Abstract
The adhesins of extraintestinal pathogenic Escherichia coli are essential for mediating direct interactions between the microbes and the host cell surfaces that they infect. Using fluorescence microscopy and gentamycin protection assays, we observed that 49 sepsis-associated E. coli (SEPEC) strains isolated from human adults adhered to and invaded Vero cells in the presence of D-mannose (100%). In addition, bacteria concentrations of approximately 2 × 107 CFU/mL were recovered from Vero cells following an invasion assay. Furthermore, PCR analysis of adhesin genes showed that 98.0% of these SEPEC strains tested positive for fimH, 69.4% for flu, 53.1% for csgA, 38.8% for mat, and 32.7% for iha. Analysis of the invasin genes showed that 16.3% of the SEPEC strains were positive for tia, 12.3% for gimB, and 10.2% for ibeA. Therefore, these data suggest that SEPEC adhesion to cell surfaces occurs through non-fimH mechanisms. Scanning electron microscopy showed the formation of microcolonies on the Vero cell surface. SEPEC invasiveness was also confirmed by the presence of intracellular bacteria, and ultrastructural analysis using electron transmission microscopy revealed bacteria inside the Vero cells. Taken together, these results demonstrate that these SEPEC strains had the ability to adhere to and invade Vero cells. Moreover, these data support the theory that renal cells may be the predominant pathway through which SEPEC enters human blood vessels.
Keywords: Adhesion, Invasiveness, Escherichia coli, Sepsis
Introduction
Escherichia coli bacteria are commonly described as the major causative agent of extraintestinal infections, such as neonatal meningitis, bacteremia, pyelonephritis, cystitis, prostatitis, and sepsis (1-4). Paradoxically, this microorganism is also a predominant facultative member of the normal human intestinal microbiota (5,6). The adhesion of pathogenic bacteria to host cells represents the first step in establishing an infection. Subsequent events include the colonization of tissues and, in certain cases, cellular invasion followed by intracellular multiplication or persistence. The adhesion process is initiated when surface structures known as adhesins bind to their specific ligands, host cell receptors or extracellular matrix proteins (4,7).
Although the occurrence of E. coli bacteremia and sepsis has increased in recent years (3,8), there are few reports detailing the mechanisms of sepsis-associated E. coli (SEPEC) pathogenesis. Furthermore, it is possible that the virulence genotypes and phylogenetic backgrounds of E. coli strains differ in diverse geographical regions (9). Recently, a study on the genetic profile of SEPEC strains reported heterogeneity in previously described extraintestinal pathogenic E. coli (ExPEC) virulence factors (10).
The contamination by ExPEC strains is usually related to the contamination of urinary or other catheters (3,11). The urinary tract is the main gateway for ExPEC, particularly in cases of sepsis (4). Urinary infections often provoke bacteremia, especially in patients that are hospitalized because of catheter contamination by ExPEC biofilms (3). ExPEC strains, such as uropathogenic E. coli (UPEC), neonatal meningitis-associated E. coli (NMEC), and SEPEC, typically share many virulence factors that promote the colonization of host surfaces, avoidance and/or subversion of host defense mechanisms, invasion and/or injury of cells and tissues and the initiation of inflammatory responses (4,9,12). In this study, we evaluated the presence of five adhesin-encoding genes (fimH, flu, csgA, mat, and iha) and three invasin-encoding genes (ibeA, tia and gimB) in 49 E. coli isolates from human sepsis patients and also investigated the adhesive and invasive abilities of these isolates in Vero cells.
Material and Methods
Bacterial strains
We analyzed a collection of 49 previously described SEPEC strains (10) maintained in our laboratories as stocks at room temperature. All strains were screened for the presence of adhesin/invasin-encoding genes and qualitatively assayed for their adhesion to and invasion of Vero cells. Based on these data, we selected four strains for further analysis using scanning (SEM) and transmission electron microscopy (TEM).
Genotyping characterization
Molecular analysis assays. PCR amplifications were performed in a final reaction volume of 30 µL. The primers used for the analyses were selected from previously published sequences: i) adhesins: type I fimbriae (fimH) (13), antigen 43 (flu) (14), curly structural gene (csgA) (7), curly regulator gene (crl) (15), meningitis-associated and temperature regulated fimbriae (mat), iron-regulated-gene-homologue adhesion (iha); ii) invasins: pathogenicity island-associated and meningitis-associated (gimB) (9), brain microvascular endothelium cell invasion (ibeA) (9), and toxigenic invasion locus in enterotoxigenic E. coli strains (tia) (9), and iii) K1 capsular polysaccharide (neuC) (16). The PCR amplifications were performed using a GeneAmp PCR System 2400 thermocycler (Applied Biosystems, USA) under the following conditions: denaturation for 5 min at 94°C, 30 cycles of 60 s at 94°C, 30 s at the annealing temperature and 60 s at 72°C, and a final extension step of 7 min at 72°C.
Adhesion and invasion assays
Qualitative adhesion assays. Monolayers of 105 Vero cells (African green monkey kidney cells) obtained from the American Type Culture Collection (ATCC, USA) were grown on coverslips (Falcon Becton Dickinson, USA) in 24-well plates in Dulbecco's modified Eagle's medium (DMEM) (Gibco-BRL, USA) containing 10% fetal bovine serum (FBS). The bacteria were grown in 3 mL trypticase soy broth for 18 h at 37°C. Vero cell monolayers were subsequently inoculated with approximately 3 × 108 bacteria/mL (tube 1 on the MacFarland scale) and incubated at 37°C for 3 h (17). All assays were performed in duplicate on different days, with or without D-mannose in the medium. We analyzed 49 SEPEC strains and used E. coli C600 as a negative control.
After 3 h of infection, each well was washed with PBS, and 0.1% Triton X-100 was added for 5 min. The coverslips were washed again, and 10 µg/mL phalloidin was added to each coverslip for 30 min in the absence of light. Following this incubation, the coverslips were washed and treated with 100 µg/mL RNase for 10 min. Propidium iodide was then added to the coverslips at 1.7 µM for 5 min. The coverslips were then analyzed by microscopy.
Scanning electron microscopy
An ultrastructural analysis using SEM was performed on four SEPEC strains (SEPEC 8, 17, 19, and 53). After 3 h of infection, Vero cells were fixed with 2% paraformaldehyde (Sigma, USA) and 2% glutaraldehyde (Electron Microscope Science, USA) in 10 mM cacodylate buffer, pH 7.4, mixed with equal volumes of cell culture medium. Subsequently, the slides were dehydrated with ethanol and immersed in propylene oxide/Epon 812 (Electron Microscope Science; ratios of 1:1 and 3:1) for 6 h. Ultrathin sections were obtained using an ultramicrotome and double-stained with 2% uranyl acetate (Fluka, Switzerland) and 0.5% lead citrate (Fluka). Finally, these sections were analyzed using a LEO-Schot Zeiss EM906 TEM at 80 kV.
Quantitative invasion assays. The bacterial invasion of Vero cells was measured using gentamycin protection assays and the enumeration of the colonies of viable intracellular bacteria (18). Prior to this assay, the minimal bactericidal concentration of gentamycin that reduced bacterial isolate counts by up to 99.9% was determined in DMEM, and gentamycin was first tested at 50 and 100 µg/mL. Because similar results were obtained independent of gentamycin concentration, a final gentamycin concentration of 100 μg/mL was used in subsequent experiments. After 3 h of infection, the cell monolayers were washed with PBS, and 1.0 mL DMEM plus 2.0% FBS and 100 µg/mL gentamycin was added to each well to kill the extracellular bacteria. The plates were incubated for 2 h at 37°C before being washed ten times with 1 mL PBS per well. The intracellular bacteria were recovered by cell lysis with Triton X-100 (1.0%), and bacterial cell invasion was determined based on the number of colony-forming units (CFU/mL) on MacConkey agar plates (19). All assays were performed in duplicate on two different days, with or without D-mannose in the medium. Our results were compared to a positive control (enteroinvasive E. coli (EIEC) serotype O124:H-) (18) and a negative control (E. coli C600).
Transmission electron microscopy. TEM analyses were performed on four SEPEC strains (SEPEC 8, 17, 19, and 53). After 3 h of infection, Vero cell monolayers were fixed with 2.5% glutaraldehyde, 4% paraformaldehyde and 10 mM calcium chloride in 100 mM cacodylate buffer, pH 7.4, for 1 h at 25°C. Next, the cells were washed in cacodylate buffer and post-fixed with 1% osmium tetroxide (OsO4) and 0.8% K4Fe(CN)6·3H2O in 100 mM cacodylate buffer for 1 h. The samples were then washed in cacodylate buffer, dehydrated in a graded series of alcohol, and embedded in Spurr resin. Ultrathin sections were obtained, stained with uranyl acetate and lead citrate, and examined using a Jeol 1200 EX TEM.
Results and Discussion
Studies demonstrating the adhesion and invasiveness of human SEPEC strains are rare (10). In contrast to diarrheagenic E. coli, UPEC and NMEC, SEPEC strains do not exhibit a well-defined molecular virulence profile (10,12), and their pathogenicity mechanisms are not clear (4). SEPEC has emerged as a distinct E. coli group that appears to display a combination of the virulence characteristics of the other E. coli groups, including diarrheagenic E. coli and other ExPEC groups (4,10). In the present study, we analyzed the bacterial adhesion and invasion properties of SEPEC strains involved in 49 cases of human sepsis at the University Hospital of the State University of Campinas, Brazil. All of these studies were performed using Vero cells to represent the urinary tract because the kidneys could be an entry point of SEPEC into the bloodstream. The three-dimensional (3-D) architecture of the extracellular matrix of SEPEC isolates undergoing adhesion was also examined, and the invasion process was visualized using TEM. Vero cells are not derived from tumors and are an analogous model for studying SEPEC infections in renal cells, as previously published (10).
Currently, the mechanisms by which E. coli attaches to surfaces are not well defined. However, some cellular and extracellular structures, such as flagella, type I fimbriae, antigen 43, curli fimbriae and exopolysaccharides (EPS), are fundamental in establishing E. coli adhesion (20-22). Flagella are important for the initial interaction with and the movement of E. coli along surfaces (21), and type I fimbriae are required for the initial adhesion of E. coli to substrates (21). Both antigen 43, an outer membrane protein, and curli fimbriae play important roles in auto-aggregation, increased adhesion (23) and the persistence of bacteria on live tissues (24,25). Additionally, EPS produced by adhered bacteria builds extracellular matrices that are responsible for the formation of 3-D micro-colonies and E. coli persistence on different substrates (21).
In our study, the genotypic analysis of virulence factor genes showed a high prevalence of adhesin-encoding genes, with 48 isolates testing positive for fimH (98.0%), 34 for flu (69.4%), 26 for csgA (53.1%), 19 for mat (38.8%), and 16 for iha (32.7%). However, a low prevalence of invasin-encoding genes was observed, and only 8 isolates were positive for tia (16.3%), 6 for gimB (12.3%) and 5 for ibeA (10.2%) (Table 1). The neuC gene, which encodes K1 capsular polysaccharide, was amplified from only 24.5% of the strains. Because epidemiological studies have linked K1 to NMEC (26,27), these data suggest a genetic relationship between some SEPEC and NMEC isolates.
Table 1. Genotypic analysis of human sepsis-associated Escherichia coli (SEPEC) strains.
| No. SEPEC strain | K1 capsular polysaccharide | Adhesins | Invasins | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| neuC | fimH | flu | csgA | crl | mat | iha | gimB | ibeA | tia | |
| 3 | - | + | - | - | + | - | - | - | - | + |
| 4 | - | + | + | - | + | - | + | - | - | - |
| 5 | + | + | - | - | + | - | - | - | - | + |
| 8 | - | + | + | - | + | - | - | - | - | - |
| 10 | - | + | - | + | + | + | - | + | + | - |
| 13 | - | + | + | - | + | - | + | - | - | - |
| 14 | - | + | + | - | + | + | - | - | + | - |
| 16 | - | + | + | - | + | + | + | - | + | - |
| 17 | - | + | + | - | + | - | + | + | - | - |
| 18 | - | + | + | + | + | - | + | + | - | - |
| 19 | - | + | - | - | + | - | - | + | - | - |
| 20 | - | + | + | + | + | - | - | - | - | - |
| 21 | - | + | + | + | + | - | - | - | - | - |
| 22 | - | + | + | - | + | - | + | - | - | - |
| 23 | - | + | - | + | + | + | - | - | + | - |
| 24 | - | + | - | + | + | + | - | + | - | - |
| 25 | - | - | + | - | + | + | + | - | + | - |
| 26 | - | + | - | + | + | + | - | - | - | - |
| 27 | + | + | + | - | + | - | - | - | - | - |
| 28 | + | + | + | - | + | + | - | - | - | - |
| 29 | + | + | - | - | + | - | - | - | - | - |
| 31 | - | + | + | + | + | + | - | - | - | - |
| 33 | - | + | + | + | + | - | - | - | - | - |
| 34 | + | + | + | - | + | + | + | - | - | + |
| 36 | + | + | + | + | + | - | - | - | - | - |
| 37 | + | + | + | + | + | + | - | - | - | - |
| 38 | - | + | + | - | + | - | + | - | - | + |
| 39 | - | + | + | + | + | + | - | - | - | - |
| 40 | - | + | + | + | + | - | - | - | - | - |
| 41 | - | + | + | + | + | + | + | - | - | - |
| 42 | - | + | + | + | + | - | - | - | - | - |
| 43 | - | + | - | + | + | + | - | - | - | - |
| 44 | - | + | - | - | - | - | - | - | - | - |
| 46 | - | + | + | + | - | - | - | - | - | - |
| 47 | + | + | - | - | + | - | - | - | - | - |
| 48 | - | + | + | + | + | + | + | - | - | - |
| 49 | + | + | + | + | + | - | - | - | - | + |
| 50 | + | + | + | + | + | + | - | + | - | + |
| 51 | + | + | + | - | + | - | - | - | - | + |
| 52 | - | + | - | + | + | + | - | - | - | - |
| 53 | - | + | + | + | + | - | - | - | - | - |
| 54 | + | + | + | + | + | - | + | - | - | + |
| 56 | - | + | + | + | + | + | + | - | - | - |
| 57 | - | + | - | - | + | - | + | - | - | - |
| 58 | - | + | + | + | + | - | + | - | - | - |
| 62 | - | + | + | + | + | + | + | - | - | - |
| 71 | - | + | - | - | + | - | - | - | - | - |
| 74 | - | + | - | - | + | - | - | - | - | - |
| 76 | - | + | + | - | + | - | - | - | - | - |
Data demonstrating the high prevalence of adhesin genes and the low prevalence of invasin genes in SEPEC strains are consistent with the literature (10), suggesting that SEPEC most likely adheres to cell surfaces by mechanisms different from those of other E. coli strains. Additionally, it is likely that SEPEC strains may express several other adhesion factors that are not yet known to be involved in their pathogenicity; these factors may also play a role in tissue specificity and the adhesion of SEPEC to epithelial and endothelial cells (data not shown).
For further adhesion and invasion assays, we analyzed four SEPEC strains using fluorescence microscopy, SEM and TEM. The strains chosen were SEPEC 8 (fimH+/flu+), SEPEC 17 (fimH+/flu+/iha+/gimB+), SEPEC 19 (fimH+/gimB+), and SEPEC 53 (fimH+/flu+/csgA+).
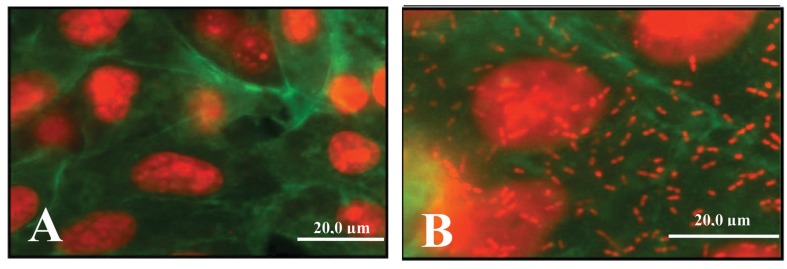
SEPEC adhesion was observed using a fluorescence assay, as shown in Figure 1.
Figure 1. The adhesion of human sepsis-associated Escherichia coli (SEPEC) strains to Vero cells was observed using a fluorescence assay. A, Negative control. B, SEPEC 8 (fimH+, flu+, csgA−, mat−, iha−, gimB−, ibeA−, tia−, neuC−) adhering to the cell surfaces.
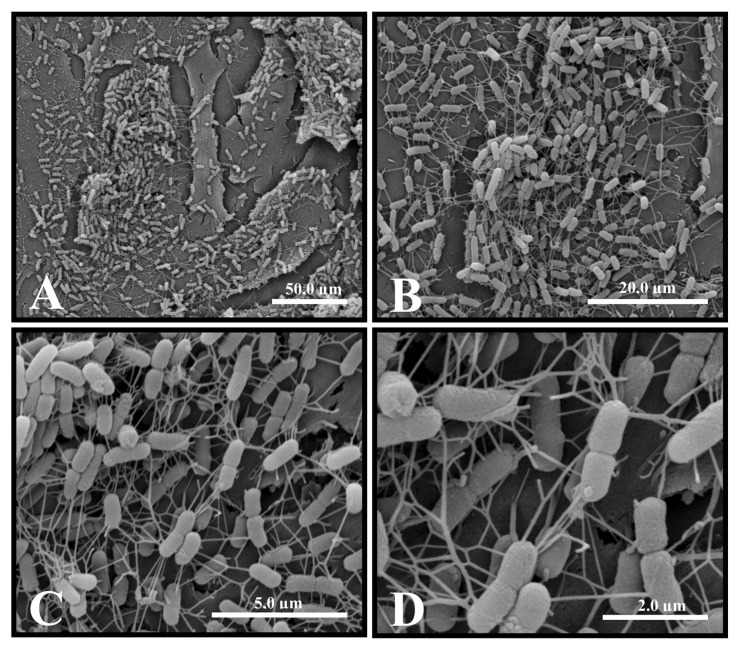
SEM revealed the formation of microcolonies surrounded by an extracellular matrix when SEPEC strains adhered to Vero cells (Figures 2 and 3). Studies have shown that adhesion is crucial for the establishment of E. coli-associated extraintestinal infections (28), such as urinary tract infections (29). In particular, bacterial adhesion is important in establishing chronic cystitis and bloodstream infections associated with catheters (3). However, there are no consistent data on SEPEC adhesion to cell surfaces.
Figure 2. Scanning electron micrograph of human sepsis-associated Escherichia coli (SEPEC) 8 (fimH+, flu+, csgA−, mat−, iha−, gimB−, ibeA−, tia−, neuC−) adhesion after 3 h of incubation with Vero cells. A, The bacteria adhered to the cell surface. B, Details of the points of microcolony formation. C, Microcolony details. D, Details of the network of extracellular matrix surrounding the microcolonies on the cell surface.
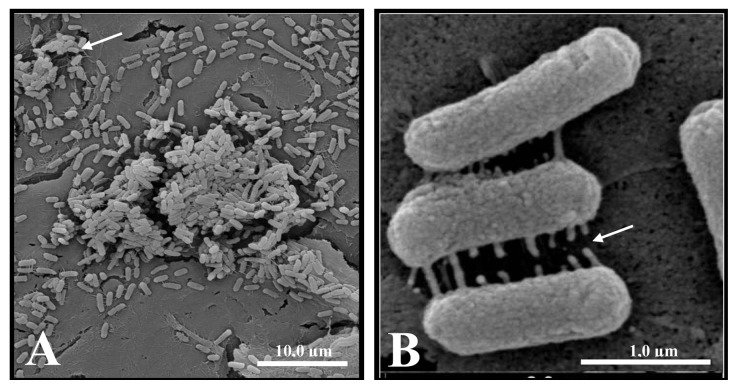
Figure 3. Scanning electron micrograph of human sepsis-associated Escherichia coli (SEPEC) 8 (fimH+, flu+, csgA−, mat−, iha−, gimB−, ibeA−, tia−, neuC−) adhesion after 3 h of incubation with Vero cells. A, Bacterial adhesion to the cell surface, with the formation of microcolonies surrounded by extracellular matrix (arrow). B, Early extracellular matrix production by bacteria adhered to the cell surface (arrow).
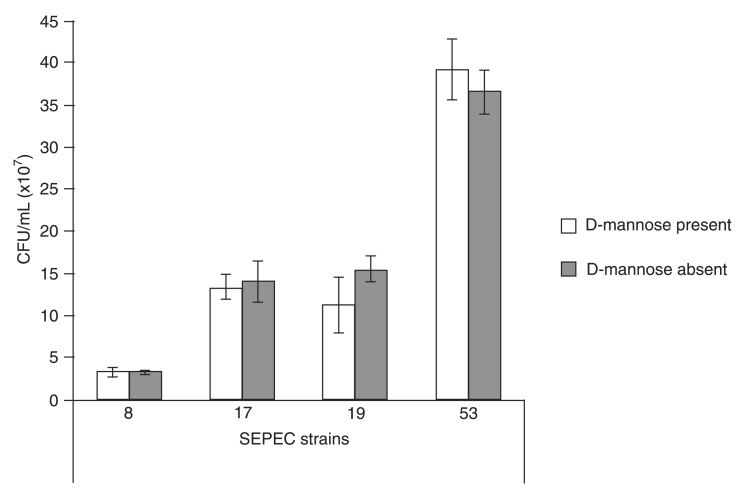
Overall, 98% of SEPEC strains were fimH-positive. Although type I fimbriae are important fimbriae used by UPEC to adhere to and invade bladder cells (13,29,30), the role of type I fimbriae in virulence is not as well defined as in other E. coli groups. However, because UPEC, SEPEC and other ExPEC groups share many virulence factors, fimH most likely has a similar function in SEPEC in the urinary environment. Thus, adhesion and invasion assays were performed in the presence of D-mannose. All SEPEC isolates (100%) adhered to and invaded cells in the presence of D-mannose (data not shown). Our invasion results were compared to those obtained for a positive control, and four SEPEC strains were subsequently chosen for ultrastructural analyses using SEM and TEM (Figure 4).
Figure 4. Colony-forming units (CFU/mL) of the human sepsis-associated Escherichia coli (SEPEC) strains chosen for transmission electron microscopy and scanning electron microscopy that were recovered from the intracellular environment. All 49 strains were recovered at higher concentrations (up to 2 × 107 CFU/mL) than the positive control (EIEC O124:H) in the presence or absence of D-mannose in the medium. The negative control, E. coli C600, did not invade Vero cells.
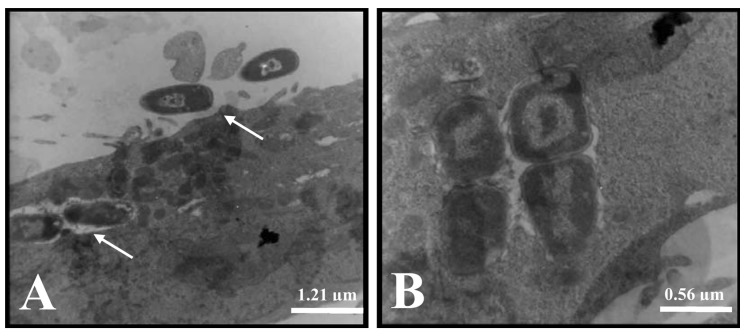
Using TEM, we observed that the bacteria adhered to many points on the cell surface, and we observed individual intracellular bacteria after 3 h of incubation with Vero cells in the presence or absence of D-mannose (Figure 5A). Additionally, bacterial subpopulations were observed, suggesting the presence of intracellular bacterial replication (Figure 5B).
Figure 5. Transmission electron micrographs of SEPEC 8 (fimH+, flu+, csgA−, mat−, iha−, gimB−, ibeA−, tia−, neuC−) adhesion and invasion after 3 h of incubation with Vero cells in the presence or absence of D-mannose. A, Bacteria that adhered to the cell surface (arrow) and intracellular bacteria in the absence of D-mannose. B, A group of four bacteria that suggests intracellular bacterial replication in the absence of D-mannose.
Thus, SEPEC strains adhered to Vero cells, formed microcolonies, produced an extracellular matrix, invaded cells, and probably replicated inside Vero cells. These findings suggest the occurrence of two simultaneous yet independent events. In fact, the adhesion to and invasion of Vero cells by SEPEC strains can be important factors involved in their pathogenicity, and these results may suggest one of the possible steps of SEPEC entry into kidney blood vessels.
Extraintestinal infections caused by E. coli, such as urinary infections, are common causes of bacteremia (3,30), a condition that always precedes sepsis. Therefore, SEPEC adhesion to host tissues, such as the kidney, could be an important factor in the course of sepsis by providing an entrance to the bloodstream. Once in the bloodstream, SEPEC strains must have favorable genetic compositions to survive in the blood and, in this way, induce sepsis (10). Some investigators have described the importance of fimH (31-33), ag43 (34,35) and csgA (25) in urinary tract and meninges infections. In addition, csgA has been shown to contribute to coagulation and blood pressure abnormalities, thereby contributing to SEPEC-induced septic shock (7).
The mechanisms by which SEPEC adhere to and invade Vero cells are not clear. However, these mechanisms suggest a gateway for SEPEC entry into the bloodstream during the course of sepsis. Although the adhesion to and invasion of eukaryotic cells by other ExPEC groups have been previously described (28,32), our data detail these processes for SEPEC clinical isolates and suggest a possible mechanism for SEPEC entry into blood vessels. We will continue searching for the factors involved in SEPEC adhesion and the relationship of these factors to the development of human sepsis. Currently, we are conducting proteomic analyses of the adhesion and invasion factors that are related to SEPEC adhesion to Vero cells to further elucidate the mechanisms involved in human sepsis.
Acknowledgments
We thank Dr. Luciano Moura Martins (Instituto Adolfo Lutz, São Paulo, SP, Brasil) and Robert Alvin Bernedo Navarro (Universidade Estadual de Campinas, Campinas, SP, Brasil) for scientific support and Ana Stella Menegon Degrossoli (Universidade Estadual de Campinas) for technical assistance. Research supported by CAPES.
References
- 1.Eisenstein BI, Jones GW. The spectrum of infections and pathogenic mechanisms of Escherichia coli. Adv Intern Med. 1988;33:231–252. [PubMed] [Google Scholar]
- 2.Kaper JB, Nataro JP, Mobley HL. Pathogenic Escherichia coli. Nat Rev Microbiol. 2004;2:123–140. doi: 10.1038/nrmicro818. [DOI] [PubMed] [Google Scholar]
- 3.Martinez JA, Soto S, Fabrega A, Almela M, Mensa J, Soriano A, et al. Relationship of phylogenetic background, biofilm production, and time to detection of growth in blood culture vials with clinical variables and prognosis associated with Escherichia coli bacteremia. J Clin Microbiol. 2006;44:1468–1474. doi: 10.1128/JCM.44.4.1468-1474.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mokady D, Gophna U, Ron EZ. Virulence factors of septicemic Escherichia coli strains. Int J Med Microbiol. 2005;295:455–462. doi: 10.1016/j.ijmm.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 5.Selander RK, Caugant DA, Whittam TS. Genetic structure and variation in natural populations of Escherichia coli. In: Neidhardt FC, Ingraham KL, Magasanik B, Low KB, Schaechter M, Umbarger HE, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington: an Society for Microbiology; 1987. pp. 1625–1648. (Editors) p. [Google Scholar]
- 6.Selander RK, Korhonen TK, Vaisanen-Rhen V, Williams PH, Pattison PE, Caugant DA. Genetic relationships and clonal structure of strains of Escherichia coli causing neonatal septicemia and meningitis. Infect Immun. 1986;52:213–222. doi: 10.1128/iai.52.1.213-222.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bian Z, Brauner A, Li Y, Normark S. Expression of and cytokine activation by Escherichia coli curli fibers in human sepsis. J Infect Dis. 2000;181:602–612. doi: 10.1086/315233. [DOI] [PubMed] [Google Scholar]
- 8.McBean M, Rajamani S. Increasing rates of hospitalization due to septicemia in the US elderly population, 1986-1997. J Infect Dis. 2001;183:596–603. doi: 10.1086/318526. [DOI] [PubMed] [Google Scholar]
- 9.Ewers C, Li G, Wilking H, Kiessling S, Alt K, Antao EM, et al. Avian pathogenic, uropathogenic, and newborn meningitis-causing Escherichia coli: how closely related are they? Int J Med Microbiol. 2007;297:163–176. doi: 10.1016/j.ijmm.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Ananias M, Yano T. Serogroups and virulence genotypes of Escherichia coli isolated from patients with sepsis. Braz J Med Biol Res. 2008;41:877–883. doi: 10.1590/s0100-879x2008001000008. [DOI] [PubMed] [Google Scholar]
- 11.Hall-Stoodley L, Costerton JW, Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nat Rev Microbiol. 2004;2:95–108. doi: 10.1038/nrmicro821. [DOI] [PubMed] [Google Scholar]
- 12.Croxen MA, Finlay BB. Molecular mechanisms of Escherichia coli pathogenicity. Nat Rev Microbiol. 2010;8:26–38. doi: 10.1038/nrmicro2265. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JR, Russo TA, Tarr PI, Carlino U, Bilge SS, Vary JC, Jr, et al. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroN (E. coli), among Escherichia coli isolates from patients with urosepsis. Infect Immun. 2000;68:3040–3047. doi: 10.1128/iai.68.5.3040-3047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang HH, Vinopal RT, Grasso D, Smets BF. High diversity among environmental Escherichia coli isolates from a bovine feedlot. Appl Environ Microbiol. 2004;70:1528–1536. doi: 10.1128/AEM.70.3.1528-1536.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maurer JJ, Brown TP, Steffens WL, Thayer SG. The occurrence of ambient temperature-regulated adhesins, curli, and the temperature-sensitive hemagglutinin tsh among avian Escherichia coli. Avian Dis. 1998;42:106–118. [PubMed] [Google Scholar]
- 16.Watt S, Lanotte P, Mereghetti L, Moulin-Schouleur M, Picard B, Quentin R. Escherichia coli strains from pregnant women and neonates: intraspecies genetic distribution and prevalence of virulence factors. J Clin Microbiol. 2003;41:1929–1935. doi: 10.1128/JCM.41.5.1929-1935.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scaletsky IC, Silva ML, Trabulsi LR. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984;45:534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peerbooms PG, Verweij AM, Maclaren DM. Vero cell invasiveness of Proteus mirabilis. Infect Immun. 1984;43:1068–1071. doi: 10.1128/iai.43.3.1068-1071.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barbosa HR, Rodrigues MFA, Campos CC, Chaves ME, Nunes I, Juliano Y, et al. Counting of viable cluster-forming and non cluster-forming bacteria: a comparison between the drop and the spread methods. Methods. 1995;22:39–50. [Google Scholar]
- 20.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: a common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 21.Danese PN, Pratt LA, Dove SL, Kolter R. The outer membrane protein, antigen 43, mediates cell-to-cell interactions within Escherichia coli biofilms. Mol Microbiol. 2000;37:424–432. doi: 10.1046/j.1365-2958.2000.02008.x. [DOI] [PubMed] [Google Scholar]
- 22.Pratt LA, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 23.Hasman H, Chakraborty T, Klemm P. Antigen-43-mediated autoaggregation of Escherichia coli is blocked by fimbriation. J Bacteriol. 1999;181:4834–4841. doi: 10.1128/jb.181.16.4834-4841.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gophna U, Barlev M, Seijffers R, Oelschlager TA, Hacker J, Ron EZ. Curli fibers mediate internalization of Escherichia coli by eukaryotic cells. Infect Immun. 2001;69:2659–2665. doi: 10.1128/IAI.69.4.2659-2665.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kikuchi T, Mizunoe Y, Takade A, Naito S, Yoshida S. Curli fibers are required for development of biofilm architecture in Escherichia coli K-12 and enhance bacterial adherence to human uroepithelial cells. Microbiol Immunol. 2005;49:875–884. doi: 10.1111/j.1348-0421.2005.tb03678.x. [DOI] [PubMed] [Google Scholar]
- 26.Achtman M, Heuzenroeder M, Kusecek B, Ochman H, Caugant D, Selander RK, et al. Clonal analysis of Escherichia coli O2:K1 isolated from diseased humans and animals. Infect Immun. 1986;51:268–276. doi: 10.1128/iai.51.1.268-276.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KS, Itabashi H, Gemski P, Sadoff J, Warren RL, Cross AS. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J Clin Invest. 1992;90:897–905. doi: 10.1172/JCI115965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramírez RM, Almanza Y. Adherence and invasion of avian pathogenic Escherichia coli to avian tracheal epithelial cells. W J Microbiol Biotech. 2009;25:1019–1023. [Google Scholar]
- 29.Tiba MR, Yano T, Leite DS. Genotypic characterization of virulence factors in Escherichia coli strains from patients with cystitis. Rev Inst Med Trop São Paulo. 2008;50:255–260. doi: 10.1590/s0036-46652008000500001. [DOI] [PubMed] [Google Scholar]
- 30.Rijavec M, Muller-Premru M, Zakotnik B, Zgur-Bertok D. Virulence factors and biofilm production among Escherichia coli strains causing bacteraemia of urinary tract origin. J Med Microbiol. 2008;57:1329–1334. doi: 10.1099/jmm.0.2008/002543-0. [DOI] [PubMed] [Google Scholar]
- 31.Connell I, Agace W, Klemm P, Schembri M, Marild S, Svanborg C. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc Natl Acad Sci U S A. 1996;93:9827–9832. doi: 10.1073/pnas.93.18.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kau AL, Hunstad DA, Hultgren SJ. Interaction of uropathogenic Escherichia coli with host uroepithelium. Curr Opin Microbiol. 2005;8:54–59. doi: 10.1016/j.mib.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Pouttu R, Puustinen T, Virkola R, Hacker J, Klemm P, Korhonen TK. Amino acid residue Ala-62 in the FimH fimbrial adhesin is critical for the adhesiveness of meningitis-associated Escherichia coli to collagens. Mol Microbiol. 1999;31:1747–1757. doi: 10.1046/j.1365-2958.1999.01311.x. [DOI] [PubMed] [Google Scholar]
- 34.Kjaergaard K, Schembri MA, Hasman H, Klemm P. Antigen 43 from Escherichia coli induces inter- and intraspecies cell aggregation and changes in colony morphology of Pseudomonas fluorescens. J Bacteriol. 2000;182:4789–4796. doi: 10.1128/jb.182.17.4789-4796.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ulett GC, Mabbett AN, Fung KC, Webb RI, Schembri MA. The role of F9 fimbriae of uropathogenic Escherichia coli in biofilm formation. Microbiology. 2007;153:2321–2331. doi: 10.1099/mic.0.2006/004648-0. [DOI] [PubMed] [Google Scholar]