Abstract
Wear particles are phagocytosed by macrophages and other inflammatory cells, resulting in cellular activation and release of proinflammatory factors, which cause periprosthetic osteolysis and subsequent aseptic loosening, the most common causes of total joint arthroplasty failure. During this pathological process, tumor necrosis factor-alpha (TNF-α) plays an important role in wear-particle-induced osteolysis. In this study, recombination adenovirus (Ad) vectors carrying both target genes [TNF-α small interfering RNA (TNF-α-siRNA) and bone morphogenetic protein 2 (BMP-2)] were synthesized and transfected into RAW264.7 macrophages and pro-osteoblastic MC3T3-E1 cells, respectively. The target gene BMP-2, expressed on pro-osteoblastic MC3T3-E1 cells and silenced by the TNF-α gene on cells, was treated with titanium (Ti) particles that were assessed by real-time PCR and Western blot. We showed that recombinant adenovirus (Ad-siTNFα-BMP-2) can induce osteoblast differentiation when treated with conditioned medium (CM) containing RAW264.7 macrophages challenged with a combination of Ti particles and Ad-siTNFα-BMP-2 (Ti-ad CM) assessed by alkaline phosphatase activity. The receptor activator of nuclear factor-κB ligand was downregulated in pro-osteoblastic MC3T3-E1 cells treated with Ti-ad CM in comparison with conditioned medium of RAW264.7 macrophages challenged with Ti particles (Ti CM). We suggest that Ad-siTNFα-BMP-2 induced osteoblast differentiation and inhibited osteoclastogenesis on a cell model of a Ti particle-induced inflammatory response, which may provide a novel approach for the treatment of periprosthetic osteolysis.
Keywords: TNF-α, BMP-2, Small interfering RNA, Titanium particles, Osteolysis
Introduction
Titanium (Ti) components have become widely used for joint replacement, and have excellent corrosion resistance, biocompatibility and a high resistance-to-weight ratio. However, wear debris that forms at prosthetic joint articulations, modular interfaces, and nonarticulating interfaces (1,2) is the main reason for prosthesis failure (3-5). In this pathological process, activated macrophages/monocytes respond to Ti particles, releasing proinflammatory mediators and cytokines including tumor necrosis factor alpha (TNF-α), interleukin (IL)-6, and IL-1β in vitro (6-8) and are present in periprosthetic soft tissue in vivo (9). Research suggests that the biological response to wear particles at the bone-implant interface is the main cause of aseptic loosening and osteolysis (10,11). The proinflammatory cytokines are thought to cause an imbalance in bone metabolism, favoring bone resorption via the induction of the receptor activator of nuclear factor-κB ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) in osteoblasts (12-14) and by blocking bone formation via inhibition of osteoblast differentiation (15-17).
Bone is a dynamic tissue that is constantly formed by osteoblasts and resorbed by osteoclasts, which maintain a dynamic balance on the principle that the amount of bone destroyed by osteoclasts is equal to the amount of bone formed by osteoblasts. Proinflammatory cytokines such as IL-1β and TNF-α can disturb the balance of bone metabolism by inhibiting bone formation and increasing bone resorption, leading to a loss of bone stock. The reduced bone stock also contributes to periprosthetic osteolysis. On the basis of these considerations, we concluded that these proinflammatory cytokines would provide a promising therapeutic target for the treatment of periprosthetic osteolysis.
Osteoblasts not only play a central role in bone formation by synthesizing several bone matrix proteins, but regulate osteoclast maturation through expression of soluble factors RANKL and M-CSF, resulting in bone resorption. Osteoblastic differentiation could inhibit osteoclast formation via downregulation of RANKL expression (18,19) and upregulation of osteoprotegerin (OPG) expression (20).
Bone morphogenetic protein 2 (BMP-2) plays an important role in regulating osteoblast differentiation and subsequent bone formation (21-23). However, TNF-α can restrain BMP-2 signaling in osteoblastic differentiation (24-26). Downregulating TNF-α expression in activated macrophages in response to Ti particles, while simultaneously upregulating BMP-2 expression to promote osteoblast differentiation, is expected to be effective in the treatment or prevention of periprosthetic ostelysis.
Therefore, we used gene therapy to prevent and/or treat aseptic loosening of prosthetic joints by constructing an adenovirus (Ad)-mediated small interfering RNA (siRNA) targeting TNF-α and, at the same time, overexpression of BMP-2 (Ad-siTNFα-BMP-2). We determined whether the recombinant adenovirus (Ad-siTNFα-BMP-2) could inhibit the expression of TNF-α in RAW264.7 cells when cultured with Ti particles. We also investigated the effects of Ad-TNF-α-siRNA-BMP-2 on osteoblastic MC3T3-E1 cells to confirm differentiation in conditioned culture media (CM).
Material and Methods
Preparation of Ti particles
Commercially pure Ti particles were obtained from Zimmer Company (USA); 90% of the Ti particles were <10 mm in diameter. The Ti particles were prepared as previously described (27). The particles were sterilized at 180°C for 6 h, followed by treating with 70% ethanol for 48 h to remove endotoxin. The particle endotoxin level in this study was lower than 0.1 EU/mL, as determined using a commercial detection kit (E-Toxate; Sigma, USA). Ti particles were sonicated and vortexed before treatment.
Construction of adenovirus-expressing TNFα-siRNA-BMP-2
Full-length BMP-2 cDNA expressed in pCDNA3.1 vector (KpnI and XbaI sites) was cloned into a pAd5E3-CMV shuttle plasmid using ClaI and SpeI sites; the plasmids were then amplified by transfection into DH5α cells, and positive clones (pAd5E3-CMV-mBMP-2-pA) were selected and confirmed by DNA miniprep and PacI digestion. To construct the pAd5E3-CMV-mBMP-2 backbone, pAd5E3-CMV-mBMP-2-pA was linearized with PacI digestion and subsequently cotransformed into BJ5183 cells with a pAd5 backbone using SwaI sites (28). Plasmids were amplified by transforming into DH5α cells followed by DNA maxiprep. To construct adenoviral vectors expressing both BMP-2 and TNF-α-siRNA, pAd5E1-hU6 TNFα siRNA-CMVeGFP were linearized with PacI digestion and subsequently cotransformed into HEK293 cells with a linearized pAd5E3-CMV-mBMP-2 backbone. Adenoviral vectors were purified with three density gradients of CsCl dialyzed with viral titers, determined by absorbance and standard plaque assays as described previously (29).
Cell culture
The murine macrophage/monocyte cell line RAW264.7 (BH-AC71; ATCC, USA) was maintained at 37°C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM; Sigma) containing 10% FBS (Hyclone, USA), 100 U/mL penicillin, and 100 U/mL streptomycin. The murine osteoblastic cell line MC3T3-E1 (ATCC) was cultured in α-minimum essential medium (Sigma) supplemented with 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin at 37°C and 5% CO2.
Collection of conditioned media
RAW264.7 cells were plated on 24-well cluster plates at a density of 1.0×105 cells in complete DMEM. After 24 h of attachment, cells were washed with PBS and stabilized in serum-free DMEM for 1 h. Then, cells were cultured separately with and without Ti particles (0.1 mg/mL) or Ti particles and Ad-siTNFα-BMP-2. After 24 h of incubation, control CM (cont CM), CM with Ti particles (Ti CM), and CM with Ti particles and Ad-siTNFα-BMP-2 (Ti-ad CM) were collected, centrifuged to remove cell debris, if any, and stored at −20°C until use. The conditioned media were made as previously described (26).
RNA isolation and real-time RT-PCR
Total RNA was extracted using Trizol (Invitrogen, USA) according to the manufacturer's instructions. The 260/280 nm absorbance ratio was measured for verification of RNA purity (NanoDrop, USA). First-strand cDNA was synthesized with 2 µg total RNA (Fermentas, Canada), and one-tenth of the cDNA was used for each PCR mixture containing EXPRESS SYBR Green (TaKaRa, Japan) and PCR Supermix (Fermentas). The reaction was subjected to a 40-cycle amplification at 95°C for 30 s, at 95°C for 5 s, and at 60°C for 30 s. Relative mRNA expression of selected genes was normalized to GAPDH and quantified using the ΔΔCT method. The sequences of the PCR primers are listed in Table 1.
Protein isolation and Western blotting
Cells were lysed in RIPA buffer (20 mM Tris-HCl, pH 7.5, 200 mM NaCl, 1% Triton X-100, 1 mM dithiothreitol) containing protease inhibitor cocktail (Roche, Switzerland). Protein concentration was measured with a bicinchoninic acid protein quantitation kit (Biovision, USA) following the manufacturer's instructions. Total protein was subjected to SDS-polyacrylamide gel electrophoresis and transferred to a PVDF membrane. The blot was probed with anti-TNF-α (Cell Signaling Technology, USA) and anti-BMP-2 (Abcam, England) primary antibodies. Anti-β-actin (CoWin Biotech, USA) was used as a loading control. Subsequently, the blots were washed in TBST (10 mM Tris-HCl, 50 mM NaCl, 0.25% Tween 20) and incubated with secondary antibody. The presence of target proteins was detected using enhanced chemiluminescence reagents (Millipore Corp., USA).
Alkaline phosphatase (ALP) activity assay
ALP activity was measured with the QuantiChrom™ Alkaline Phosphatase Assay Kit (BioAssay Systems, USA). Briefly, the culture medium was removed. Cells were rinsed twice with PBS and lysed in 150 µL/well RIPA buffer. Lysates were harvested, and clarified by centrifugation at 12,000 g at 4°C for 10 min. The supernatants were incubated with 3.7 mM 4-nitrophenyl phosphate in 100 mM diethanolamine, pH 9.8, containing 0.1% Triton X-100 at 37°C for 5 min. The amount of released 4-nitrophenolate was determined photometrically at 405 nm. Enzyme activity was calculated according to the manufacturer's recommendations.
ELISA
RAW264.7 cells were incubated with Ti particles (0.1 mg/mL) for 24 h. Ti CM was collected to determine the concentration of TNF-α. A mouse TNF-α ELISA kit (AMEKO, USA) was used for quantitative measurement following the manufacturer's recommendations.
Statistical analysis
Data are reported as means±SE for at least triplicate determinations. Differences between groups were analyzed using analysis of variance. Statistical significance was defined as P<0.05. Statistical analyses were performed using SPSS version 17.0 (IBM, USA).
Results
Effects of Ad-siTNFα-BMP-2 and Ti particles on MC3T3-E1 viability
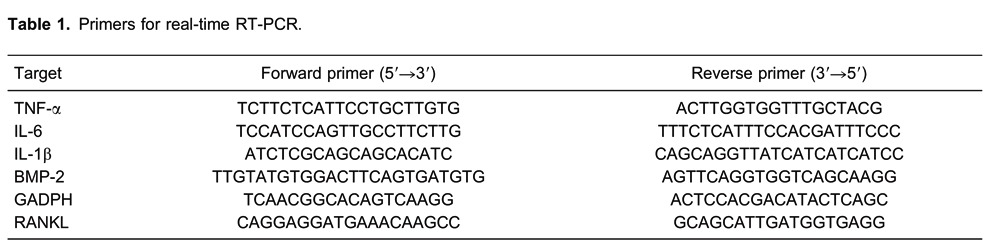
Cell viability shown by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay was not significantly different among RAW264.7 and MC3T3-E1 cells treated with different multiplicity of infection (MOI) of Ad-siTNFα-BMP-2 (30, 50, and 70 MOI) for 48 h (Figure 1A and B). Furthermore, 0.1 mg/mL Ti particles and 50 MOI Ad-siTNFα-BMP-2 made no difference in the viability of RAW264.7 cells at each time point (Figure 1C). Similarly, 50 MOI Ad-siTNFα-BMP-2 had no effect on the viability of MC3T3-E1 cells (Figure 1D). The results suggested that both Ti particles and Ad-siTNFα-BMP-2 were not toxic to RAW264.7 cells, and Ad-siTNFα-BMP-2 was not toxic to MC3T3-E1 cells.
Figure 1. Effect of Ad-siTNFα-BMP-2 on cell viability. A, RAW264.7 cells (A) and MC3T3-E1 cells (B) were treated with different MOIs of Ad-siTNFα-BMP-2 for 48 h. C, RAW264.7 cells were treated with Ti and 50 MOI Ad-siTNFα-BMP-2 for 24-72 h. D, MC3T3-E1 cells were treated with 50 MOI Ad-siTNFα-BMP-2 for 24-72 h. The cellular activity was estimated by MTT assay. Data are reported as means±SD. MOI: multiplicity of infection.
Effects of suppression of Ad-siTNFα-BMP-2 on Ti particle-induced inflammatory factor
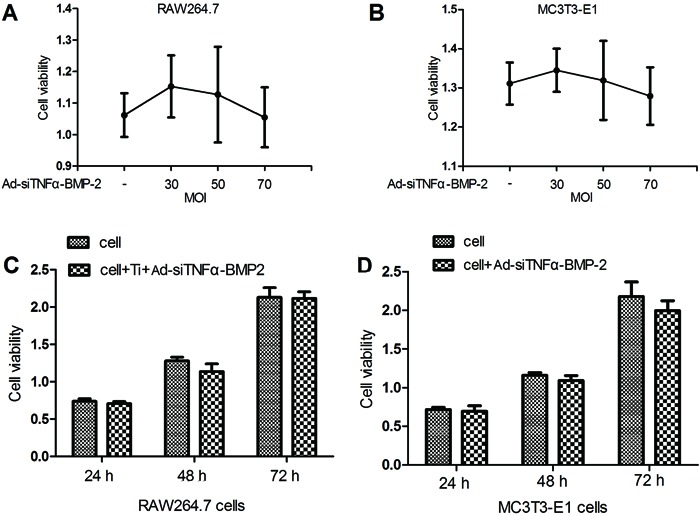
Detection showed that the expression of TNF-α by real-time RT-PCR was significantly increased in RAW264.7 cells treated with different doses of Ti particles (0.05, 0.1, 0.15 mg/mL), and the result indicated that expression of TNF-α was highest in RAW264.7 cells treated with 0.1 mg/mL Ti particles (data not shown). We believed that 0.1 mg/mL Ti particles was the optimum dose-induced TNF-α expression for our experimental conditions. Our results showed that TNF-α mRNA expression at 50 MOI was significantly reduced after 48 h (Figure 2A). The same result was observed for TNF-α protein levels, as shown by Western blot analysis after 48 h. Ad-siTNFα-BMP-2 at 50 MOI significantly reduced expression of TNF-α protein levels (Figure 2B). These results (Figure 2A and B) confirmed that Ad-siTNFα-BMP-2 reduced TNF-α expression in RAW264.7 cells treated with 0.1 mg/mL Ti particles.
Figure 2. Suppression effects of Ad-siTNFα-BMP-2 on titanium particle-induced inflammatory factor. A, RAW264.7 cells were plated on 6-well cluster plates at a density of 4×105 cells/well. After 24 h, RAW264.7 cells were treated with or without Ti particles in the presence or absence of Ad-siTNFα-BMP-2 for 48 h. There was a significant reduction of TNF-α mRNA expression at 50 MOI . B, TNF-α protein levels detected by Western blot after 48 h. There was significant reduction of IL-6 (C) and IL-1β (D) mRNA expression by Ad-siTNFα-BMP-2 at 50 MOI. E, TNF-α protein levels detected by ELISA when treated with 0.1 mg/mL Ti particles or a combination of 0.1 mg/mL Ti particles and 50 MOI Ad-siTNFα-BMP-2 for 24 h. Data are reported as means±SD. Similar results were obtained in three independent experiments. MOI: multiplicity of infection. *P<0.05, compared to the cultures with Ti particles only; +P<0.05, compared to 30 MOI (one-way ANOVA).
We also detected inflammatory factors IL-1β and IL-6. The results showed that downregulation of TNF-α mRNA reduced mRNA expression of inflammatory cytokines such as IL-6 (Figure 2C) and IL-1β (Figure 2D). It is suggested that TNF-α can promote IL-6 and IL-1β mRNA expression.
Detection of TNF-α protein levels by ELISA was performed for assessment of RAW264.7 cells treated with 0.1 mg/mL Ti particles or a combination of 0.1 mg/mL Ti particles and 50 MOI Ad-siTNFα-BMP-2 for 24 h (Figure 2E).
BMP-2 expression in MC3T3-E1 cells treated with Ad-siTNFα-BMP-2
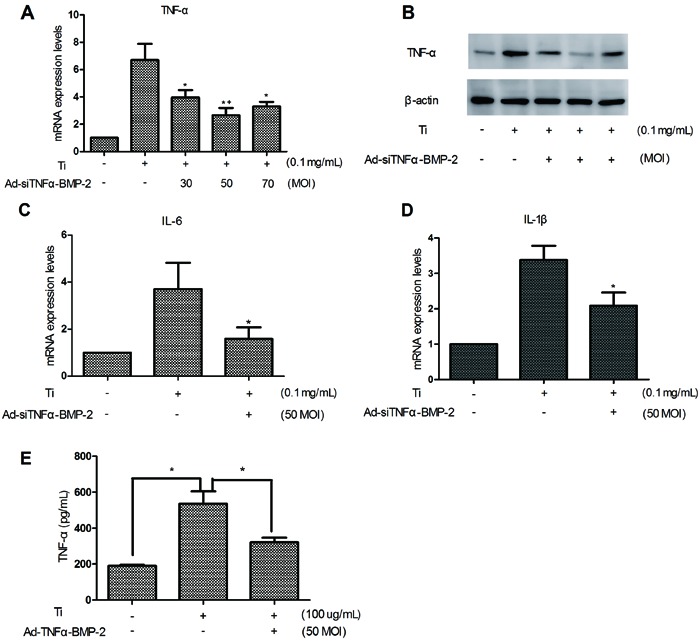
We detected the expression of BMP-2 mRNA by real-time PCR, which was significantly increased in MC3T3-E1 cells treated with different MOI (30, 50, and 70). Our results showed that the MOI of Ad-siTNFα-BMP-2 was determined to be 50 (Figure 3A). The same result was shown for TNF-α protein levels by the detection of Western blots after 48 h (Figure 3C). Expression of BMP-2 was analyzed at 2, 4, and 6 days in MC3T3-E1 cells treated with 50 MOI Ad-siTNFα-BMP-2 by real-time RT-PCR (Figure 3B).
Figure 3. BMP-2 expression in MC3T3-E1 treated with Ad-siTNFα-BMP-2. A, MC3T3-E1 cells were plated on 6-well cluster plates at a density of 4×105 cells/well. After 24 h, MC3T3-E1 cells were treated with Ad-siTNFα-BMP-2 for 48 h. There was a significant increase of BMP-2 mRNA expression. B, Expression of BMP-2 was analyzed at 2, 4, and 6 days in MC3T3-E1 cells treated with 50 MOI Ad-siTNFα-BMP-2. C, BMP-2 protein levels detected by Western blot after 48 h. Data are reported as means±SD. Similar results were obtained in three independent experiments. MOI: multiplicity of infection. *P<0.01, compared to control (one-way ANOVA).
Induction of osteoblast differentiation and inhibition of osteoclastogenesis by transduction of MC3T3-E1 cells with Ad-siTNFα-BMP-2 and different conditioned media
ALP activity, a marker of the early period of osteoblastic differentiation, was assessed at 2, 4, and 6 days in MC3T3-E1 cells treated with Ad-siTNFα-BMP-2 using an ALP kit (Figure 4A). The result indicated that Ad-siTNFα-BMP-2 could induce osteoblast differentiation.
Figure 4. Induction of osteoblast differentiation evaluated by a combination of treatment with conditioned medium (CM; Ti CM and Ti-ad CM) and Ad-siTNFα-BMP-2. Inhibition of RANKL mRNA expression in MC3T3-E1 cells was evaluated by a combination of treatment with CM (Ti CM and Ti-ad CM). Data are reported as means±SD. Similar results were obtained in three independent experiments. A, MC3T3-E1 cells were plated on 24-well cluster plates at a density of 1×105 cells/well. After 24 h, MC3T3-E1 cells were treated with Ad-siTNFα-BMP-2 (50 MOI), ALP activity levels were assessed for 2-6 days. *P<0.05, compared to the treatment with Ad-siTNFα-BMP-2 (50 MOI) at 2 days. B, ALP activity was assessed in MC3T3-E1 cells treated with Ad-siTNFα-BMP-2 (50 MOI) and CM [cont (control) CM, Ti CM and Ti-ad CM] at 6 days. *P<0.05, compared to the treatment with a combination cont CM and Ad-siTNFα-BMP-2. C, MC3T3-E1 cells were treated with CM (cont CM, Ti CM and Ti-ad CM) for 24 h. RANKL mRNA expression was assessed by real-time RT-PCR. MOI: multiplicity of infection, ND: not detected. *P<0.05, compared to the treatment with Ti CM (one-way ANOVA).
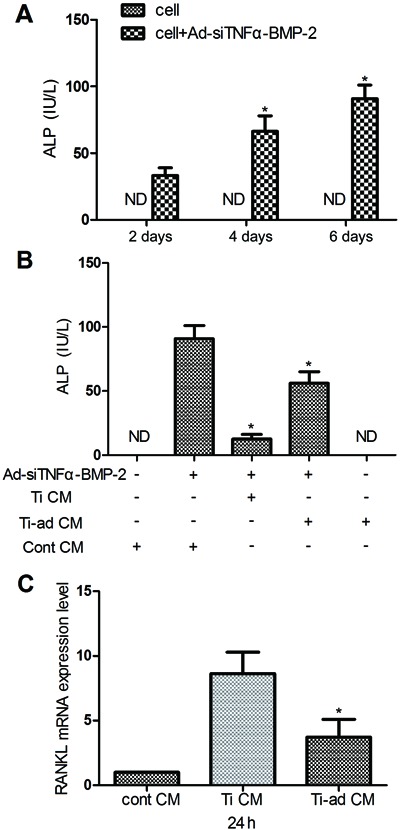
Furthermore, Ad-siTNFα-BMP-2 induced osteoblast differentiation when treated with different CM (cont CM, Ti CM, or Ti-ad CM), and assessed by levels of ALP activity (Figure 4B). The results suggest that Ad-siTNFα-BMP-2 might compensate Ti particle-induced osteolysis through promoting osteoblast differentiation in vivo.
RANKL is a member of the TNF family that is essential for osteoclastogenesis (30). We detected expression of RANKL mRNA in MC3T3-E1 cells treated with different CM (cont CM, Ti CM, and Ti-ad CM) by real-time PCR (Figure 4C). The result indicated that Ad-siTNFα-BMP-2 might inhibit Ti particle-induced osteoclastogenesis.
Discussion
Currently, there is no effective treatment for aseptic joint loosening apart from reoperation. Reoperated patients suffer a serious physical and psychological trauma and economic burden; in addition, there are high risks of operation and of subsequent multiple perioperative complications for elderly patients who undergo revision. Subsequently, there is a research focus on approaches for taking advantage of nonsurgical methods to cure aseptic joint loosening. However, there is still no recognized effective treatment because the mechanism of aseptic joint loosening is not completely clear. Despite all this, metal particle-induced proinflammatory cytokines such as IL-1β and TNF-α are considered to be important factors for stimulating osteoclastogenesis.
Cytokines such as TNF-α, IL-1β, and IL-6 are involved in osteoclastogenesis through induced RANKL expression in osteoblasts. In this study, Ad-siTNFα-BMP-2 could inhibit Ti particles induced to increase TNF-α in macrophages and simultaneously could also downregulate IL-1β and IL-6 expression in cultures with Ti particles. Our results showed that Ad-siTNFα-BMP-2 effectively reduced expression of RANKL in MC3T3-E1 cells by downregulating expression of these proinflammatory cytokines from RAW264.7 cells in response to Ti particles. Downregulation of TNF-α levels was important for expression of RANKL, which was critical for osteoclast differentiation and function. Furthermore, TNF-α and RANKL support osteoclast survival (31,32). Ad-siTNFα-BMP-2 directly and indirectly downregulated TNF-α and RANKL expression in our experiment. It is speculated that Ad-siTNFα-BMP-2 might reduce Ti particle-induced bone resorption.
ALP activity is a marker of the early period of osteoblastic differentiation. In our study, Ad-siTNFα-BMP-2 promoted pro-osteoblast differentiation into osteoblasts, which contributed to the increased number of osteoblasts and bone formation. Furthermore, promotion of osteoblast differentiation reduced RANKL expression and inhibited the ability of the cells to support osteoclast differentiation (18,19); osteoblast expression of the RANKL/OPG ratio was much higher in less mature osteoblasts than in mature ones (19). Simultaneously, TNF-α induced apoptosis much easier in less mature osteoblasts than in mature ones (32). Hence, Ad-siTNFα-BMP-2 may promote osteoblast differentiation, contribute to osteoclast differentiation inhibition, and increase bone formation.
TNF-α, IL-6, and IL-1β can inhibit osteoblast differentiation (15-17) and TNF-α has been shown to inhibit BMP-2 signaling pathways (24-26). In this study, macrophages challenged in culture with media containing titanium particles (Ti CM) inhibited Ad-siTNFα-BMP-2-induced osteoblast differentiation. Ad-siTNFα-BMP-2 induced osteoblast differentiation in macrophage cultures in conditioned medium including Ti particles and Ad-siTNFα-BMP-2 (Ti-ad CM). The results suggested that Ad-siTNFα-BMP-2 was expected to induce osteoblast differentiation in a cell model of wear particle-induced inflammation, which was an essential part of bone formation that compensated resorbed bone matrix to maintain its structural integrity.
Orthopedic gene therapy had its origins in the early 1990s in attempts to deliver genes to joints (33,34). The aim of the present study was to design intra- and periarticular tissues that can promote synthesis of anti-arthritic gene products, thereby providing a sustained, local therapy for individual arthritic joints. This approach is attractive because joints are discrete, accessible cavities that can be readily injected. Interference RNA is an almost standard method for the knockdown of any target gene of interest in vitro, exploring a naturally occurring catalytic mechanism. The downregulation of pathologically relevant genes that are aberrantly expressed in a given disease will offer novel therapeutic approaches. siRNA therapeutics has developed rapidly, and already there are clinical trials ongoing or planned. Meanwhile, BMP-2 is approved for clinical use to help induce osteogenesis (35-37). The long journey from the identification of BMP-2 in demineralized bone fractions to US Food and Drug Administration approval for use in a singular orthopedic application has been completed. It has been demonstrated to be safe, efficacious, and cost-effective, leading to increased patient satisfaction and improved clinical outcomes (38). Therefore, Ad-siTNFα-BMP-2 may provide a promising therapeutic approach for the treatment of periprosthetic osteolysis.
Our results also emphasized the need for more effective treatment methods. Further evidence of the effect of Ad-siTNFα-BMP-2 on particle-induced osteolysis is required in in vivo experiments on animal models. This would involve the creation of an animal model of aseptic loosening, and would then involve the local administration of an intra-articular injection of recombinant adenovirus in order to detect the effect of recombinant adenovirus on Ti particle-induced proinflammatory cytokine expression and periprosthetic osteolysis. Our data may aid in the continued advances in research for the prevention and/or treatment of particle-induced osteolysis.
Acknowledgments
Research supported by the National Natural Science Foundation of China (NSFC, #81060146).
Footnotes
First published online October 18, 2013.
References
- 1.Harris WH. The problem is osteolysis. Clin Orthop Relat Res. 1995;311:46–53. [PubMed] [Google Scholar]
- 2.Goldring SR, Clark CR, Wright TM. The problem in total joint arthroplasty: aseptic loosening. J Bone Joint Surg Am. 1993;75:799–801. doi: 10.2106/00004623-199306000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Kadoya Y, Kobayashi A, Ohashi H. Wear and osteolysis in total joint replacements. Acta Orthop Scand Suppl. 1998;278:1–16. [PubMed] [Google Scholar]
- 4.Friedman RJ, Black J, Galante JO, Jacobs JJ, Skinner HB. Current concepts in orthopaedic biomaterials and implant fixation. Instr Course Lect. 1994;43:233–255. [PubMed] [Google Scholar]
- 5.Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;393:66–70. doi: 10.1097/00003086-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hallab NJ, Jacobs JJ. Biologic effects of implant debris. Bull NYU Hosp Jt Dis. 2009;67:182–188. [PubMed] [Google Scholar]
- 7.Kaufman AM, Alabre CI, Rubash HE, Shanbhag AS. Human macrophage response to UHMWPE, TiAlV, CoCr, and alumina particles: analysis of multiple cytokines using protein arrays. J Biomed Mater Res A. 2008;84:464–474. doi: 10.1002/jbm.a.31467. [DOI] [PubMed] [Google Scholar]
- 8.Liu F, Zhu Z, Mao Y, Liu M, Tang T, Qiu S. Inhibition of titanium particle-induced osteoclastogenesis through inactivation of NFATc1 by VIVIT peptide. Biomaterials. 2009;30:1756–1762. doi: 10.1016/j.biomaterials.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 9.Shanbhag AS, Jacobs JJ, Black J, Galante JO, Glant TT. Cellular mediators secreted by interfacial membranes obtained at revision total hip arthroplasty. J Arthroplasty. 1995;10:498–506. doi: 10.1016/S0883-5403(05)80152-4. [DOI] [PubMed] [Google Scholar]
- 10.Jacobs JJ, Roebuck KA, Archibeck M, Hallab NJ, Glant TT. Osteolysis: basic science. Clin Orthop Relat Res. 2001;393:71–77. doi: 10.1097/00003086-200112000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials. 2005;26:1271–1286. doi: 10.1016/j.biomaterials.2004.04.035. [DOI] [PubMed] [Google Scholar]
- 12.Boyce BF, Li P, Yao Z, Zhang Q, Badell IR, Schwarz EM, et al. TNF-alpha and pathologic bone resorption. Keio J Med. 2005;54:127–131. doi: 10.2302/kjm.54.127. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka Y, Nakayamada S, Okada Y. Osteoblasts and osteoclasts in bone remodeling and inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:325–328. doi: 10.2174/1568010054022015. [DOI] [PubMed] [Google Scholar]
- 14.Mori T, Miyamoto T, Yoshida H, Asakawa M, Kawasumi M, Kobayashi T, et al. IL-1beta and TNFalpha-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int Immunol. 2011;23:701–712. doi: 10.1093/intimm/dxr077. [DOI] [PubMed] [Google Scholar]
- 15.Hikiji H, Shin WS, Koizumi T, Takato T, Susami T, Koizumi Y, et al. Peroxynitrite production by TNF-alpha and IL-1beta: implication for suppression of osteoblastic differentiation. Am J Physiol Endocrinol Metab. 2000;278:E1031–E1037. doi: 10.1152/ajpendo.2000.278.6.E1031. [DOI] [PubMed] [Google Scholar]
- 16.Gilbert L, He X, Farmer P, Boden S, Kozlowski M, Rubin J, et al. Inhibition of osteoblast differentiation by tumor necrosis factor-alpha. Endocrinology. 2000;141:3956–3964. doi: 10.1210/en.141.11.3956. [DOI] [PubMed] [Google Scholar]
- 17.Peruzzi B, Cappariello A, Del Fattore A, Rucci N, De Benedetti F, Teti A. c-Src and IL-6 inhibit osteoblast differentiation and integrate IGFBP5 signalling. Nat Commun. 2012;3:630–630. doi: 10.1038/ncomms1651. [DOI] [PubMed] [Google Scholar]
- 18.Gori F, Hofbauer LC, Dunstan CR, Spelsberg TC, Khosla S, Riggs BL. The expression of osteoprotegerin and RANK ligand and the support of osteoclast formation by stromal-osteoblast lineage cells is developmentally regulated. Endocrinology. 2000;141:4768–4776. doi: 10.1210/en.141.12.4768. [DOI] [PubMed] [Google Scholar]
- 19.Atkins GJ, Kostakis P, Pan B, Farrugia A, Gronthos S, Evdokiou A, et al. RANKL expression is related to the differentiation state of human osteoblasts. J Bone Miner Res. 2003;18:1088–1098. doi: 10.1359/jbmr.2003.18.6.1088. [DOI] [PubMed] [Google Scholar]
- 20.Thomas GP, Baker SU, Eisman JA, Gardiner EM. Changing RANKL/OPG mRNA expression in differentiating murine primary osteoblasts. J Endocrinol. 2001;170:451–460. doi: 10.1677/joe.0.1700451. [DOI] [PubMed] [Google Scholar]
- 21.Yamaguchi A, Katagiri T, Ikeda T, Wozney JM, Rosen V, Wang EA, et al. Recombinant human bone morphogenetic protein-2 stimulates osteoblastic maturation and inhibits myogenic differentiation in vitro . J Cell Biol. 1991;113:681–687. doi: 10.1083/jcb.113.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kang Q, Sun MH, Cheng H, Peng Y, Montag AG, Deyrup AT, et al. Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 2004;11:1312–1320. doi: 10.1038/sj.gt.3302298. [DOI] [PubMed] [Google Scholar]
- 23.Castro-Govea Y, Cervantes-Kardasch VH, Borrego-Soto G, Martinez-Rodriguez HG, Espinoza-Juarez M, Romero-Diaz V, et al. Human bone morphogenetic protein 2-transduced mesenchymal stem cells improve bone regeneration in a model of mandible distraction surgery. J Craniofac Surg. 2012;23:392–396. doi: 10.1097/SCS.0b013e318240fe9b. [DOI] [PubMed] [Google Scholar]
- 24.Gilbert L, He X, Farmer P, Rubin J, Drissi H, van Wijnen AJ, et al. Expression of the osteoblast differentiation factor RUNX2 (Cbfa1/AML3/Pebp2alpha A) is inhibited by tumor necrosis factor-alpha. J Biol Chem. 2002;277:2695–2701. doi: 10.1074/jbc.M106339200. [DOI] [PubMed] [Google Scholar]
- 25.Kaneki H, Guo R, Chen D, Yao Z, Schwarz EM, Zhang YE, et al. Tumor necrosis factor promotes Runx2 degradation through up-regulation of Smurf1 and Smurf2 in osteoblasts. J Biol Chem. 2006;281:4326–4333. doi: 10.1074/jbc.M509430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee SS, Sharma AR, Choi BS, Jung JS, Chang JD, Park S, et al. The effect of TNFalpha secreted from macrophages activated by titanium particles on osteogenic activity regulated by WNT/BMP signaling in osteoprogenitor cells. Biomaterials. 2012;33:4251–4263. doi: 10.1016/j.biomaterials.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Rakshit DS, Ly K, Sengupta TK, Nestor BJ, Sculco TP, Ivashkiv LB, et al. Wear debris inhibition of anti-osteoclastogenic signaling by interleukin-6 and interferon-gamma. Mechanistic insights and implications for periprosthetic osteolysis. J Bone Joint Surg Am. 2006;88:788–799. doi: 10.2106/JBJS.E.00711. [DOI] [PubMed] [Google Scholar]
- 28.Anderson RD, Haskell RE, Xia H, Roessler BJ, Davidson BL. A simple method for the rapid generation of recombinant adenovirus vectors. Gene Ther. 2000;7:1034–1038. doi: 10.1038/sj.gt.3301197. [DOI] [PubMed] [Google Scholar]
- 29.Palmer GD, Gouze E, Gouze JN, Betz OB, Evans CH, Ghivizzani SC. Gene transfer to articular chondrocytes with recombinant adenovirus. Methods Mol Biol. 2003;215:235–246. doi: 10.1385/1-59259-345-3:235. [DOI] [PubMed] [Google Scholar]
- 30.Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K, et al. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun. 1998;253:395–400. doi: 10.1006/bbrc.1998.9788. [DOI] [PubMed] [Google Scholar]
- 31.Blair HC, Robinson LJ, Zaidi M. Osteoclast signalling pathways. Biochem Biophys Res Commun. 2005;328:728–738. doi: 10.1016/j.bbrc.2004.11.077. [DOI] [PubMed] [Google Scholar]
- 32.Xing L, Boyce BF. Regulation of apoptosis in osteoclasts and osteoblastic cells. Biochem Biophys Res Commun. 2005;328:709–720. doi: 10.1016/j.bbrc.2004.11.072. [DOI] [PubMed] [Google Scholar]
- 33.Bandara G, Robbins PD, Georgescu HI, Mueller GM, Glorioso JC, Evans CH. Gene transfer to synoviocytes: prospects for gene treatment of arthritis. DNA Cell Biol. 1992;11:227–231. doi: 10.1089/dna.1992.11.227. [DOI] [PubMed] [Google Scholar]
- 34.Bandara G, Mueller GM, Galea-Lauri J, Tindal MH, Georgescu HI, Suchanek MK, et al. Intraarticular expression of biologically active interleukin 1-receptor-antagonist protein by ex vivo gene transfer. Proc Natl Acad Sci U S A. 1993;90:10764–10768. doi: 10.1073/pnas.90.22.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salgado AJ, Coutinho OP, Reis RL. Bone tissue engineering: state of the art and future trends. Macromol Biosci. 2004;4:743–765. doi: 10.1002/mabi.200400026. [DOI] [PubMed] [Google Scholar]
- 37.Yilgor P, Hasirci N, Hasirci V. Sequential BMP-2/BMP-7 delivery from polyester nanocapsules. J Biomed Mater Res A. 2010;93:528–536. doi: 10.1002/jbm.a.32520. [DOI] [PubMed] [Google Scholar]
- 38.Khan SN, Lane JM. The use of recombinant human bone morphogenetic protein-2 (rhBMP-2) in orthopaedic applications. Expert Opin Biol Ther. 2004;4:741–748. doi: 10.1517/14712598.4.5.741. [DOI] [PubMed] [Google Scholar]


