Abstract
A dendritic cell (DC)-based vaccine strategy could reduce the risk of recurrence and improve the survival of breast cancer patients. However, while therapy-induced apoptosis of hepatocellular and colorectal carcinoma cells can enhance maturation and antigen presentation of DCs, whether this effect occurs in breast cancer is currently unknown. In the present study, we investigated the effect of doxorubicin (ADM)-induced apoptotic MCF-7 breast cancer cells on the activation of DCs. ADM-induced apoptotic MCF-7 cells could effectively induce immature DC (iDC) maturation. The mean fluorescence intensity (MFI) of DC maturity marker CD83 was 23.3 in the ADM-induced apoptotic MCF-7 cell group compared with 8.5 in the MCF-7 cell group. The MFI of DC co-stimulatory marker CD86 and HLA-DR were also increased after iDCs were treated with ADM-induced apoptotic MCF-7 cells. Furthermore, the proliferating autologous T-lymphocytes increased from 14.2 to 40.3% after incubated with DCs induced by apoptotic MCF-7 cells. The secretion of interferon-γ by these T-lymphocytes was also increased. In addition, cell-cell interaction between apoptotic MCF-7 cells and iDCs, but not soluble factors released by apoptotic MCF-7 cells, was crucial for the maturation of iDCs. These findings constitute a novel in vitro DC-based vaccine strategy for the treatment of breast cancer by ADM-induced apoptotic MCF-7 cells.
Keywords: Breast cancer, Dendritic cells, Immunotherapy, Apoptosis, Doxorubicin
Introduction
Breast cancer accounts for more than 10% of the total cancer incidence among women and is the second most common type of cancer and the fifth most common cause of cancer death in the world. Traditional therapies for breast cancer include surgery, chemotherapy, radiotherapy, and hormone therapy. While breast cancer cure rates with current multimodality therapy have improved, an estimated 20 to 30% of patients will have a recurrence of their disease. Immunotherapy has the potential for systemic, specific killing of residual and drug-resistant tumor cells. Thus, a safe, effective breast cancer vaccine strategy may significantly reduce this recurrence risk and improve survival (1).
Dendritic cell (DC)-based immunotherapy is a promising approach currently under investigation in different cancers. DCs are the body's most effective antigen-presenting cells. The main function of the DC is to process antigen and present it on its surface to stimulate T-lymphocytes. Thus, DCs isolated from breast cancer patients can be activated in vitro with the desired tumor antigens. These activated DCs can then be reintroduced into patients to activate or boost the immune response. This procedure effectively initiates a cytotoxic response against tumor antigens (2). A recent study has shown that dying hepatocellular and colorectal carcinoma cells, induced by chemotherapy or radiotherapy, can enhance the maturation and antigen presentation of DCs (3). However, whether apoptotic breast cancer cells can induce DC activation is currently unknown.
Doxorubicin (adriamycin, ADM) is a well-established drug for the treatment of breast cancer and the aim of the current study was to investigate ADM-treated breast cancer cells as inducers of DC activation in vitro. When treated with ADM to induce apoptosis, the human breast cancer cell line MCF-7 was shown to effectively induce the maturation of healthy donor-derived immature DCs (iDCs) and subsequently stimulate autologous T-lymphocytes to proliferate and secrete interferon-γ (IFN-γ) in vitro. These findings contribute to the development of a novel DC-based vaccine strategy for breast cancer.
Material and Methods
Cell lines and culture
The human breast cancer cell line MCF-7 was obtained from the American Type Culture Collection (USA). Cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS; Sijiqing Biological Engineering Materials Co., China) at 37°C in a 5% CO2 atmosphere. MCF-7 cells were treated with 0, 1, 2, or 5 µg/mL ADM (Zhejiang Hisun Pharmaceutical, China) for 24 h and then subjected to flow cytometric analysis (FACS) for the determination of apoptosis.
Generation of iDCs in vitro
With written informed consent and approval of the Committee of Basic Research at the Fourth Military Medical University, peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy donors by density centrifugation using Ficoll-Paque Plus (DH184-1; Dingguo Biotechnology, China). After erythrocyte lysis, PBMCs were cultured in complete RPMI 1640 medium for 2 h at 37°C and then monocytes were isolated by adherence to plastic culture flasks and incubated with 100 ng/mL recombinant human granulocyte/macrophage colony stimulating factor (GM-CSF) (PeproTech, USA) and 10 ng/mL recombinant human IL-4 (PeproTech) for 6 days.
Co-culture of iDCs with ADM-treated MCF-7 cells
After 24-h treatment with ADM (5 µg/mL) and extensive washing, MCF-7 cells were co-cultured with iDCs at a ratio of 2:1 in RPMI 1640 supplemented with GM-CSF (100 ng/mL), IL-4 (10 ng/mL) and 10% FBS for 24 h. As a control, iDCs were co-cultured with untreated MCF-7 cells for 24 h. As a positive control, mature DCs were generated by further stimulating iDCs with 1 µg/mL lipopolysaccharide (LPS; Sigma-Aldrich, USA) for 24 h. The co-culture of iDCs with ADM-treated MCF-7 cells was carried out either together in the same compartment or separately in 0.4-µM pore-sized filter Transwell inserts on a 24-well culture plate (Corning Costar, USA). The phenotype of the iDCs was then analyzed by FACS.
T-lymphocyte proliferation and activation assay
After 24 h of co-culture with ADM-treated or untreated MCF-7 cells, DCs were co-cultured with autologous T-lymphocytes at a ratio of 1:10. T-lymphocytes were sorted from PBMCs by negative selection using Pan T-Cell Isolation Kit magnetic beads (Miltenyi Biotec GmbH, Germany) and were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE, Invitrogen) according to manufacturer instructions. After 5 days of co-culture, T-lymphocyte proliferation was detected by FACS measurement of CFSE fluorescence intensity and supernatants were collected for the measurement of secreted IFN-γ by ELISA.
Flow cytometry analysis
ADM-treated MCF-7 cells were stained with annexin V-FITC and propidium iodide (PI) using an annexin V:FITC Apoptosis Detection Kit I (BD Biosciences, USA) according to manufacturer instructions and then analyzed using a BD flow cytometer (FacsCalibur™) with the CELLQuest™ software to determine the extent of apoptosis. To determine the maturity of DCs, 5 × 105 cells were stained with APC-conjugated anti-CD11c and either FITC-conjugated anti-HLA-DR, anti-CD86 or anti-CD83 antibodies for 30 min on ice in PBS containing 2% FBS, and were then washed and analyzed using a FacsCalibur™ apparatus with CELLQuest™ software. Dead cells were excluded by PI gating, and DCs were gated on the CD11c+ population. All antibodies were from BD Biosciences.
Detection of IFN-γ
Supernatants collected at the end of the culture period (see T-lymphocyte proliferation and activation assay) were tested using IFN-γ ELISA kits (BD Biosciences) according to manufacturer instructions.
Statistical analyses
Data are reported as means ± SD. All statistical analyses were performed by one-way ANOVA and P < 0.05 was considered to be statistically significant.
Results
ADM induces apoptosis of MCF-7 cells in a dose-dependent manner
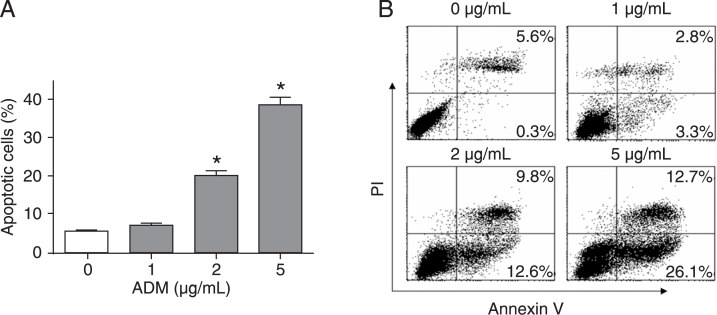
To obtain apoptotic MCF-7 cells, the cell line was treated with 0, 1, 2, or 5 µg/mL ADM for 24 h and then examined for apoptosis by FACS analysis. ADM effectively induced the apoptosis of MCF-7 cells in a dose-dependent manner (Figure 1A). With an increase in the dose of ADM, more MCF-7 cells underwent apoptosis (annexin V+), including early apoptosis (annexin V+/PI-, lower right quadrant) and late apoptosis (annexin V+/PI+, upper right quadrant) (Figure 1B). Thus, MCF-7 cells were treated with 5 µg/mL ADM (denoted as APO) in all subsequent experiments.
Figure 1. Doxorubicin (ADM) induces apoptosis of MCF-7 cells in a dose-dependent manner. MCF-7 cells were treated with 0, 1, 2, or 5 µg/mL ADM for 24 h and then subjected to annexin V-FITC and propidium iodide (PI) staining followed by flow cytometry analysis of apoptosis. Early apoptotic cells were defined as annexin V+/PI-, while late apoptotic cells were double-positive. A, The percentage of apoptotic cells is the sum of the percentage of annexin V+/PI- and double-positive cells. Data are reported as means ± SD for three independent experiments. *P < 0.05 vs the 0 µg/mL ADM group (one-way ANOVA). B, Representative data from three independent experiments.
ADM-induced apoptotic MCF-7 cells induce iDC maturation
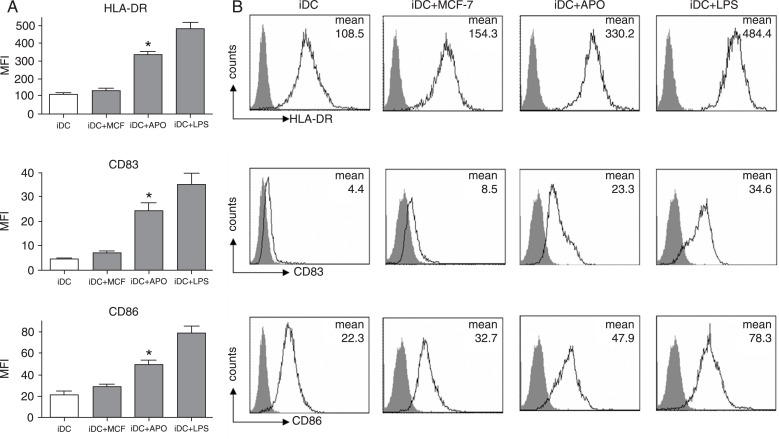
The ability of ADM-induced apoptotic MCF-7 cells to effectively induce iDC maturation was investigated using iDCs obtained after the incubation of PBMCs from healthy donors with 100 ng/mL GM-CSF and 10 ng/mL IL-4 for 6 days. Then, iDCs were cultured with ADM-induced apoptotic MCF-7 cells, untreated MCF-7 cells, LPS or with no addition for 24 h, followed by FACS analysis of DC-surface expression of the maturity marker CD83, co-stimulatory marker CD86 and HLA-DR. As shown in Figure 2A and B, iDCs expressed intermediate levels of CD86 and HLA-DR but were negative for CD83. After co-culture with ADM-induced apoptotic MCF-7 cells (iDCs+APO) for 24 h, DC expression of CD83, CD86 and HLA-DR was significantly increased to levels similar to those found in the classic LPS-induced iDC maturation model (iDCs+LPS). In contrast, surface expression of CD83, CD86 and HLA-DR by iDCs co-cultured with untreated MCF-7 (iDCs+MCF-7) was similar to that of iDCs cultured alone. Thus, ADM-induced apoptotic MCF-7 cells can effectively induce iDC maturation.
Figure 2. Doxorubicin (ADM)-treated apoptotic MCF-7 cells induce immature dendritic cell (iDC) maturation. Peripheral blood mononuclear cells from healthy donors were incubated with 100 ng/mL GM-CSF and 10 ng/mL IL-4 for 6 days and were differentiated into iDCs. Apoptotic MCF-7 cells were extensively washed after treatment with ADM (5 µg/mL) for 24 h and were co-cultured with iDCs for a further 24 h (iDCs+APO). Then, the CD11c-positive DC population was gated and the DC-surface expression of CD83, CD86 and HLA-DR was analyzed by FACS. As a control, iDCs were co-cultured with MCF-7 cells for 24 h (iDCs+MCF-7). As a positive control, mature DCs were generated by further stimulating iDCs with 1 µg/mL lipopolysaccharide for 24 h (iDC+LPS). GM-CSF = granulocyte/macrophage colony-stimulating factor. A, Data are reported as mean fluorescence intensity (MFI) ± SD for three independent experiments. *P < 0.05 vs the iDC+MCF-7 group (one-way ANOVA). B, Representative data of MFI from three independent experiments. The gray histograms represent staining with the corresponding isotype-matched control antibodies.
DCs induced by apoptotic MCF-7 cells stimulate the proliferation and activation of T-lymphocytes
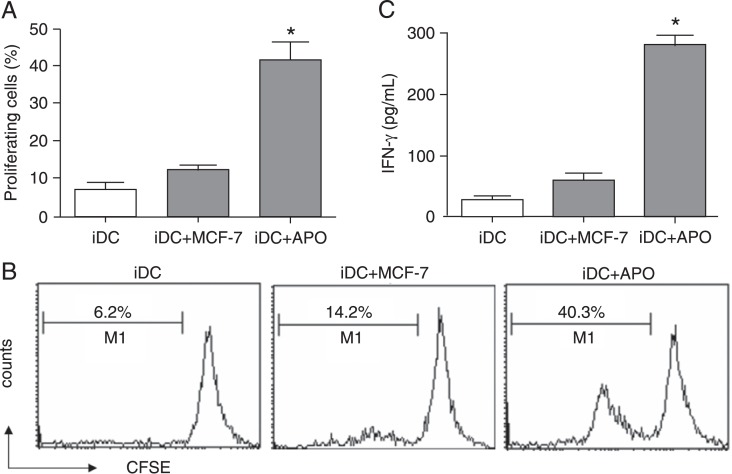
DCs induced by apoptotic MCF-7 cells were then examined for the ability to stimulate T-lymphocyte proliferation and activation. Autologous T-lymphocytes were obtained from the PBMCs of the DC donor by negative selection and were labeled with CFSE dye. Subsequently, the T-lymphocytes were co-cultured with the DCs induced by apoptotic MCF-7 cells (iDCs+APO) or untreated MCF-7 cells (iDCs+MCF-7). After 5 days of co-culture, as a measure of T-lymphocyte proliferation, CFSE dilution was analyzed by flow cytometry. As shown in Figure 3A and B, DCs induced by apoptotic MCF-7 cells could effectively stimulate T-lymphocyte proliferation as compared to control DCs (iDCs+MCF-7).
Figure 3. Immature dendritic cells (iDCs) co-cultured with doxorubicin (ADM)-treated apoptotic MCF-7 cells enhance autologous T-lymphocyte proliferation and interferon-γ (IFN-γ) release. Autologous T-lymphocytes were obtained from peripheral blood mononuclear cells of the DC donor by negative selection and were labeled with CFSE dye. Subsequently, the T-lymphocytes were co-cultured with the DCs induced by apoptotic MCF-7 cells (iDC+APO) or untreated MCF-7 cells (iDC+MCF-7) for 5 days. A, The proliferation of T-lymphocytes was assessed by analyzing CFSE dilution by flow cytometry. Data are reported as means ± SD for three independent experiments. B, Representative data from three independent experiments. The percentage of proliferated T-lymphocytes is indicated. C, ELISA was carried out to detect IFN-γ secretion in the supernatants. CFSE = carboxyfluorescein diacetate succinimidyl ester. Data are reported as means ± SD for three independent experiments. *P < 0.05 vs the iDC+MCF-7 group (one-way ANOVA).
As a measure of T-lymphocyte activation, the secretion of IFN-γ by T-lymphocytes stimulated with DCs induced by apoptotic MCF-7 cells (iDCs+APO) or untreated MCF-7 cells (iDCs+MCF-7) was examined. ELISA showed that, compared to DCs cultured with untreated MCF-7 (iDCs+MCF-7), DCs induced by apoptotic MCF-7 cells (iDCs+APO) caused a significant increase in IFN-γ secretion and could therefore activate T-lymphocytes effectively (Figure 3C).
Cell-cell interaction is crucial for the maturation of iDCs by apoptotic MCF-7 cells
To determine whether soluble factors released by apoptotic MCF-7 cells during co-culture were involved in DC maturation, iDCs were separated from apoptotic MCF-7 cells using 24-well Costar-Transwells with a membrane pore size of 0.4 µM, which enables soluble factor diffusion while blocking direct cell-to-cell contact. As shown in Figure 4, the ability of ADM-induced apoptotic MCF-7 cells to promote iDC maturation was abrogated when the iDCs were separated from apoptotic MCF-7 cells by a membrane, suggesting that the cell-cell interaction between apoptotic MCF-7 cells and iDCs was crucial for the maturation of iDCs.
Figure 4. Cell-cell interaction is crucial for maturation induction of immature dendritic cells (iDCs) by apoptotic MCF-7 cells. The co-culture of iDCs with doxorubicin (ADM)-treated MCF-7 cells was performed either together in the same compartment or separated by 0.4-µM pore-sized filter Transwell inserts on a 24-well culture plate for 24 h. Then, the CD11c-positive DC population was gated and the DC-surface expression of CD83, CD86 and HLA-DR was analyzed by FACS. Data are reported as mean fluorescence intensity (MFI) ± SD for three independent experiments. *P < 0.05 vs Transwell co-culture group (one-way ANOVA).

Discussion
DCs are central to the initiation of tumor-specific immune responses. However, the tumor microenvironment compromises DC activation and limits the success of DC-based therapies. Therefore, removing the DCs from a cancer patient's immunosuppressive milieu and priming in vitro has the potential for more effective DC activation (4). Diverse DC-based vaccine strategies are currently being developed both at the preclinical stage and in clinical trials. For example, DCs loaded with peptides derived from breast cancer tumor-associated antigens (TAAs), such as HER2, CEA and MUC-1, are being tested in this strategy (5-7). However, DC vaccines loaded with peptides are limited in their application, as they will be effective only in patients with specific HLA subtypes, depending on the TAA epitope chosen. Another DC-based vaccine approach being explored in breast cancer uses viral vectors carrying TAA genes (such as CEA and MUC-1) along with co-stimulatory molecule genes (B7.1, ICAM-1, LFA-3) (8,9). However, since peptide or viral vaccines only target one or two antigens, any surviving tumor cell clones could, theoretically, easily down-regulate the expression of those target antigens and evade the immune response. Thus, using whole-cell vaccines is a promising approach because a broad array of TAAs are processed and presented by DCs, thereby allowing the simultaneous stimulation of helper T-lymphocytes and cytotoxic T-lymphocytes, and minimizing immune escape. Since tumor cells are poorly immunogenic, approaches that potentiate immunogenicity, such as transfecting an immunostimulatory gene like B7.1 or CCL21 into breast cancer cells are currently being investigated (10,11).
In the current study, treatment with ADM potentiated the immunogenicity of the MCF-7 breast cancer cell line, leading to the induction of iDC maturation and activation in vitro. These findings could contribute to a novel DC-based vaccine strategy for the treatment of breast cancer. Although this whole-cell vaccine could also derive from a patient's own tumor samples, the MCF-7 cell line can be readily produced on a larger scale and is easier to standardize. While tumor cells also die in a non-immunogenic fashion in response to many chemotherapeutic and cell-damaging agents, cancer cells succumbing to anthracyclines (such as doxorubicin and mitoxantrone), oxaliplatin or ionizing radiation can elicit vigorous anticancer immune responses (12,13). Anthracyclines are the most potent inducers of immunogenic cell death, not only in CT26 tumors, but also in EL4 thymomas and MCA205 sarcomas (12,13).
Our data have shown that the ability of ADM-induced apoptotic MCF-7 cells to promote iDC maturation was abrogated when the iDCs were separated from apoptotic MCF-7 cells by a membrane. This suggests that cell-cell interaction between apoptotic MCF-7 cells and iDCs, but not soluble factors released by apoptotic MCF-7 cells, is crucial for the maturation of iDCs. One possible explanation is that ADM may regulate the exposure of calreticulin/ERp57 complexes on the cell surface of apoptotic MCF-7 cells, which stimulates the uptake of antigen by DCs and maturation. It has been reported that anthracyclines, oxaliplatin and ionizing radiation have the potential to trigger immunogenic cell death by regulating the translocation of calreticulin/ERp57 complexes to the plasma membrane of tumor cells (13,14). Calreticulin/ERp57 is considered to be an “eat-me” signal that is required for DCs to engulf dying tumor cells and thereafter induce T-cell activation against tumors (13,15,16).
ADM has been used in the clinic as therapy for breast cancer for many years and it is interesting to note that not only does it induce MCF-7 cell apoptosis, but it also regulates tumor cell expression of NDRG2 and CD24, which are involved in neoplasm metastasis (Jin Zheng et al., unpublished data).
In the current study, a novel in vitro DC-based vaccine strategy against breast cancer has been developed using ADM-induced apoptotic MCF-7 cells. Further studies in vivo are warranted to determine whether such a DC vaccine could promote the patients' immune response and become a more effective approach to controlling breast cancer in the clinic. In view of the complex in vivo environment in which ADM may have a cytotoxic effect on immune cells, whether ADM also induces DC activation in vivo through breast cancer cell apoptosis remains to be elucidated in animal experiments.
Acknowledgments
Research supported by the National Natural Science Foundation of China (#81072973, #30971763, #30973442).
References
- 1.Soliman H. Developing an effective breast cancer vaccine. Cancer Control. 2010;17:183–190. doi: 10.1177/107327481001700307. [DOI] [PubMed] [Google Scholar]
- 2.Palucka K, Ueno H, Fay J, Banchereau J. Dendritic cells and immunity against cancer. J Intern Med. 2011;269:64–73. doi: 10.1111/j.1365-2796.2010.02317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 4.Apetoh L, Locher C, Ghiringhelli F, Kroemer G, Zitvogel L. Harnessing dendritic cells in cancer. Semin Immunol. 2011;23:42–49. doi: 10.1016/j.smim.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Vlad AM, Finn OJ. Glycoprotein tumor antigens for immunotherapy of breast cancer. Breast Dis. 2004;20:73–79. doi: 10.3233/bd-2004-20109. [DOI] [PubMed] [Google Scholar]
- 6.Mittendorf EA, Holmes JP, Ponniah S, Peoples GE. The E75 HER2/neu peptide vaccine. Cancer Immunol Immunother. 2008;57:1511–1521. doi: 10.1007/s00262-008-0540-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brossart P, Wirths S, Stuhler G, Reichardt VL, Kanz L, Brugger W. Induction of cytotoxic T-lymphocyte responses in vivo after vaccinations with peptide-pulsed dendritic cells. Blood. 2000;96:3102–3108. [PubMed] [Google Scholar]
- 8.Garnett CT, Greiner JW, Tsang KY, Kudo-Saito C, Grosenbach DW, Chakraborty M, et al. TRICOM vector based cancer vaccines. Curr Pharm Des. 2006;12:351–361. doi: 10.2174/138161206775201929. [DOI] [PubMed] [Google Scholar]
- 9.Gulley JL, Arlen PM, Tsang KY, Yokokawa J, Palena C, Poole DJ, et al. Pilot study of vaccination with recombinant CEA-MUC-1-TRICOM poxviral-based vaccines in patients with metastatic carcinoma. Clin Cancer Res. 2008;14:3060–3069. doi: 10.1158/1078-0432.CCR-08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dols A, Smith JW, Meijer SL, Fox BA, Hu HM, Walker E, et al. Vaccination of women with metastatic breast cancer, using a costimulatory gene (CD80)-modified, HLA-A2-matched, allogeneic, breast cancer cell line: clinical and immunological results. Hum Gene Ther. 2003;14:1117–1123. doi: 10.1089/104303403322124828. [DOI] [PubMed] [Google Scholar]
- 11.Riedl K, Baratelli F, Batra RK, Yang SC, Luo J, Escuadro B, et al. Overexpression of CCL-21/secondary lymphoid tissue chemokine in human dendritic cells augments chemotactic activities for lymphocytes and antigen presenting cells. Mol Cancer. 2003;2:35. doi: 10.1186/1476-4598-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Casares N, Pequignot MO, Tesniere A, Ghiringhelli F, Roux S, Chaput N, et al. Caspase-dependent immunogenicity of doxorubicin-induced tumor cell death. J Exp Med. 2005;202:1691–1701. doi: 10.1084/jem.20050915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Obeid M, Tesniere A, Ghiringhelli F, Fimia GM, Apetoh L, Perfettini JL, et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat Med. 2007;13:54–61. doi: 10.1038/nm1523. [DOI] [PubMed] [Google Scholar]
- 14.Panaretakis T, Joza N, Modjtahedi N, Tesniere A, Vitale I, Durchschlag M, et al. The co-translocation of ERp57 and calreticulin determines the immunogenicity of cell death. Cell Death Differ. 2008;15:1499–1509. doi: 10.1038/cdd.2008.67. [DOI] [PubMed] [Google Scholar]
- 15.Obeid M, Panaretakis T, Tesniere A, Joza N, Tufi R, Apetoh L, et al. Leveraging the immune system during chemotherapy: moving calreticulin to the cell surface converts apoptotic death from “silent” to immunogenic. Cancer Res. 2007;67:7941–7944. doi: 10.1158/0008-5472.CAN-07-1622. [DOI] [PubMed] [Google Scholar]
- 16.Obeid M, Panaretakis T, Joza N, Tufi R, Tesniere A, van Endert P, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–1850. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]