Abstract
The objective of this study was to observe possible interactions between the renin-angiotensin and nitrergic systems in chronic hypoxia-induced pulmonary hypertension in newborn piglets. Thirteen chronically instrumented newborn piglets (6.3 ± 0.9 days; 2369 ± 491 g) were randomly assigned to receive saline (placebo, P) or the AT1 receptor (AT1-R) blocker L-158,809 (L) during 6 days of hypoxia (FiO2 = 0.12). During hypoxia, pulmonary arterial pressure (Ppa; P < 0.0001), pulmonary vascular resistance (PVR; P < 0.02) and the pulmonary to systemic vascular resistance ratio (PVR/SVR; P < 0.05) were significantly attenuated in the L (N = 7) group compared to the P group (N = 6). Western blot analysis of lung proteins showed a significant decrease of endothelial NOS (eNOS) in both P and L animals, and of AT1-R in P animals during hypoxia compared to normoxic animals (C group, N = 5; P < 0.01 for all groups). AT1-R tended to decrease in L animals. Inducible NOS (iNOS) did not differ among P, L, and C animals and iNOS immunohistochemical staining in macrophages was significantly more intense in L than in P animals (P < 0.01). The vascular endothelium showed moderate or strong eNOS and AT1-R staining. Macrophages and pneumocytes showed moderate or strong iNOS and AT1-R staining, but C animals showed weak iNOS and AT1-R staining. Macrophages of L and P animals showed moderate and weak AT2-R staining, respectively, but the endothelium of all groups only showed weak staining. In conclusion, pulmonary hypertension induced by chronic hypoxia in newborn piglets is partially attenuated by AT1-R blockade. We suggest that AT1-R blockade might act through AT2-R and/or Mas receptors and the nitrergic system in the lungs of hypoxemic newborn piglets.
Keywords: Newborn animals, Pulmonary hypertension, Angiotensin II, Angiotensin receptors, Hypoxia
Introduction
Pulmonary hypertension induced by chronic hypoxia is a complex process of sustained elevation of pulmonary artery pressure involving vasoconstriction and pulmonary vessel remodeling. These events have been consistently observed in experimental animal models and in adult humans (1), but in newborns and in neonatal animal models, only one study showed involvement of the renin-angiotensin system (RAS) (2). They are multifactorial processes involving different mediators such as endothelin and angiotensin II (Ang II) and nitric oxide. Ang II is a powerful pulmonary vasoconstrictor and promoter of vascular smooth muscle cell (VSMC) growth in humans and animals. Ang II can act through the angiotensin type 1 receptor (AT1-R) to induce mitogenesis, stimulate DNA synthesis, promote cellular proliferation in cultured rat VSMC, and to enhance growth factors such as platelet-derived growth factor and transforming-growth factor β (3-5). The mechanisms of action of Ang II involve binding to AT1-R, as demonstrated in different studies reporting attenuation of pulmonary hypertension by AT1-R blockers and transient up-regulation of AT1-R in adult rats exposed to chronic hypoxia (6,7). Moreover, we have demonstrated that pulmonary vasoconstriction induced by acute hypoxia was significantly attenuated by the AT1-R blocker losartan in piglets (2). Therefore, it is possible that the RAS participates in the mechanisms leading to pulmonary hypertension induced by chronic hypoxia (4,5).
It is well known that AT1-R blockade stimulates nitric oxide (NO) production via AT2-R, a process that may result in the attenuation of pulmonary hypertension induced by chronic hypoxia (8,9). One possible endothelial mechanism of regulation of the pulmonary vascular tone during chronic hypoxia is by decreasing endothelial NO synthase (eNOS) expression, a hypothesis supported by the fact that eNOS expression is significantly lower in newborn piglets exposed to chronic hypoxia compared to a control group (10). However, the interactions between the RAS and nitrergic systems in the pulmonary vasculature have not been studied in human or animal newborns with pulmonary hypertension induced by chronic hypoxia. Asphyxiated newborn infants are predisposed to persistent pulmonary hypertension, and these mechanisms could be partially involved in this condition (11).
We propose, as a working hypothesis, that pulmonary hypertension induced by chronic hypoxia in newborn piglets is mediated by increased circulating Ang II levels, associated with down-regulation of eNOS. As a consequence, hypoxic pulmonary hypertension can be ameliorated by pretreatment with the AT1-R blocker L-158,809 through increased NO production.
Material and Methods
Animal preparation
Thirteen newborn piglets (age = 6.3 ± 0.9 days; weight = 2369 ± 491 g) were anesthetized intraperitoneally (ip), with 2% (w/v) isoflurane and 50 µg/kg fentanyl. The piglets were paralyzed with an ip injection of 0.4 mg/kg pancuronium bromide. A 3.5-mm tube was placed in the trachea and the piglet was mechanically ventilated with a time-cycled, pressure-limited infant ventilator (Sechrist, Model IV-100 B Infant Ventilator, USA) with peak inspiratory pressure set at 12 cmH2O, positive end-expiratory pressure set at 2 cmH2O, with a respiratory rate of 30 breaths/min, an inspiratory time of 0.5 s, and FiO2 = 0.50 during surgery. The femoral artery was cannulated with a 3.5-French umbilical catheter and the femoral vein with a 5-French Swan-Ganz thermodilution catheter. The Swan-Ganz catheter was advanced under fluoroscopic guidance into the pulmonary artery, and was maintained patent with a heparin lock (1000 U/mL). Both catheters were fixed in a pouch located on the back of the animal. The piglets received cefoxitin intravenously at 100 mg·kg−1·day−1 and acetaminophen (10 mg/kg orally) immediately after surgery and thereafter as needed.
The femoral artery catheter was used for mean systemic arterial pressure (Psa) measurements and for blood sampling for the determination of arterial blood gases (ABG), pH, and hemoglobin. The Swan-Ganz catheter was used to measure pulmonary artery (Ppa) and wedge pressure (Pwp), right atrial pressure (Rap), and cardiac output (CO). CO was measured by thermodilution using a CO computer (Model 95510-1, Edwards Laboratory, USA). Vascular pressures (Ppa, Pwp, Rap, Psa) were measured with pressure transducers (Model P23-ID, Gould Instruments, USA) and Grass pre-amplifiers (Model 7D, Grass Instrument, USA). All signals were simultaneously digitized with a frequency of 100 Hz using an analog-to-digital board (AT - CODAS, Dataq Instruments Inc., USA) and stored in a personal computer for analysis. ABG and pH were analyzed with a blood gas analyzer (model 238, Ciba-Corning, England) and corrected for core temperature. Hemoglobin was measured with a CO-oximeter (model 482 - Instrumentation Laboratory, USA).
Study protocol
The studies were started 48-72 h after surgery. Thirteen unanesthetized and chronically instrumented newborn piglets were randomly assigned to receive saline as placebo (P group; N = 6), or the AT1-R antagonist L-158,809 (L group; N = 7). The animals were placed in a sling inside a Plexiglas chamber, in a thermoneutral environment (26°-28°C) with 70% relative humidity, where CO2 was scavenged with soda lime, and the CO2 tension was kept at less than 6 mmHg. Piglets were fed ad libitum with newborn piglet milk during the study. All hemodynamic measurements were made during quiet sleep and evaluated by behavioral assessment. Heparinized saline was infused continuously through the pulmonary artery catheter (10 IU/mL) at a rate of 5 mL/h throughout the study.
After a 30-min period of stabilization, the hemodynamic measurements (Psa, Ppa, Pwp, Rap, CO), and blood sampling for ABG were obtained and referred to as baseline normoxia values. Saline or L-158,809 (1 mg·kg−1·day−1, ip) was administered to the animals immediately after the measurements. The potassium salt of L-158,809 was prepared at 3 mg/mL in 15% (w/v) saturated sodium bicarbonate solution and the dose was selected on the basis of a study performed in adult pigs (12). After obtaining the baseline values, the oxygen concentration was reduced to 12% and kept at this level for 6 days, allowing free movement of the animals. The hemodynamic measurements and blood sampling were repeated twice on the second and sixth days of continuous hypoxia. Systemic vascular (SVR) and pulmonary vascular (PVR) resistances were calculated from the hemodynamic data. Five healthy newborn piglets were used as controls for immunohistochemistry and for immunoblotting studies (C group).
Handling and care of the animals were in accordance with the guidelines of the National Institute of Health, and the study protocol was approved by the Animal Care and Use Committee of the University of Miami.
Pathology and immunohistochemistry
Piglets were sacrificed with Euthanasia® solution immediately after completion of the experiment and the lungs were removed en bloc from the thorax. One lung was inflated through the trachea with 4% (w/v) paraformaldehyde in 0.1 M sodium phosphate buffer, pH 7.4, at room temperature, using a column pressure of 20 cmH2O for 30 min, and was then immersion-fixed in the same solution for at least 1 week. The other lung was processed for paraffin embedding.
The fixed lung was dehydrated, cleared with xylene and paraffin-embedded. Five-micrometer sections were cut, mounted on gelatin-chromalum-coated glass slides, and used for immunohistochemistry. All sections from groups C, P, and L were immunostained simultaneously for each of the primary antibodies used. Antigen recovery from dewaxed lung sections was carried out using a microwave oven, and immunostaining was performed as described elsewhere (13,14). Sections were then incubated with rabbit anti-AT1-R (Santa Cruz Biotechnology Inc., USA), goat anti-AT2 (Santa Cruz Biotechnology), mouse anti-eNOS (clone 3, BD Transduction Laboratories, USA), mouse anti-iNOS (clone 6, BD Transduction Laboratories), or rabbit anti-smooth muscle anti-α-actin (RDI Inc., USA) antibodies. Controls were prepared by incubating sections overnight with anti-AT1-R or anti-AT2-R antibodies previously adsorbed with their respective immunogens at 1:10 (w/v) and 1:5 (w/v) in blocking buffer, respectively. Primary antibodies replaced blocking buffer in additional controls. Antibodies were detected with a secondary biotinylated antibody and the Elite ABC kit (Vector, USA), using 3,3′-diaminobenzidine (Pierce Biotechnology Inc., USA) as the chromogen. Sections were analyzed and photographed with a model BX60-F3 microscope, a PM-35DX camera and a PM30 exposure control module (Olympus, Japan). Images were digitized using a Sprintscan 35 Plus Polaroid, and plates were mounted using the Photoshop 7.0 software.
Immunohistochemically stained sections were evaluated and scored blindly and independently by two investigators (ARM, SGR). A numerical value (modal score) ranging from 0 to 3 was assigned by each investigator to each slide on the basis of intensity and distribution of the stain, for the following variables: arterial and venular endothelium, capillaries, macrophages, alveolar interstitium, alveolar cells (type I and II pneumocytes), and bronchial epithelium. A zero score was assigned if no stain was present, grade 1 was assigned if there was mild staining, grade 2 if there was moderate staining, and grade 3 if staining was strong (15,16).
Western blotting analysis
Frozen lungs were homogenized (1 g lung to 9 mL buffer) in a water-ice bath, in 20 mM Tris-HCl buffer, pH 7.4, containing 0.32 M sucrose, 1% (w/v) sodium dodecyl sulfate (SDS), 10 mM disodium ethylenediamine tetra-acetic acid, 1 mM benzamidine, 0.3 mM phenylmethanesulfonylfluoride, 0.3 µM aprotinine, and 2 mM β-mercaptoethanol, using a Potter-Elvehjem homogenizer (Glas-Col, USA) at 1000 rpm. Homogenates were subjected to SDS-PAGE in 7-20% gradient gels using a discontinuous system (17). Proteins on the gel were transferred to nitrocellulose membranes (Hybond ECL, Amersham Biosciences, England) (18), which were used for the immunodetection of eNOS, inducible NOS (iNOS) and AT1-R, using the same primary antibodies as those employed for immunohistochemistry. Immunoblots were developed and documented using an enhanced chemiluminescence kit (Amersham Biosciences) and Hyperfilm ECL (Amersham Biosciences), respectively. Controls were obtained using primary antibodies pre-adsorbed with their respective immunogens, and also omitting the primary antibodies. Immunoblotting images were quantified using the Gel-Pro Analyzer version 3.1 software (Media Cybernetics, USA). Integrated optical densities (IOD) for the specific protein bands were determined, and the ratio IOD/µg homogenate protein applied to the gel was calculated.
Statistical analyses
Repeated measures analysis of variance (ANOVA) was used to compare the hemodynamic patterns of response of the P and L groups, both over time (time-treatment interaction) and independent of time (overall group difference) for the dependent variables. One-way ANOVA was used to compare Western blot data among the P, L, and C groups, and the Tukey multiple comparison test was used as post hoc analysis. The Mann-Whitney rank sum test was used to compare the immunohistochemical scores. The consistency between two investigators scoring immunohistochemical results was analyzed using the weighed κ coefficient that ranged from 0.7 to 0.85, thus indicating good agreement. Data are reported as means ± SD. P < 0.05 was considered to be statistically significant.
Results
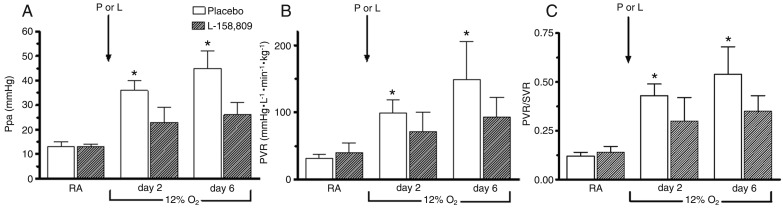
There were no statistically significant differences in weight gain or hemoglobin between the P and L groups at any time in room air or 12% O2. There were no significant differences in ABG or acid-base values in room air/normoxia and hypoxia or in systemic hemodynamic responses (Psa, SVR, and CO) between the P and L groups. There was a statistically significant difference from normoxia to hypoxia in PaO2 values in both groups (Table 1). A significant attenuation of Ppa (P < 0.0001) and PVR (P < 0.02), and a decrease in the PVR/SVR ratio were observed during chronic hypoxia after AT1 receptor blockade (Figure 1), suggesting a selective action on the pulmonary circulation.
Table 1. Effect of hypoxia and treatment with an angiotensin II type 1 blocker on newborn piglets.
| Variables | Group | Normoxia | Chronic hypoxia | |
|---|---|---|---|---|
| 2 days | 6 days | |||
| pH | P | 7.47 ± 0.03 | 7.51 ± 0.02 | 7.48 ± 0.04 |
| L | 7.41 ± 0.05 | 7.48 ± 0.04 | 7.50 ± 0.06 | |
| PaCO2 (mmHg) | P | 39 ± 6 | 30 ± 2 | 30 ± 4 |
| L | 42 ± 5 | 30 ± 2 | 31 ± 4 | |
| PaO2 (mmHg) | P | 94 ± 8 | 40 ± 9* | 42 ± 7* |
| L | 103 ± 8 | 47 ± 9* | 45 ± 7* | |
| BE (mM) | P | 5 ± 4 | 2 ± 2 | 1 ± 5 |
| L | 3 ± 5 | 1 ± 3 | 3 ± 4 | |
| Psa (mmHg) | P | 71 ± 6 | 78 ± 9 | 79 ± 16 |
| L | 75 ± 5 | 68 ± 6 | 70 ± 8 | |
| SVR (mmHg·L−1·min−1·kg−1) | P | 254 ± 26 | 234 ± 56 | 284 ± 89 |
| L | 299 ± 69 | 239 ± 31 | 263 ± 51 | |
| CO (L·min−1·kg−1) | P | 0.28 ± 0.03 | 0.32 ± 0.04 | 0.28 ± 0.07 |
| L | 0.24 ± 0.04 | 0.27 ± 0.02 | 0.25 ± 0.04 | |
Data are reported as means ± SD. BE = base excess; Psa = mean systemic arterial pressure; SVR = systemic vascular resistance; CO = cardiac output; P = placebo group (N = 6); L = L-158,809 group (N = 7). There were no significant differences in arterial blood gas or acid-base values in room air/normoxia and hypoxia or in systemic hemodynamics and CO responses between groups. There was a statistically significant difference from normoxia to hypoxia in PaO2 values in both groups (*P < 0.05, Mann-Whitney U-test).
Figure 1. A, Pulmonary artery pressure (Ppa); B, pulmonary vascular resistance (PVR), and C, PVR/SVR ratio in normoxia and on the second and sixth days of hypoxia in the P and L groups. Pulmonary hypertension induced by chronic hypoxia was markedly attenuated after AT1 receptor blockade. SVR = systemic vascular resistance; P = placebo group; L = L-158,809 group; RA = room air. A significant decrease in the PVR/SVR ratio was observed during AT1 receptor blockade. Bars represent the mean ± SD value. *P < 0.05 for significant difference between the P and L groups (repeated measures ANOVA).
Immunohistochemistry
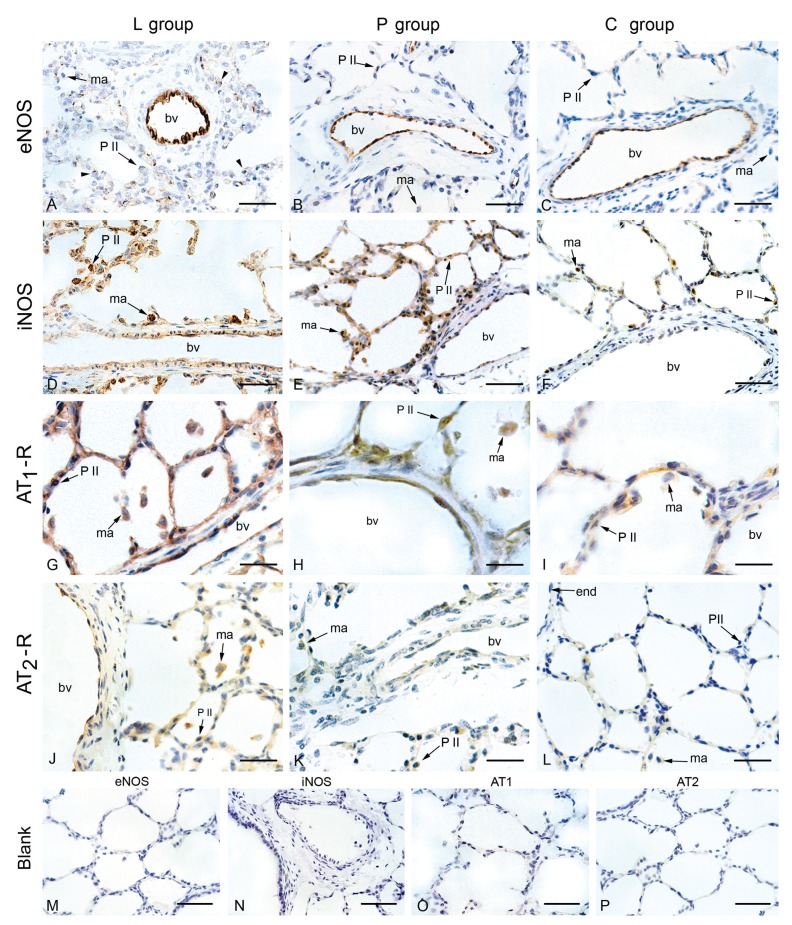
The endothelium of blood vessels with a diameter up to 600 µm exhibited a moderate (groups L and P; Figure 2A and B, respectively) or strong (control; Figure 2C) staining by anti-eNOS antibody. However, modal scores for eNOS staining were not significantly different among the three groups (Table 2).
Figure 2. Immunohistochemical localization of eNOS, iNOS, AT1-R and AT2-R in the lungs of the hypoxic groups L (L-158,809-treated) and P (placebo) that were submitted to 6 days of hypoxia and of normoxic piglets (group C, control). A-C, eNOS stained the endothelium of blood vessels (bv) in all groups; D-F, iNOS staining of the endothelium, alveolar and interstitial macrophages (ma) was observed in hypoxic (L and P groups) but not in normoxic animals; G-I, AT1-R was expressed in the endothelium of all groups; macrophages and type II pneumocytes (P II) were moderately stained in the groups L and P, and were weakly stained in normoxic (group C) animals; J-L, a weak AT2-R staining was observed in the endothelium (end), whereas macrophages in the P and L groups exhibited weak or moderate staining, respectively; M-P, immunohistochemistry controls for eNOS and iNOS were carried out omitting the respective primary antibodies (blank); controls for AT1-R and AT2-R were obtained by pre-adsorbing the primary antibodies with their respective peptide immunogens; none of the controls were stained, indicating that detection was specific. Bars = 4 µm (A-F); 2 µm (G-L) and 10 µm (M-P). eNOS = endothelial nitric oxide synthase; iNOS = inducible nitric oxide synthase, AT1-R = AT1 receptor; AT2-R = AT2 receptor.
Table 2. Immunohistochemical quantification by modal scores of eNOS, iNOS, AT1-R, and AT2-R.
| Structure | eNOS | iNOS | AT1-R | AT2-R | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | P | L | C | P | L | C | P | L | C | P | L | |
| Endothelium | 3 | 2 | 2 | 0 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 1 |
| Pneumo II | 0 | 0 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 1 | 1 |
| Macrophages | 0 | 0 | 0 | 0 | 1* | 2* | 1 | 2 | 2 | 0 | 1** | 2** |
0 = not detectable, 1 = mild/weak, 2 = moderate, and 3 = strong staining. eNOS = endothelial nitric oxide synthase; iNOS = inducible NOS; AT1-R = AT1 receptor; AT2-R = AT2 receptor; C = control group (N = 5); P = placebo group (N = 6); L = L-158,809 group (N = 7). There was a significant difference between the P and L groups for iNOS (*P < 0.01) and AT2-R (**P < 0.02) in macrophages (Mann-Whitney rank sum test).
The endothelium of the pulmonary arteries of the L and P groups was weakly stained (grade 1; Figure 2D,E) by anti-iNOS antibody, whereas that of the control group was essentially not stained (grade 0). Alveolar and interstitial macrophages were detected in lower numbers and showed grade 2 staining in the L group (Figure 2D) and grade 1 staining in the P group (Figure 2E), and this difference in macrophage staining was significant (P < 0.01). The control group (Figure 2F) exhibited very few macrophages that stained grade 0 (Table 2).
AT1-R immunostaining was detected in the endothelium and alveolar interstitium of all groups, with no statistically significant differences among the three groups. Macrophages and type II pneumocytes were moderately stained in groups L and P, and were weakly stained in the C group (Figure 2G-I; Table 2).
A mild AT2-R immunoreactivity was detected in the alveolar septa (grade 1) in all sections of the L group. Macrophages of the P and L groups exhibited weak (grade 1) or moderate (grade 2) staining, respectively (Figure 2J,K). The P group significantly differed from the L group (P < 0.02), where only half the sections showed a weak macrophage staining for AT2-R expression (grade 1), and a weak staining in the endothelium and alveolar septa (Figure 2K). The C group showed a very weak or absent expression of AT2-R (Figure 2L). Immunohistochemical blanks showed no staining for any of the antibodies used (Figure 2M-P).
Western blotting of eNOS, iNOS and AT1 receptors
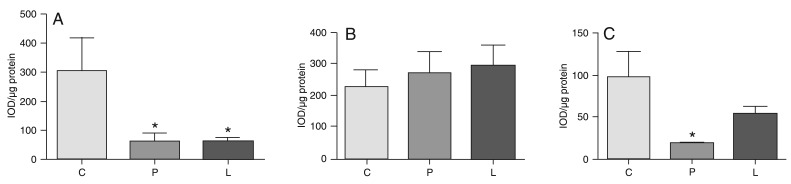
Hypoxia elicited a significant (P < 0.01) decrease of immunoreactive eNOS lung protein of both the P and L groups relative to the normoxia C group levels, as measured by immunoblotting (Figure 3A). Expression of iNOS protein did not significantly differ among the C, P and L groups (Figure 3B). Although AT1-R expression in the L group was numerically smaller than in the C group (Figure 3C), the difference was not statistically significant. The P group significantly differed from the C group (P < 0.001), but not from the L group. These data are consistent with the interpretation of a tendency to down-regulation of AT1-R in the L group as compared to the normoxia C group.
Figure 3. Western blot analysis of the expression of endothelial nitric oxide synthase (eNOS; Panel A), inducible nitric oxide synthase (iNOS; Panel B) and AT1 receptor (AT1-R; Panel C) in piglet lungs submitted (groups L and P) or not (group C) to 6 days of hypoxia. Data are reported as integrated optical density (IOD) per µg protein (mean ± SD), expressed in arbitrary units. C = control group; P = placebo group; L = L-158,809 group. *P < 0.01 compared to group C (one-way ANOVA and Tukey multiple comparison as a post hoc test).
Discussion
Pulmonary hypertension induced by chronic hypoxia in newborn piglets was markedly attenuated by L-158,809, a highly potent and selective nonpeptide AT1-R blocker (19). The fact that the increase in the PVR/SVR ratio was significantly attenuated during chronic hypoxia in the L group suggests the occurrence of a selective pulmonary vasodilatation after AT1-R blockade.
The attenuation of the pulmonary hypertension of newborn piglets exposed to hypoxia for 6 days is consistent with previous studies examining the role of the RAS during the development of chronic hypoxic pulmonary hypertension. Adult rats were exposed to 7 or 14 days of hypoxia, showing attenuation of pulmonary hypertension after AT1-R blockade (6,20). In another study using adult rats exposed for 2-6 weeks to a 10% oxygen atmosphere in a normobaric chamber, olmesartan medoxomil (an AT1-R blocker) significantly reduced the induction of hypoxic cor pulmonale not only on echocardiographic observations but also in brain natriuretic peptide, transforming growth factor β and endothelin gene expressions in molecular studies; systolic blood pressure was independent of olmesartan medoxomil (21). The same investigators reported that olmesartan still seems to block a potential positive feedback loop of the Ang II-AT1-R system, which may lead to attenuation of pro-inflammatory signals in the mouse lung, that are associated with hypoxic pulmonary hypertension (22). These observations were confirmed in adult humans when the RAS was not previously activated by dehydration or sodium depletion (23). In addition, we have demonstrated that pulmonary vasoconstriction induced by acute hypoxia in newborn piglets was significantly attenuated by the AT1-R blocker losartan (2).
It is important to consider possible mechanisms underlying pulmonary hypertension and the mechanisms related to the effects of the AT1-R antagonist, since hypoxic pulmonary hypertension was attenuated by AT1-R blockade in the present study. The association between the exposure of newborn piglets to chronic hypoxia and impairment of the endothelium-dependent vasodilatation ability related to the decreased eNOS expression has been very well demonstrated (10). It should be pointed out that eNOS expression did not return to control values after 6 days of AT1-R blockade.
Concerning other sources of NO production, iNOS protein expression was not significantly different among groups in the present study. However, there was a statistically significant difference in iNOS immunohistochemical expression in modal macrophage scores. Probably this difference was not detected in terms of protein expression because the Western blot data were assessed in the whole lung homogenate, and the iNOS expression was only different between the P and L groups at the level of macrophages.
Inducible NOS, in contrast to eNOS, has been primarily related to the immune function of macrophages. Inducible NOS is induced by a variety of stimuli such as cytokines, inflammatory factors, and hypoxia, which result in the release of much higher amounts of NO by very complex mechanisms involving different modulating factors such as hypoxia-induced factor 1, interleukin 1-β and Ang II (24-27). However, in agreement with the data of the present study, some studies have shown that iNOS is up-regulated in the pulmonary vasculature and in cardiac tissue in different animal models and in humans exposed to hypoxia, suggesting that hypoxia down-regulates eNOS activity and expression and up-regulates iNOS. This may represent an alternative adaptive mechanism counteracting the effects of hypoxia in the cardiovascular system. This is not necessarily a favorable adaptive mechanism because excess iNOS can induce the production of reactive oxygen species such as nitrotyrosine, facilitating tissue damage and hypotension (26,28-30).
Concerning AT1-R protein expression, the present study did not show the transient up-regulation observed in rodents (6). On the contrary, only a down-regulation was demonstrable in the placebo group compared to normoxic controls. This could be related to species differences and the duration of hypoxia to which the animals were submitted.
Analyzing the mechanisms that could explain the effects of AT1-R blockade, several studies have shown that AT1-R blockade acts through increased NO production via the AT2-R, demonstrating the relationship between the RAS and nitrergic systems in different models, including the pulmonary circulation, causing vasodilatation (8,9,31-33). Furthermore, increased Ang II can be converted to angiotensin-(1-7) by the action of angiotensin-converting enzyme-2. Angiotensin-(1-7) acts through the Mas receptors (34,35) resulting in pulmonary vasodilatation and thus can counteract the action of Ang II, increasing the release of NO (36,37).
The mild immunoreactive expression of AT2-R observed here in the P and L groups, significantly more intense in the L group, is unexpected during the neonatal period (6,38,39) and may represent a compensatory mechanism due to hypoxic pulmonary hypertension, perhaps enhanced by the AT1-R blockade in the L group.
In conclusion, pulmonary hypertension induced by chronic hypoxia was partially attenuated by AT1-R blockade in newborn piglets. The chronic hypoxic pulmonary hypertension could be explained by down-regulated eNOS expression. We suggest that AT1-R blockade attenuated the hypoxic pulmonary hypertension acting through AT2-R and/or Mas receptors and the nitrergic system in the lungs of hypoxemic newborn piglets.
Acknowledgments
We wish to thank Silvia Helena Henriques Camelo and Carlos Devia for help with data collection, and Adriana Pelegrini-da-Silva for the immunohistochemical studies. Research supported by the University of Miami: Project New Born. Merck Research Laboratories provided L-158,809 in powder form. J.S. Camelo Jr. received a scholarship from FAPESP.
References
- 1.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 2.Camelo JS, Jr, Hehre D, Devia C, Camelo SH, Bancalari E, Suguihara C. The role of angiotensin II receptor-1 blockade in the hypoxic pulmonary vasoconstriction response in newborn piglets. Neonatology. 2008;93:263–268. doi: 10.1159/000111879. [DOI] [PubMed] [Google Scholar]
- 3.Marshall RP, McAnulty RJ, Laurent GJ. Angiotensin II is mitogenic for human lung fibroblasts via activation of the type 1 receptor. Am J Respir Crit Care Med. 2000;161:1999–2004. doi: 10.1164/ajrccm.161.6.9907004. [DOI] [PubMed] [Google Scholar]
- 4.Jeffery TK, Wanstall JC. Pulmonary vascular remodeling: a target for therapeutic intervention in pulmonary hypertension. Pharmacol Ther. 2001;92:1–20. doi: 10.1016/s0163-7258(01)00157-7. [DOI] [PubMed] [Google Scholar]
- 5.Solari V, Puri P. Genetic polymorphisms of angiotensin system genes in congenital diaphragmatic hernia associated with persistent pulmonary hypertension. J Pediatr Surg. 2004;39:302–306. doi: 10.1016/j.jpedsurg.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Morrell NW, Morris KG, Stenmark KR. Role of angiotensin-converting enzyme and angiotensin II in development of hypoxic pulmonary hypertension. Am J Physiol. 1995;269:H1186–H1194. doi: 10.1152/ajpheart.1995.269.4.H1186. [DOI] [PubMed] [Google Scholar]
- 7.Chassagne C, Eddahibi S, Adamy C, Rideau D, Marotte F, Dubois-Rande JL, et al. Modulation of angiotensin II receptor expression during development and regression of hypoxic pulmonary hypertension. Am J Respir Cell Mol Biol. 2000;22:323–332. doi: 10.1165/ajrcmb.22.3.3701. [DOI] [PubMed] [Google Scholar]
- 8.Kalinowski L, Matys T, Chabielska E, Buczko W, Malinski T. Angiotensin II AT1 receptor antagonists inhibit platelet adhesion and aggregation by nitric oxide release. Hypertension. 2002;40:521–527. doi: 10.1161/01.hyp.0000034745.98129.ec. [DOI] [PubMed] [Google Scholar]
- 9.Ye S, Zhong H, Duong VN, Campese VM. Losartan reduces central and peripheral sympathetic nerve activity in a rat model of neurogenic hypertension. Hypertension. 2002;39:1101–1106. doi: 10.1161/01.hyp.0000018590.26853.c7. [DOI] [PubMed] [Google Scholar]
- 10.Fike CD, Aschner JL, Zhang Y, Kaplowitz MR. Impaired NO signaling in small pulmonary arteries of chronically hypoxic newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1244–L1254. doi: 10.1152/ajplung.00345.2003. [DOI] [PubMed] [Google Scholar]
- 11.Lapointe A, Barrington KJ. Pulmonary hypertension and the asphyxiated newborn. J Pediatr. 2011;158:e19–e24. doi: 10.1016/j.jpeds.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Huckle WR, Drag MD, Acker WR, Powers M, McFall RC, Holder DJ, et al. Effects of subtype-selective and balanced angiotensin II receptor antagonists in a porcine coronary artery model of vascular restenosis. Circulation. 1996;93:1009–1019. doi: 10.1161/01.cir.93.5.1009. [DOI] [PubMed] [Google Scholar]
- 13.Martins AR, Zanella CA, Zucchi FC, Dombroski TC, Costa ET, Guethe LM, et al. Immunolocalization of nitric oxide synthase isoforms in human archival and rat tissues, and cultured cells. J Neurosci Methods. 2011;198:16–22. doi: 10.1016/j.jneumeth.2011.02.024. [DOI] [PubMed] [Google Scholar]
- 14.Martins AR, Dias MM, Vasconcelos TM, Caldo H, Costa MC, Chimelli L, et al. Microwave-stimulated recovery of myosin-V immunoreactivity from formalin-fixed, paraffin-embedded human CNS. J Neurosci Methods. 1999;92:25–29. doi: 10.1016/s0165-0270(99)00090-4. [DOI] [PubMed] [Google Scholar]
- 15.Mirza MH, Oliver JL, Seahorn TL, Hosgood G, Moore RM. Detection and comparison of nitric oxide in clinically normal horses and those with naturally acquired small intestinal strangulation obstruction. Can J Vet Res. 1999;63:230–240. [PMC free article] [PubMed] [Google Scholar]
- 16.Tilelli CQ, Martins AR, Larson RE, Garcia-Cairasco N. Immunohistochemical localization of myosin Va in the adult rat brain. Neuroscience. 2003;121:573–586. doi: 10.1016/s0306-4522(03)00546-3. [DOI] [PubMed] [Google Scholar]
- 17.Laemmli UK, Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973;80:575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- 18.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fulton GJ, Davies MG, Barber L, Svendsen E, Hagen PO. Localized versus systemic angiotensin II receptor inhibition of intimal hyperplasia in experimental vein grafts by the specific angiotensin II receptor inhibitor L158,809. Surgery. 1998;123:218–227. [PubMed] [Google Scholar]
- 20.Zhao L, al-Tubuly R, Sebkhi A, Owji AA, Nunez DJ, Wilkins MR. Angiotensin II receptor expression and inhibition in the chronically hypoxic rat lung. Br J Pharmacol. 1996;119:1217–1222. doi: 10.1111/j.1476-5381.1996.tb16025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamoto T, Harasawa H, Akimoto K, Hirata H, Kaneko H, Kaneko N, et al. Effects of olmesartan medoxomil as an angiotensin II-receptor blocker in chronic hypoxic rats. Eur J Pharmacol. 2005;528:43–51. doi: 10.1016/j.ejphar.2005.10.063. [DOI] [PubMed] [Google Scholar]
- 22.Tanabe Y, Morikawa Y, Kato T, Kanai S, Watakabe T, Nishijima A, et al. Effects of olmesartan, an AT1 receptor antagonist, on hypoxia-induced activation of ERK1/2 and pro-inflammatory signals in the mouse lung. Naunyn Schmiedebergs Arch Pharmacol. 2006;374:235–248. doi: 10.1007/s00210-006-0110-1. [DOI] [PubMed] [Google Scholar]
- 23.Cargill RI, Lipworth BJ. Lisinopril attenuates acute hypoxic pulmonary vasoconstriction in humans. Chest. 1996;109:424–429. doi: 10.1378/chest.109.2.424. [DOI] [PubMed] [Google Scholar]
- 24.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2011 doi: 10.1093/eurheartj/ehr304. [ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angele MK, Schwacha MG, Smail N, Catania RA, Ayala A, Cioffi WG, et al. Hypoxemia in the absence of blood loss upregulates iNOS expression and activity in macrophages. Am J Physiol. 1999;276:C285–C290. doi: 10.1152/ajpcell.1999.276.2.C285. [DOI] [PubMed] [Google Scholar]
- 26.Strunk V, Hahnenkamp K, Schneuing M, Fischer LG, Rich GF. Selective iNOS inhibition prevents hypotension in septic rats while preserving endothelium-dependent vasodilation. Anesth Analg. 2001;92:681–687. doi: 10.1097/00000539-200103000-00025. [DOI] [PubMed] [Google Scholar]
- 27.Jiang B, Xu S, Hou X, Pimentel DR, Cohen RA. Angiotensin II differentially regulates interleukin-1-beta-inducible NO synthase (iNOS) and vascular cell adhesion molecule-1 (VCAM-1) expression: role of p38 MAPK. J Biol Chem. 2004;279:20363–20368. doi: 10.1074/jbc.M314172200. [DOI] [PubMed] [Google Scholar]
- 28.Palmer LA, Semenza GL, Stoler MH, Johns RA. Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cells via HIF-1. Am J Physiol. 1998;274:L212–L219. doi: 10.1152/ajplung.1998.274.2.L212. [DOI] [PubMed] [Google Scholar]
- 29.Aikio O, Vuopala K, Pokela ML, Andersson S, Hallman M. Nitrotyrosine and NO synthases in infants with respiratory failure: influence of inhaled NO. Pediatr Pulmonol. 2003;35:8–16. doi: 10.1002/ppul.10222. [DOI] [PubMed] [Google Scholar]
- 30.Shehata SM, Sharma HS, Mooi WJ, Tibboel D. Pulmonary hypertension in human newborns with congenital diaphragmatic hernia is associated with decreased vascular expression of nitric-oxide synthase. Cell Biochem Biophys. 2006;44:147–155. doi: 10.1385/CBB:44:1:147. [DOI] [PubMed] [Google Scholar]
- 31.Abadir PM, Carey RM, Siragy HM. Angiotensin AT2 receptors directly stimulate renal nitric oxide in bradykinin B2-receptor-null mice. Hypertension. 2003;42:600–604. doi: 10.1161/01.HYP.0000090323.58122.5C. [DOI] [PubMed] [Google Scholar]
- 32.Thai H, Wollmuth J, Goldman S, Gaballa M. Angiotensin subtype 1 receptor (AT1) blockade improves vasorelaxation in heart failure by up-regulation of endothelial nitric-oxide synthase via activation of the AT2 receptor. J Pharmacol Exp Ther. 2003;307:1171–1178. doi: 10.1124/jpet.103.054916. [DOI] [PubMed] [Google Scholar]
- 33.Olson S, Oeckler R, Li X, Du L, Traganos F, Zhao X, et al. Angiotensin II stimulates nitric oxide production in pulmonary artery endothelium via the type 2 receptor. Am J Physiol Lung Cell Mol Physiol. 2004;287:L559–L568. doi: 10.1152/ajplung.00312.2003. [DOI] [PubMed] [Google Scholar]
- 34.Santos RA, Simoes e Silva AC, Maric C, Silva DM, Machado RP, de Buhr I, et al. Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Natl Acad Sci U S A. 2003;100:8258–8263. doi: 10.1073/pnas.1432869100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Santos RA, Ferreira AJ, Simoes e Silva AC. Recent advances in the angiotensin-converting enzyme 2-angiotensin(1-7)-Mas axis. Exp Physiol. 2008;93:519–527. doi: 10.1113/expphysiol.2008.042002. [DOI] [PubMed] [Google Scholar]
- 36.Kuba K, Imai Y, Ohto-Nakanishi T, Penninger JM. Trilogy of ACE2: a peptidase in the renin-angiotensin system, a SARS receptor, and a partner for amino acid transporters. Pharmacol Ther. 2010;128:119–128. doi: 10.1016/j.pharmthera.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shenoy V, Qi Y, Katovich MJ, Raizada MK. ACE2, a promising therapeutic target for pulmonary hypertension. Curr Opin Pharmacol. 2011;11:150–155. doi: 10.1016/j.coph.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Treml B, Neu N, Kleinsasser A, Gritsch C, Finsterwalder T, Geiger R, et al. Recombinant angiotensin-converting enzyme 2 improves pulmonary blood flow and oxygenation in lipopolysaccharide-induced lung injury in piglets. Crit Care Med. 2010;38:596–601. doi: 10.1097/CCM.0b013e3181c03009. [DOI] [PubMed] [Google Scholar]
- 39.Cox BE, Liu XT, Fluharty SJ, Rosenfeld CR. Vessel-specific regulation of angiotensin II receptor subtypes during ovine development. Pediatr Res. 2005;57:124–132. doi: 10.1203/01.PDR.0000148067.07899.B9. [DOI] [PubMed] [Google Scholar]