi: 10.1002/14651858.CD003907.pub4. [DOI] [PubMed] [Google Scholar]
- 37.Haastert K, Ying Z, Grothe C, Gomez-Pinilla F. The effects of FGF-2 gene therapy combined with voluntary exercise on axonal regeneration across peripheral nerve gaps. Neurosci Lett. 2008;443:179–183. doi: 10.1016/j.neulet.2008.07.087. [DOI] [PMC free article] [PubMed] [Google Scholar]