Abstract
REGγ is a proteasome activator that facilitates the degradation of small peptides. Abnormally high expression of REGγ has been observed in thyroid carcinomas. The purpose of the present study was to explore the role of REGγ in poorly differentiated thyroid carcinoma (PDTC). For this purpose, small interfering RNA (siRNA) was introduced to down-regulate the level of REGγ in the PDTC cell line SW579. Down-regulation of REGγ at the mRNA and protein levels was confirmed by RT-PCR and Western blot analyses. FACS analysis revealed cell cycle arrest at the G1/S transition, the MTT assay showed inhibition of cell proliferation, and the Transwell assay showed restricted cell invasion. Furthermore, the expression of the p21 protein was increased, the expression of proliferating cell nuclear antigen (PCNA) protein decreased, and the expression of the p27 protein was unchanged as shown by Western blot analyses. REGγ plays a critical role in the cell cycle, proliferation and invasion of SW579 cells. The alteration of p21 and PCNA proteins related to the down-regulation of REGγ suggests that p21 and PCNA participate in the process of REGγ regulation of cell cycle progression and cell proliferation. Thus, targeting REGγ has a therapeutic potential in the management of PDTC patients.
Keywords: REGγ, PDTC, Cell cycle, Cell proliferation, SW579 cell line, Thyroid carcinoma
Introduction
Proteasomes are large proteolytic complexes found throughout the eukaryotic cell that destroy damaged or misfolded proteins as well as critical regulatory proteins controlling fundamental cellular processes such as cell cycle progression, transcription and cell signaling (1,2). Proteasomes are composed of a 20S catalytic core and a regulatory particle, which can be the regulatory 19S particle or a member of the REG (11S) family (3,4). The 19S regulator contains an ATPase subunit, which recognizes and degrades ubiquitin-protein conjugates in an ATP-dependent manner when binding to the 20S proteasome. In contrast, the REG family is an ATP- and ubiquitin-independent proteasome activator. The members of the REG family include REGα, REGβ, and REGγ, which share approximately 35% amino acid similarity (4,5). REGγ has been demonstrated in worms, insects and higher animals, whereas REGα and REGβ has been detected only in vertebrates (6). REGα and REGβ are distributed throughout the cell compartments, whereas REGγ is present only inside nuclei (7). REGα and REGβ are highly expressed in immune cells and are thought to play an important role in the production of major histocompatibility complex class I antigenic peptides (8); however, the function of REGγ is unknown. REGγ has been characterized only in terms of its ability to degrade small peptide model substrates, but recent evidence demonstrated that REGγ is also responsible for the degradation of some proteins, such as the steroid receptor co-activator 3 (9), hepatitis C virus core protein (10) and Smad ubiquitination regulatory factor 1 (11). The degradation of these proteins does not depend on ATP and ubiquitin, which suggests a new mechanism for protein degradation by proteasomes. Additionally, REGγ was identified as a novel regulator of Cajal body integrity (12) and is involved in the control of nuclear trafficking of splicing factors (13). Studies of REGγ expression in tumor tissues demonstrated that REGγ increased progressively from normal tissue to benign neoplasms and then to malignant tissues in thyroid and colorectal cancers, suggesting an important role for REGγ in tumorigenesis (14,15). These new findings prompted us to reassess the biochemical properties and physiological functions of REGγ in tumors.
Poorly differentiated thyroid carcinoma (PDTC), derived from follicular cells, is an aggressive thyroid tumor with high malignancy; its histopathological and biological aggressiveness is intermediate between well-differentiated thyroid carcinomas and anaplastic thyroid cancer (16). PDTC accounts for up to 10% of all thyroid cancers and has a higher incidence in Europe than in the United States (17,18). Over 50% of PDTCs have regional nodal metastases, and therefore total thyroidectomy is necessary for these patients. However, the use of radioactive iodine, external-beam radiation therapy, and chemotherapy is controversial. The aggressive nature of PDTC produces a rapid and fatal patient outcome despite appropriate treatment (17-19). More studies of the carcinogenesis of PDTC are necessary.
Deregulated cell cycle and proliferation are at the origin of carcinogenesis, and together with invasion they represent hallmarks of malignancy. Here we investigate how REGγ affects the cell cycle, cell proliferation and invasion in poorly differentiated thyroid carcinoma cells by reducing the level of the REGγ protein. p21, p27, and proliferating cell nuclear antigen (PCNA) proteins are critical regulators of cell cycle and cell proliferation. The degradation of the p21 protein was shown to be regulated by REGγ in other carcinoma cell lines (20). p21 and PCNA proteins frequently interact with each other (21,22). Cell cycle progression is controlled by a set of cyclin-dependent kinases (CDKs) that are inhibited by two classes of CDK inhibitors; p21 and p27 belong to the same class, so we also investigated the alterations in the levels of p21, p27 and PCNA proteins in poorly differentiated thyroid carcinoma cells resulting from reduced levels of the REGγ protein.
Material and Methods
Cell lines
The SW579 cell line, derived from a poorly differentiated human thyroid carcinoma and possessing nuclear features of papillary carcinoma and squamous differentiation, was purchased from the American Type Culture Collection (USA).
Reagents
Rabbit polyclonal anti-REGγ was purchased from Zymed (USA). Mouse monoclonal anti-p21 was purchased from BD (USA). Rabbit polyclonal anti-p27, mouse monoclonal anti-PCNA and rabbit polyclonal anti-β-actin were purchased from Santa Cruz (USA). Culture medium L-15 was purchased from Invitrogen (USA). Fetal calf serum was purchased from Gibco (USA). Lipofectamine™ 2000 was purchased from Invitrogen. The RT-PCR kit was purchased from Dingguo Co. (China).
Vectors expressing small interfering RNA (siRNA) for REGγ (pREGγ) and scrambled vector (pSV) were constructed using pGenesil-1 plasmids by GeneSil Co. (China). The sequence of siRNA oligonucleotide duplex is as follows (structure: BamHI + sense + loop + antisense + termination signal + SalI + HindIII): pREGγ 5′-AACTCAGATCCAC TCTGACAT-3′: pREGγ-A (sense) 5′-GATCCGCTCAG ATCCACTCTGACATTTCAAGACGATGTCAGAGTGG ATCTGAGTTTTTTGTCGACA-3′; pREGγ-B (antisense) 3′-GCGAGTCTAGGTGAGACTGTAAAGTTCTGCTAC AGTCTCACCTAGACTCAAAAAACAGCTGTTCGA-5′. pSV 5′-GACTTCATAAGGCGCATGC-3′: pSV-A (sense) 5′-GATCCGACTTCATAAGGCGCATGCTTCAAGACGG CATGCGCCTTATGAAGTCTTTTTTGTCGACA-3′; pSV-B (antisense) 3′-GCTGAAGTATTCCGCGTACGAAGTTCTG CCGTACGCGGAATACTTCAGAAAAAACAGCTGTT CGA-5′.
Cell culture and plasmid transfection
SW579 cells were grown in L-15 medium supplemented with 10% fetal calf serum in a humidified atmosphere containing 5% CO2 at 37°C. They were transfected with the eukaryotic expression vector pREGγ or pSV using Lipofectamine™ 2000 according to the manufacturer protocol. Briefly, 80% confluent cells grown on 100-mm dishes were washed three times with serum-free medium before the addition of plasmid DNA and Lipofectamine™ 2000 at a 1:20-ratio. Transfected cells were maintained in serum-containing media for 24 h, G418-resistant colonies were selected and screened, and the stable cells were digested with 0.15% trypsin and then grown and harvested for further experiments.
Reverse transcription-PCR analysis
Total cellular RNA was extracted from the parental cells (mock), cells treated with pSV and cells treated with pREGγ using Trizol reagent according to the manufacturer protocol. RNA concentrations were determined spectrophotometrically and equal amounts of total RNA (2.5 µg) were reverse transcribed using oligo primer and reverse transcriptase for 60 min at 42°C. The following primers designed using the Primer Premier 5.0 software were used: REGγ sense, 5′-CCCTGGCCTCCCAAAGTGCT-3′ and REGγ antisense, 5′-TCGGCCACTGCACTCCAACC-3′; β-actin sense, 5′-GCATCCTGACCCTGAAGTACC-3′, and β-actin antisense, 5′-TCGGCCACTGCACTCCAACC-3′. The PCR conditions were as follows: 94°C for 5 min, followed by 30 cycles of 94°C for 1 min, 57°C for 1 min (58°C for β-actin) and 71°C for 1 min. The final extension was at 72°C for 7 min. PCR products were resolved on 1% agarose gel, visualized with ethidium bromide, and photographed under UV light. Each experiment was repeated three times and each repetition gave similar results.
Western blot analysis
Protein extracts were obtained from the parental cells (mock), cells treated with pSV and cells treated with pREGγ using Tris-buffered lysis. Protein was determined using a bicinchoninic acid assay (BCA Protein Assay Kit, Pierce, USA). Thirty micrograms of protein from each sample was subjected to 10% SDS-PAGE gel electrophoresis and transferred to a PVDF membrane (Bio-Rad, USA). The membrane was blocked with 5% non-fat dry milk in PBS (phosphate-buffered saline) for 1 h. After incubation with primary antibodies in blocking solution overnight at 4°C, membranes were then washed three times with T-PBS (PBS with Tween-20). Horseradish peroxidase-conjugated secondary antibodies were used, and the membranes were developed using an enhanced chemiluminescence protocol (Bio-Rad). β-actin served as a load control. Each experiment was repeated three times and each repetition gave similar results.
Flow cytometry analysis
Parental cells (mock), cells treated with pSV and cells treated with pREGγ were seeded on 24-well tissue culture plates (1 × 105). Cells were fixed gently by adding 80% ethanol and placing them in a freezer for 2 h and then analyzed by flow cytometry (FacsCalibur; BD). Each experiment was repeated three times and each repetition gave similar results.
MTT proliferation assay (cell growth curve)
For the analysis of cell growth rate, parental cells (mock), cells treated with pSV and cells treated with pREGγ were plated onto 96-well plates at a density of 3 × 103 cells/well, and viable cells were counted from day 1 to day 7. Cells were stained with 20 µL sterile MTT dye at 37°C for 4 h, the culture medium was removed and 150 µL dimethylsulfoxide was thoroughly mixed with the cells for 10 min for day 1 to day 7 groups at 1-day intervals. Spectrometric absorbance at 490 nm was measured with a microplate reader (Multi-skan spectrum, Thermo Electron Corporation, USA). Each experiment was repeated three times and each repetition gave similar results.
Transwell assay
Transwell invasion was measured by the invasion of cells through matrigel-coated (BD) transwell inserts (Costar, USA). Briefly, 24-well transwell inserts with 8-mm pore size were coated with matrigel (1:8) in cold serum-free L-15 culture medium. Parental cells (mock), cells treated with pSV and cells treated with pREGγ were trypsinized, and 100 µL of the cell suspension (5 × 105 cells/mL) was added to the upper compartment, and cell culture medium with 10% fetal calf serum was added to the lower compartment. After a 24-h incubation period, the inserts were inverted and stained with hematoxylin. Five fields were chosen randomly, the number of cells was counted and the mean value was calculated. Each experiment was repeated three times and each repetition gave similar results.
Statistical analysis
Data were analyzed statistically by ANOVA using the SPSS 15.0 software. Data are reported as means ± SD of three independent experiments, and a P value less than 0.05 was considered to be statistically significant.
Results
Knockdown of REGγ in SW579 cells upon siRNA treatment causes the reduction of REGγ mRNA and protein
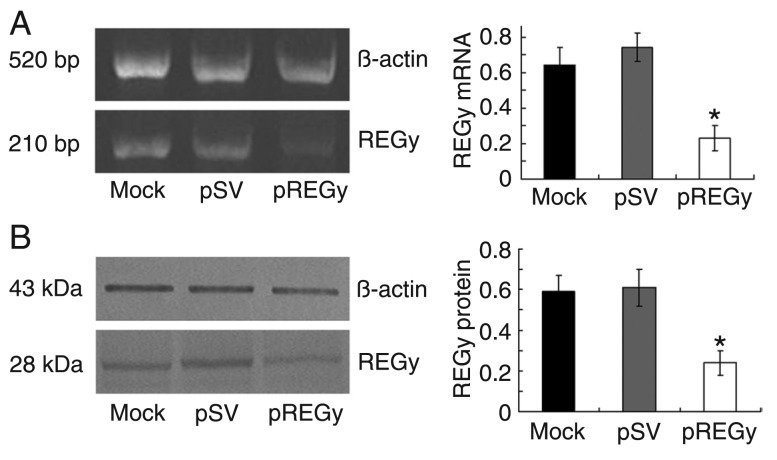
To reduce the expression levels of REGγ, the cells were transfected with an siRNA vector targeting REGγ (pREGγ); cells transfected with scrambled vector (pSV) and untransfected cells (mock) served as controls. The RT-PCR analysis showed significantly decreased levels of REGγ mRNA in pREGγ groups compared to pSV groups or in mock groups; β-actin activity was analyzed to serve as a loading control (Figure 1A). Western blot analysis was performed to determine the expression of the REGγ protein; as shown in Figure 1B, there was a significant decrease in the expression of the REGγ protein in pREGγ groups compared to pSV groups or mock groups. The percentage of remaining expression was about 38% in relation to the 100% control. β-actin activity was analyzed to serve as a loading control. Taken together, these results demonstrate the efficient down-regulation of REGγ upon treatment with siRNA targeting this protein.
Figure 1. Small interfering RNA knockdown of REGγ mRNA and protein expression in SW579 cells. A, Total RNA was extracted from parental cells (mock), cells treated with scrambled vector (pSV) and cells treated with proteasome activator (pREGγ). Semi-quantitative RT-PCR was performed according to standard protocols. β-actin was used as an internal control. Data are reported as means ± SD of three independent experiments. *P < 0.05 compared to other groups (ANOVA). B, Total proteins were extracted from SW579 parental cells (mock), cells treated with pSV and cells treated with pREGγ. Western blot analysis was performed to detect REGγ expression. β-actin was used as an internal control. Data are reported as means ± SD of three independent experiments. *P < 0.05 compared to other groups (ANOVA).
RNAi-mediated down-regulation of REGγ inhibits SW579 cell proliferation
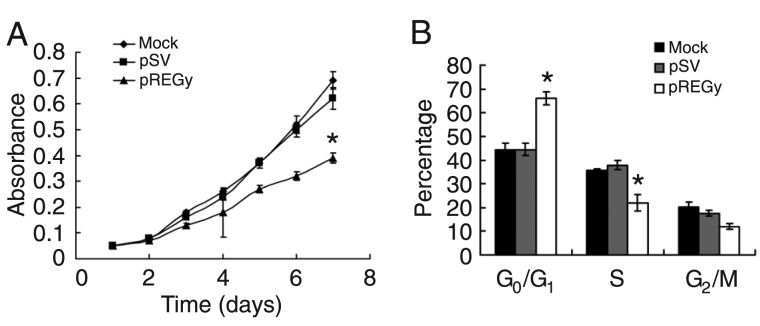
To confirm the role of REGγ in cell proliferation in the SW579 cell line, the cells were transfected with pREGγ; cells transfected with pSV and untransfected cells (mock) served as controls. We measured cell growth capability by the MTT assay, and the growth curve showed that cell proliferation was significantly inhibited in pREGγ groups compared to pSV groups or mock groups (Figure 2A).
Figure 2. Small interfering RNA knockdown of REGγ decreases cell proliferation and halts the cell cycle in the G0/G1 phase. A, Parental cells (mock), cells treated with scrambled vector (pSV) and cells treated with proteasome activator (pREGγ) were maintained in 96-well plates. Absorbance was measured at different times (1-7 days). Data are reported as means ± SD of spectrometric absorbance of three independent experiments. *P < 0.05 compared to other groups (ANOVA). B, The cell cycles of parental cells (mock), cells treated with pSV and cells treated with pREGγ were analyzed by flow cytometry. Each bar indicates the distribution of the cell cycles. Data are reported as means ± SD of three independent experiments. *P < 0.05 compared to other groups (ANOVA).
RNAi-mediated down-regulation of REGγ impedes the cell cycle in SW579 cells
To confirm the role of REGγ in the cell cycle of SW579 cells, we transfected the cells with pREGγ; cells transfected with pSV and untransfected cells (mock) served as controls. Then, we measured the cell cycle using flow cytometry. The results showed that the percentage of cells arrested at the G0/G1 phase in the pREGγ group was remarkably higher than in the pSV or mock groups (Figure 2B).
RNAi-mediated down-regulation of REGγ is accompanied by the simultaneous up-regulation of the p21 protein and down-regulation of the PCNA protein
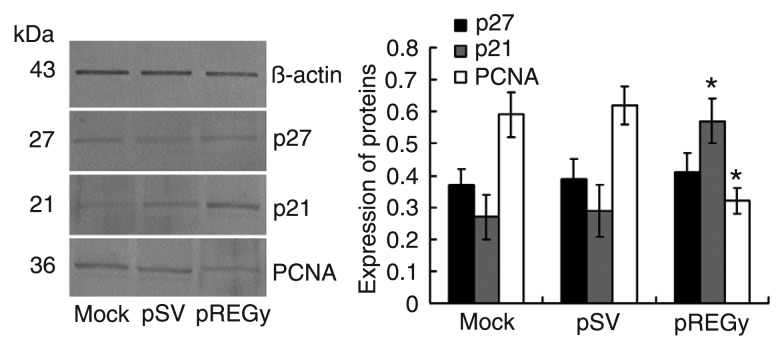
To confirm the role of REGγ in regulating the protein levels of p21, p27 and PCNA in SW579, we transfected the cells with pREGγ; cells transfected with pSV and untransfected cells (mock) served as controls. Western blot analysis showed that there were significantly increased levels of the p21 protein and significantly decreased levels of the PCNA protein in the pREGγ groups compared to the pSV or mock groups, but the level of the p27 protein in the pREGγ groups showed no significant change (Figure 3).
Figure 3. Small interfering RNA knockdown of REGγ increases p21 and decreases proliferating cell nuclear antigen (PCNA) protein expression. Total proteins were extracted from parental cells (mock), cells treated with scrambled vector (pSV) and cells treated with proteasome activator (pREGγ). Expression of p21, p27 and PCNA proteins was assessed by Western blot. β-actin was used as an internal control. Data are reported as means ± SD of three independent experiments. *P < 0.05 compared to other groups (ANOVA).
Down-regulation of REGγ restricts invasion of SW579 cells
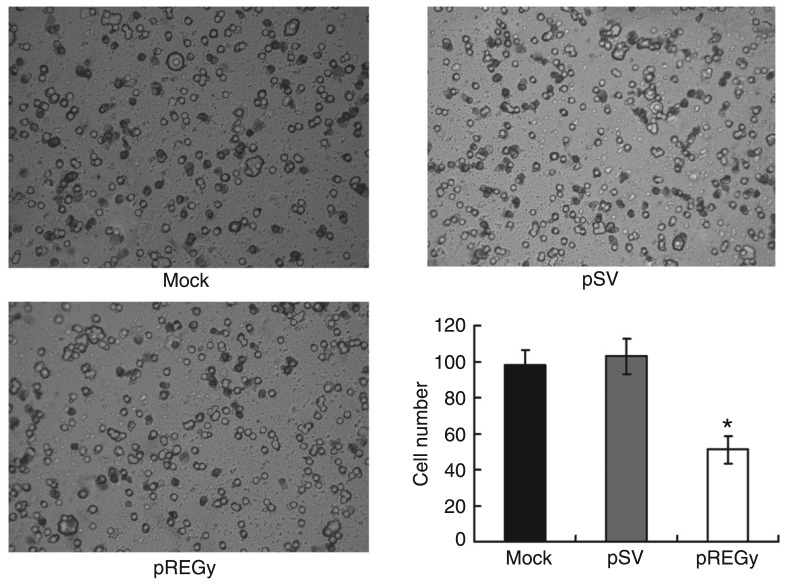
To confirm the role of REGγ in regulating the invasion of the SW579 cell line, cells were transfected with pREGγ; cells transfected with pSV and untransfected (mock) served as controls. The transwell assay showed that the invasion of pREGγ groups was significantly restricted compared to the pSV or mock groups (Figure 4).
Figure 4. Small interfering RNA knockdown of REGγ decreases cellular inva-siveness. Parental cells (mock), cells treated with scrambled vector (pSV) and cells treated with proteasome activator (pREGγ) were added to transwell inserts and the number of invasive cells was observed and counted. Data are reported as means ± SD of three independent experiments. *P < 0.05 compared to other groups (ANOVA).
Discussion
REGγ (also known as PA28γ and PSME3) is a proteasome activator and REGγ-proteasome-mediated protein degradation may regulate fundamental cellular processes such as cell cycle progression (20,23). In the present study, we introduced siRNA sequences into the poorly differentiated human thyroid carcinoma cell line SW579 to down-regulate the expression of REGγ in order to assess its effects on cell cycle progression, cell proliferation, and invasion regulation. RT-PCR assays were used to detect the expression of REGγ mRNA; Western blot analyses were used to detect the expression of REGγ, p21, p27 and PCNA proteins; FACS was used to detect the state of the cell cycle; MTT assays were used to detect cell proliferation, and transwell invasion assays were used to detect cell invasion.
Previous studies have shown that knockdown of REGγ in Drosophila cells inhibited cell proliferation (24); REGγ knockout mice displayed modest growth retardation, and the lack of REGγ in mouse embryonic fibroblasts prevented G1/S phase progression (25,26); REGγ could promote breast carcinoma cell growth and cell cycle progression (27). Our study corroborates these observations by demonstrating that the cell cycle progression was dramatically arrested and cell proliferation was markedly inhibited by the down-regulated expression of REGγ in SW579 cells. Additionally, more cells with REGγ depletion were arrested at the G0/G1 phase in our experiment, in agreement with two independent studies on mouse embryonic fibroblasts (25,26). However, another study (20) showed that REGγ depletion leads to cell cycle arrest at the G2/M phase in TPA-treated cells, indicating that different cell types may use different mechanisms.
p21 is a broad-spectrum CDK inhibitor that plays a central role in regulating the cell cycle in many types of cells (28). p21 has two distinct inhibitory effects on the entry of the cell from the G1 phase into S phase: one is to inhibit the G1-phase cyclins/CDK complexes, and the other is to directly block DNA synthesis via an interaction with PCNA (29-32). In the present study, the level of the p21 protein was markedly increased by siRNA interference of REGγ, consistent with the phenomenon that more cells with REGγ depletion were arrested at the G0/G1 phase than cells transfected with pSV and cells untransfected. Previous studies have shown that the depletion of REGγ following siRNA interference leads to a striking increase in p21 protein levels in TPC cells, but the knockdown of REGγ had only a relatively slight effect on p21 levels in MCF-7 cells and had no effect in HepG2 and 3T3-L1 cells (20). It is interesting that REGγ-dependent p21 regulation is cell-type specific.
p27 is also a CDK inhibitor that plays an important role in protecting cells from excessive proliferation by regulating CDK activity, thus inhibiting the G1/S transition (22). The levels of the p27 protein are increased in quiescent cells and rapidly decrease after stimulation with mitogens. Constitutive expression of p27 in cultured cells causes cell cycle arrest in the G1 phase (21,33). Therefore, we investigated the expression of p27 in SW579 cells and did not observe any change in the expression of the p27 protein with the depletion of REGγ. Thus, also taking into account previous reports that the REGγ-proteasome pathway in charge of proteolytic turnover of p21 was not involved in p27 (20,23), we suggest that p21, but not p27, plays an important role in the REGγ-related control of cell cycle progression in SW579 cells.
PCNA is a factor for DNA polymerase delta, which is synthesized during the late G1 to S phase of the cell cycle and has been shown to be a cyclin (34,35). PCNA was initially identified as an auto-antigen in patients with systemic lupus erythematosus (36), as was REGγ (also called Ki antigen) (37), which suggests a possible relationship between PCNA and REGγ. Previous studies have indicated a significant positive correlation between REGγ and PCNA expression in papillary thyroid cancer, multinodular goiter, and normal thyroid tissues (14). In the present study, the levels of the PCNA protein were down-regulated following the decline in the levels of REGγ, suggesting that REGγ may be involved in the regulatory machinery of proliferation in thyroid cancer cells, like PCNA. It is well known that p21 has a close partnership with PCNA. The N-terminal half of PCNA participates in the binding to p21, the C-terminal domain of p21 is involved in the binding of PCNA, and the C-terminal domain of p21 is sufficient to displace DNA replication enzymes from PCNA, thereby blocking processive DNA synthesis (30). Additionally, p21 can promote the proteasome-dependent degradation of the PCNA protein and thus inhibit PCNA-dependent DNA replication in adult cardiomyocytes, but p27 could not (38). In the present study, a decline in the levels of the PCNA protein was also observed when the level of REGγ was down-regulated by REGγ siRNA interference, and the level of the p21 protein was up-regulated. Because there is no direct evidence that REGγ is involved in the degradation of the PCNA protein, we infer that p21 promoted PCNA protein degradation, which, combined with its inactivation of the PCNA protein, contributes to the arrest of the cell cycle at the G1/S phase transition and the inhibition of cell proliferation in SW579 cells.
The invasion of carcinoma cells is the characteristic of malignancy. Previous studies have found that all thyroid papillary carcinoma tissues obviously express higher levels of REGγ than the adjacent normal areas, but the highest intensity of REGγ expression was observed in peripheral cells inside the cancer capsule or invading the capsular region. REGγ was also highly expressed in the whole area of the anaplastic carcinoma mass, but REGγ was expressed at its highest level in the most poorly differentiated cancer cells, such as squamous metaplasia (14). These phenomena suggest that REGγ is implicated in the invasion and histological differentiation of thyroid carcinoma. In the present study, the invasion of SW579 cells was restricted by the depletion of REGγ following siRNA interference. We postulate that the REGγ-controlled invasion of carcinoma cells is probably due to the role of REGγ in regulating the degradation of relevant proteins, but the specific mechanism is still unclear.
Down-regulation of REGγ using siRNA interference successfully inhibited cell proliferation and arrested the cell cycle in SW579 cells, and also restricted invasion of SW579 cells. Simultaneously, the expression of the p21 protein was up-regulated and the expression of the PCNA protein was down-regulated, contributing to the G1/S phase transition of the cell cycle and to proliferation of SW579 cells. These results suggest that REGγ could be a molecular target in therapeutic applications for the treatment of patients with PDTC.
Acknowledgments
Research supported by the National Natural Science Foundation of China (Grant #30670811).
References
- 1.Goldberg AL. Protein degradation and protection against misfolded or damaged proteins. Nature. 2003;426:895–899. doi: 10.1038/nature02263. [DOI] [PubMed] [Google Scholar]
- 2.Varshavsky A. Regulated protein degradation. Trends Biochem Sci. 2005;30:283–286. doi: 10.1016/j.tibs.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Dubiel W, Pratt G, Ferrell K, Rechsteiner M. Purification of an 11 S regulator of the multicatalytic protease. J Biol Chem. 1992;267:22369–22377. [PubMed] [Google Scholar]
- 4.Ma CP, Slaughter CA, DeMartino GN. Identification, purification, and characterization of a protein activator (PA28) of the 20 S proteasome (macropain) J Biol Chem. 1992;267:10515–10523. [PubMed] [Google Scholar]
- 5.Realini C, Jensen CC, Zhang Z, Johnston SC, Knowlton JR, Hill CP, et al. Characterization of recombinant REGalpha, REGbeta, and REGgamma proteasome activators. J Biol Chem. 1997;272:25483–25492. doi: 10.1074/jbc.272.41.25483. [DOI] [PubMed] [Google Scholar]
- 6.Masson P, Andersson O, Petersen UM, Young P. Identification and characterization of a Drosophila nuclear proteasome regulator. A homolog of human 11 S REGgamma (PA28gamma) J Biol Chem. 2001;276:1383–1390. doi: 10.1074/jbc.M007379200. [DOI] [PubMed] [Google Scholar]
- 7.Wojcik C, Tanaka K, Paweletz N, Naab U, Wilk S. Proteasome activator (PA28) subunits, alpha, beta and gamma (Ki antigen) in NT2 neuronal precursor cells and HeLa S3 cells. Eur J Cell Biol. 1998;77:151–160. doi: 10.1016/s0171-9335(98)80083-6. [DOI] [PubMed] [Google Scholar]
- 8.Groettrup M, Soza A, Eggers M, Kuehn L, Dick TP, Schild H, et al. A role for the proteasome regulator PA28alpha in antigen presentation. Nature. 1996;381:166–168. doi: 10.1038/381166a0. [DOI] [PubMed] [Google Scholar]
- 9.Li X, Lonard DM, Jung SY, Malovannaya A, Feng Q, Qin J, et al. The SRC-3/AIB1 coactivator is degraded in a ubiquitin-and ATP-independent manner by the REGgamma proteasome. Cell. 2006;124:381–392. doi: 10.1016/j.cell.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 10.Moriishi K, Okabayashi T, Nakai K, Moriya K, Koike K, Murata S, et al. Proteasome activator PA28gamma-dependent nuclear retention and degradation of hepatitis C virus core protein. J Virol. 2003;77:10237–10249. doi: 10.1128/JVI.77.19.10237-10249.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nie J, Wu M, Wang J, Xing G, He F, Zhang L. REGgamma proteasome mediates degradation of the ubiquitin ligase Smurf1. FEBS Lett. 2010;584:3021–3027. doi: 10.1016/j.febslet.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 12.Cioce M, Boulon S, Matera AG, Lamond AI. UV-induced fragmentation of Cajal bodies. J Cell Biol. 2006;175:401–413. doi: 10.1083/jcb.200604099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldin V, Militello M, Thomas Y, Doucet C, Fic W, Boireau S, et al. A novel role for PA28gamma-proteasome in nuclear speckle organization and SR protein trafficking. Mol Biol Cell. 2008;19:1706–1716. doi: 10.1091/mbc.E07-07-0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Okamura T, Taniguchi S, Ohkura T, Yoshida A, Shimizu H, Sakai M, et al. Abnormally high expression of proteasome activator-gamma in thyroid neoplasm. J Clin Endocrinol Metab. 2003;88:1374–1383. doi: 10.1210/jc.2002-021413. [DOI] [PubMed] [Google Scholar]
- 15.Roessler M, Rollinger W, Mantovani-Endl L, Hagmann ML, Palme S, Berndt P, et al. Identification of PSME3 as a novel serum tumor marker for colorectal cancer by combining two-dimensional polyacrylamide gel electrophoresis with a strictly mass spectrometry-based approach for data analysis. Mol Cell Proteomics. 2006;5:2092–2101. doi: 10.1074/mcp.M600118-MCP200. [DOI] [PubMed] [Google Scholar]
- 16.Pilotti S, Collini P, Manzari A, Marubini E, Rilke F. Poorly differentiated forms of papillary thyroid carcinoma: distinctive entities or morphological patterns? Semin Diagn Pathol. 1995;12:249–255. [PubMed] [Google Scholar]
- 17.Burman KD, Ringel MD, Wartofsky L. Unusual types of thyroid neoplasms. Endocrinol Metab Clin North Am. 1996;25:49–68. doi: 10.1016/s0889-8529(05)70312-1. [DOI] [PubMed] [Google Scholar]
- 18.Sywak M, Pasieka JL, Ogilvie T. A review of thyroid cancer with intermediate differentiation. J Surg Oncol. 2004;86:44–54. doi: 10.1002/jso.20044. [DOI] [PubMed] [Google Scholar]
- 19.Wreesmann VB, Ghossein RA, Patel SG, Harris CP, Schnaser EA, Shaha AR, et al. Genome-wide appraisal of thyroid cancer progression. Am J Pathol. 2002;161:1549–1556. doi: 10.1016/S0002-9440(10)64433-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Amazit L, Long W, Lonard DM, Monaco JJ, O'Malley BW. Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol Cell. 2007;26:831–842. doi: 10.1016/j.molcel.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 21.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–704. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 22.Lloyd RV, Erickson LA, Jin L, Kulig E, Qian X, Cheville JC, et al. p27kip1: a multifunctional cyclin-dependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol. 1999;154:313–323. doi: 10.1016/S0002-9440(10)65277-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen X, Barton LF, Chi Y, Clurman BE, Roberts JM. Ubiquitin-independent degradation of cell-cycle inhibitors by the REGgamma proteasome. Mol Cell. 2007;26:843–852. doi: 10.1016/j.molcel.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masson P, Lundgren J, Young P. Drosophila proteasome regulator REGgamma: transcriptional activation by DNA replication-related factor DREF and evidence for a role in cell cycle progression. J Mol Biol. 2003;327:1001–1012. doi: 10.1016/s0022-2836(03)00188-8. [DOI] [PubMed] [Google Scholar]
- 25.Murata S, Kawahara H, Tohma S, Yamamoto K, Kasahara M, Nabeshima Y, et al. Growth retardation in mice lacking the proteasome activator PA28gamma. J Biol Chem. 1999;274:38211–38215. doi: 10.1074/jbc.274.53.38211. [DOI] [PubMed] [Google Scholar]
- 26.Barton LF, Runnels HA, Schell TD, Cho Y, Gibbons R, Tevethia SS, et al. Immune defects in 28-kDa proteasome activator gamma-deficient mice. J Immunol. 2004;172:3948–3954. doi: 10.4049/jimmunol.172.6.3948. [DOI] [PubMed] [Google Scholar]
- 27.Tian M, Xiaoyi W, Xiaotao L, Guosheng R. Proteasomes reactivator REG gamma enchances oncogenicity of MDA-MB-231 cell line via promoting cell proliferation and inhibiting apoptosis. Cell Mol Biol. 2009;55(Suppl):OL1121–OL1131. [PubMed] [Google Scholar]
- 28.Weinberg WC, Denning MF. P21Waf1 control of epithelial cell cycle and cell fate. Crit Rev Oral Biol Med. 2002;13:453–464. doi: 10.1177/154411130201300603. [DOI] [PubMed] [Google Scholar]
- 29.Chen J, Jackson PK, Kirschner MW, Dutta A. Separate domains of p21 involved in the inhibition of Cdk kinase and PCNA. Nature. 1995;374:386–388. doi: 10.1038/374386a0. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Peters R, Saha P, Lee P, Theodoras A, Pagano M, et al. A 39 amino acid fragment of the cell cycle regulator p21 is sufficient to bind PCNA and partially inhibit DNA replication in vivo. Nucleic Acids Res. 1996;24:1727–1733. doi: 10.1093/nar/24.9.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo Y, Hurwitz J, Massague J. Cell-cycle inhibition by independent CDK and PCNA binding domains in p21Cip1. Nature. 1995;375:159–161. doi: 10.1038/375159a0. [DOI] [PubMed] [Google Scholar]
- 32.Goubin F, Ducommun B. Identification of binding domains on the p21Cip1 cyclin-dependent kinase inhibitor. Oncogene. 1995;10:2281–2287. [PubMed] [Google Scholar]
- 33.Toyoshima H, Hunter T. p27, a novel inhibitor of G1 cyclin-Cdk protein kinase activity, is related to p21. Cell. 1994;78:67–74. doi: 10.1016/0092-8674(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 34.Bravo R, Frank R, Blundell PA, Donald-Bravo H. Cyclin/PCNA is the auxiliary protein of DNA polymerase-delta. Nature. 1987;326:515–517. doi: 10.1038/326515a0. [DOI] [PubMed] [Google Scholar]
- 35.Prelich G, Tan CK, Kostura M, Mathews MB, So AG, Downey KM, et al. Functional identity of proliferating cell nuclear antigen and a DNA polymerase-delta auxiliary protein. Nature. 1987;326:517–520. doi: 10.1038/326517a0. [DOI] [PubMed] [Google Scholar]
- 36.Miyachi K, Fritzler MJ, Tan EM. Autoantibody to a nuclear antigen in proliferating cells. J Immunol. 1978;121:2228–2234. [PubMed] [Google Scholar]
- 37.Nikaido T, Shimada K, Shibata M, Hata M, Sakamoto M, Takasaki Y, et al. Cloning and nucleotide sequence of cDNA for Ki antigen, a highly conserved nuclear protein detected with sera from patients with systemic lupus erythematosus. Clin Exp Immunol. 1990;79:209–214. doi: 10.1111/j.1365-2249.1990.tb05180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engel FB, Hauck L, Boehm M, Nabel EG, Dietz R, von Harsdorf R. p21(CIP1) Controls proliferating cell nuclear antigen level in adult cardiomyocytes. Mol Cell Biol. 2003;23:555–565. doi: 10.1128/MCB.23.2.555-565.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]