Abstract
The phosphorylation of cardiac troponin I (cTnI) plays an important role in the contractile dysfunction associated with heart failure. Human cardiac troponin I-interacting kinase (TNNI3K) is a novel cardiac-specific functional kinase that can bind to cTnI in a yeast two-hybrid screen. The purpose of this study was to investigate whether TNNI3K can phosphorylate cTnI at specific sites and to examine whether the phosphorylation of cTnI caused by TNNI3K can regulate cardiac myofilament contractile function. Co-immunoprecipitation was performed to confirm that TNNI3K could interact with cTnI. Kinase assays further indicated that TNNI3K did not phosphorylate cTnI at Ser23/24 and Ser44, but directly phosphorylated Ser43 and Thr143 in vitro. The results obtained for adult rat cardiomyocytes also indicated that enhanced phosphorylation of cTnI at Ser43 and Thr143 correlated with rTNNI3K (rat TNNI3K) overexpression, and phosphorylation was reduced when rTNNI3K was knocked down. To determine the contractile function modulated by TNNI3K-mediated phosphorylation of cTnI, cardiomyocyte contraction was studied in adult rat ventricular myocytes. The contraction of cardiomyocytes increased with rTNNI3K overexpression and decreased with rTNNI3K knockdown. We conclude that TNNI3K may be a novel mediator of cTnI phosphorylation and contribute to the regulation of cardiac myofilament contraction function.
Keywords: TNNI3K, cTnI, Phosphorylation, Contraction function
Introduction
The human cardiac troponin I-interacting kinase (TNNI3K) gene expresses a novel cardiac-specific functional kinase. It was first cloned from an adult heart cDNA library based on large-scale expressed sequence tag sequencing 1. The TNNI3K protein contains three domains, including seven N-terminal ankyrin repeats, a protein kinase (PK) domain that contains motifs conserved in both serine/threonine and tyrosine PKs, and a C-terminal Ser-rich domain. TNNI3K belongs to a new family of kinases, called the mixed lineage kinase family in the tyrosine kinase-like group, based on a sequence comparison of the catalytic domain together with knowledge of sequence similarity and domain structures outside the catalytic domain 2. Numerous studies have demonstrated that TNNI3K can phosphorylate several substrates and undergo autophosphorylation 3,4. The TNNI3K C-terminal Ser-rich domain was previously used as bait to perform a yeast two-hybrid screen of a human cardiac library, and cardiac troponin I (cTnI) was identified as a TNNI3K-interacting protein 1.
Troponin, in conjunction with tropomyosin, functions as a molecular switch and regulates muscle contraction in response to changes in the intracellular Ca2+ concentration. Troponin consists of three subunits, including the Ca2+-binding subunit troponin C, the tropomyosin-binding subunit T, and the inhibitory subunit troponin I 5. cTnI plays a key role in the regulation of cardiac muscle contraction 6,7. As a major physiological mechanism for altering myofilament properties, the phosphorylation of cTnI stimulates a conformational change of troponin and regulates cardiomyocyte contractility 8,9. In addition to phosphorylation at specific serine and threonine residues by several different kinases, cTnI has a major role in the dynamic modulation of contractile function, which may causally contribute to the contractile dysfunction associated with heart failure 10-12.
In this study, we performed co-immunoprecipitation analysis to further confirm the in vivo interaction between TNNI3K and cTnI. We focused on TNNI3K-mediated phosphorylation of cTnI and identified the pertinent phosphorylation sites by kinase analysis, site-directed mutagenesis, and Western blot. It is of great interest to determine the functional consequences of TNNI3K-mediated cTnI phosphorylation in rat left ventricular myocytes.
Material and Methods
Plasmids and adenovirus vectors
The pcDNA4-Xpress/TNNI3Kmut, pcDNA6-Flag/cTnI, and pcDNA4-Xpress/TNNI3K expression vectors were prepared as previously described 1. The AdEasy System was used to prepare adenoviruses with constitutively active rat TNNI3K (Ad.Flag-rTNNI3K). Ad.EGFP was purchased from Vector Gene Technology Co., Ltd. (China) and used as a control. The small hairpin RNA (shRNA), which targeted rat TNNI3K (NCBI Accession NM_181769.1), was designed and cloned into RNA interference (RNAi) adenovirus vectors (Ad.rTNNI3KRNAi) by Genechem Co., Ltd., (China). The adenovirus vector containing the rat TNNI3K targeting shRNA was named Ad.rTNNI3KRNAi, and a negative control adenovirus vector containing negative control shRNA (Ad.rTNNI3Knc) was constructed by Genechem.
Cell culture and transient transfection
The human embryonic kidney cell line HEK293T was obtained from the Cell Resource Center, IBMS, CAMS/PUMC and cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin in a humidified incubator at 37°C, 5% CO2, and 95% O2. Transient transfection of plasmids was performed using the Lipofectamine™ 2000 reagent (Invitrogen, USA) according to manufacturer protocol. For co-immunoprecipitation, 2 × 107 cells at approximately 70% confluence were co-transfected with 8 µg each of pcDNA4-Xpress-TNNI3K and pcDNA6-Flag or pcDNA6-Flag-cTnI. For the kinase assay, 2 × 107 cells at approximately 70% confluence were co-transfected with pcDNA6-cTnI and pcDNA4-Xpress, pcDNA4-Xpress-TNNI3K, or pcDNA4-Xpress-TNNI3Kmut (8 µg of each vector).
Co-immunoprecipitation analysis
For co-immunoprecipitation experiments, 293T cells were harvested 72 h after transfection by first washing the cells grown on a 60-mm dish twice with cold PBS and then lysing the cells with 0.5 mL cold immunoprecipitation buffer [1% NP-40, 0.25% deoxycholate, 2 mM EGTA, 1 mM EDTA, 150 mM NaCl, 50 mM Tris-HCl, pH 7.5, and 5 g/L protease inhibitor cocktail tablets (Roche, USA)]. Cellular debris was pelleted by centrifugation at 12,000 g for 15 min, and the lysate was incubated with the corresponding antisera for 1-2 h at 4°C under rotation. The lysates were then incubated with 2 µg anti-Flag antibody (Cell Signaling Technology, USA) for 6 h at 4°C and then mixed with 20 µL protein A-agarose beads (Vigorous Biotechnology Beijing Co., Ltd., China) for an additional 3 h. Immunoprecipitates were collected by centrifugation and washed twice in the immunoprecipitation buffer to remove unbound protein, boiled in sample buffer, centrifuged, and then removed from the beads. Immunoprecipitated proteins were subjected to 12% SDS-PAGE, and immunoblotting was performed with the anti-Xpress antibody (Invitrogen). To prepare total cell lysates, cells from a 60-mm dish were lysed in 0.5 mL cold immunoprecipitation buffer. Total protein (80 µg) was separated on 12% SDS-PAGE and immunoblotting was performed with the anti-Xpress, anti-Flag, and anti-GAPDH antibodies (Zhongshan Goldenbridge, China).
In vitro kinase assay
For the kinase assay, 293T cells were transfected with expression plasmids. After 72 h, cells from a 60-mm dish were washed twice with cold PBS and then lysed in 0.5 mL cold lysis buffer (1% NP-40, 0.25% deoxycholate, 1 mM EGTA, 1 mM EDTA, 150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 1 mM Na3VO4, 100 mM NaF, 1 mM β-glycerophosphate, and 5 g/L Protease Inhibitor Cocktail Tablets). Xpress-tagged TNNI3K and Flag-tagged cTnI were co-immunoprecipitated using the anti-Flag antibody and protein A-agarose beads and then washed three times with lysis buffer and two times with kinase reaction buffer (25 mM Tris-HCl, pH 7.5, 10 mM MgCl2, 10 mM MnCl2, 5 mM β-glycerophosphate, and 2 mM DTT). To initiate the reactions, 20 µL phosphorylation mix containing ATP was added to the samples and the mixture was incubated at 37°C for 30 min. Reactions were terminated by adding SDS-PAGE sample buffer and boiling for 10 min. After boiling, the samples were centrifuged and the beads were removed. Samples were separated on 12% SDS-PAGE. Western blots were performed using anti-p-cTnI (Cell Signaling Technology), anti-p-cTnI (Thr143; cat: ab58546, Abcam, UK), and anti-p-cTnI (Ser43; cat: ab59420, Abcam) antibodies. Total cell lysates were prepared as described above.
Isolation of adult rat ventricular myocytes
Isolated left ventricular myocytes were prepared from adult rat hearts as previously described 13. Sprague- Dawley rats weighing approximately 180 g were obtained from the Department of Laboratory Animals, Hebei Medical University. All experimental protocols using animals were approved by the Animal Ethics Review Committee at FuWai Hospital and Cardiovascular Institute. All experiments conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication No. 85-23, revised in 1996).
Culture of adult rat cardiac myocytes
The entire culture procedure was performed under a class II laminar flow hood. Laminin solution [10 mg/mL mouse laminin (Invitrogen) in PBS] was used to coat the tissue culture-treated dishes and incubated overnight at 37°C. Freshly isolated cardiac myocytes were suspended in the basal culture (CCT) medium, which was modified medium 199 supplemented with 2 mM L-carnitine, 10 µM cytosine-D-arabinofuranoside, and 5 mM taurine 13. After the myocytes were pelleted by gravity for 10 min, the supernatant was aspirated and the myocytes were washed two more times using the same protocol. The myocytes were then plated at a density of 1 × 105 cells per 35 mm dish in CCT medium containing 1% penicillin-streptomycin. After 2-h incubation in a 5% CO2 incubator at 37°C, the medium was changed to FBS-free CCT medium.
RNAi and adenoviral infection
For RNAi, adult rat cardiac myocytes were transfected with adenovirus rTNNI3KRNAi and incubated for 24 h. The Ad.rTNNI3Knc, which contained a negative control shRNA, was used as a negative control. Nontransfected cells were used as a blank control. Cardiomyocytes were infected with a control adenovirus (Ad.EGFP), Ad.Flag-rTNNI3K, or Ad.rTNNI3KRNAi for 24 h. Adenovirus-directed gene transfer was initiated after 2 h of culture. The culture medium was aspirated together with unattached myocytes, and a half-volume (e.g., 1 mL for a 35-mm Petri dish) of the FBS-free CCT medium containing an appropriate titer of gene-carrying adenovirus was added to the dish. Cells were incubated in the FBS-free medium in the presence of Ad.Flag-rTNNI3K (20, 40, 60, 80, or 100 multiplicity of infection [MOI]) virus or Ad.rTNNI3KRNAi virus (40, 60, 80, or 100 MOI) or Ad-EGFP control virus (100 MOI) for 6 h. Subsequently, an additional half-volume of FBS-free CCT was added.
Functional studies
Cardiomyocyte contractility was measured using a video-based edge detection system (Ion Optix Co., USA). Myocytes were placed on the stage of an inverted microscope, superfused with Tyrode solution containing a Ca2+-concentration of 1.8 mM at a flow rate of 1.8 mL/min, and electrically stimulated at 1 Hz at room temperature. Cell length was monitored from the bright-field image by an optical edge-tracking method. The contraction amplitude was measured as the percentage of shortening of cell length.
Western blot analysis of the relationship between TNNI3K protein levels and cTnI phosphorylation levels
The anti-Flag, anti-p-cTnI (Ser43), and anti-p-cTnI (Thr143) antibodies were used for Western blot analysis. Cardiomyocytes infected with Ad.rTNNI3K on a 60-mm dish were washed twice with cold PBS and lysed in RAPI buffer containing 1 mM PMSF, 1 mM Na3VO4, and 100 mM NaF. Cell debris was pelleted by centrifugation at 12,000 g for 15 min and the supernatant was then collected. Protein concentrations of each sample were measured using the bicinchoninic acid method (BCA kit, Applygen, China). Equal volumes of 5X SDS-PAGE loading buffer were added to each sample and boiled at 100°C for 10 min. Total protein (80 µg) was subjected to 12% SDS-PAGE and electrophoretically transferred onto a nitrocellulose membrane. The membranes were probed with mouse monoclonal anti-Flag or rabbit polyclonal anti-p-cTnI (Ser43) or anti-p-cTnI (Thr143) antibodies, all at 1:1000 dilutions, for 2 h at room temperature. After washing, the membrane was incubated with horseradish peroxidase (HRP)-conjugated secondary anti-mouse or anti-rabbit antibodies, respectively (1:2000 dilution), for 1 h at room temperature. For loading controls and normalization, the same membranes were re-blocked with 5% nonfat dry milk in Tris-buffer saline containing 0.05% Tween-20 and then washed and incubated with a monoclonal anti-GAPDH antibody (1:1000) overnight at 4°C. The blots were subsequently blotted with an HRP-conjugated anti-mouse secondary antibody. Immunoreactive proteins were visualized using enhanced chemiluminescence detection reagent by exposure to Eastman Kodak X-ray films (Kodak, USA). Evaluation of the expression of specific proteins was performed using Alpha Imager Software (Alpha Innotech, USA) by quantifying the pixels of electronic images.
Quantitative real-time reverse transcription polymerase chain reaction (RT-PCR) analysis
Quantitative real-time RT-PCR analysis was used to determine the level of rTNNI3K mRNA after cells had been transfected with RNAi against rTNNI3K for 24 h. Total RNA was extracted using Trizol solution, quantified by spectrophotometry, and then reverse-transcribed using SuperScript III (Invitrogen). Relative gene expression levels were determined from threshold cycle values and normalized to GAPDH. Quantification of specific RNA transcripts was performed by SYBR Green real-time PCR using Opticon 2 (Bio-Rad, USA). The primer sequences for rTNNI3K and GAPDH were as follows: rTNNI3K forward primer: 5′-CACCTTCCTCTTCTTCCGATT-3′, rTNNI3K reverse primer: 5′-CTGTCCTCAAAGTTGCTGTCG-3′; GAPDH forward primer: 5′-CAACGACCCCTTCATTGACCT-3′, GAPDH reverse primer: 5′-CAGTAGACTCCACGACATACTC-3′.
Statistical analysis
Data are reported as means ± SE and were compared by one-way analysis of variance (ANOVA) in a two-sided test. Differences were considered to be statistically significant at P < 0.05. All other data were analyzed by SPSS v.13.0 (SPSS, Inc., USA).
Results
Identification of the interaction between cTnI and TNNI3K
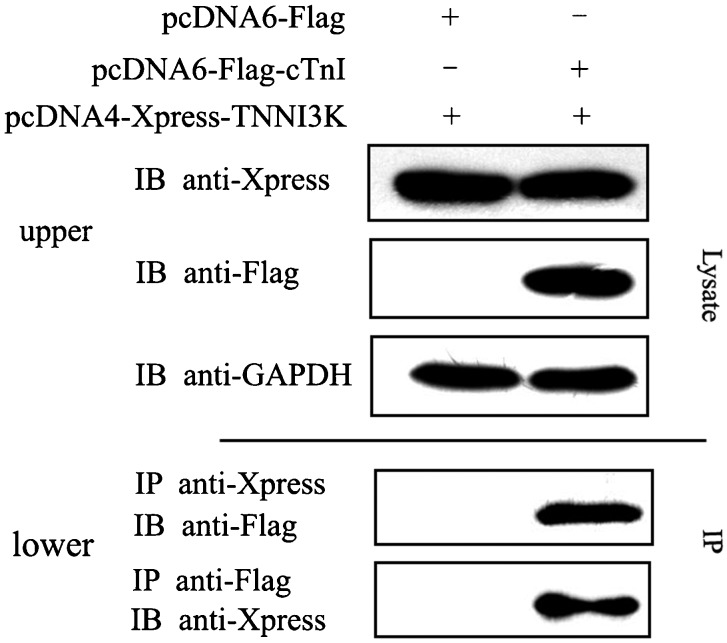
The TNNI3K C-terminal Ser-rich domain was used as bait to perform a yeast two-hybrid screen of a human cardiac library, which previously identified cTnI as one of TNNI3K-interacting proteins 1. To further confirm the interaction between cTnI and TNNI3K, we co-transfected HEK293T cells with Xpress-tagged TNNI3K and Flag-tagged cTnI or Flag-tagged empty control vector, incubated the cells for 72 h, and then lysed the cells. The lysates were co-immunoprecipitated with anti-Xpress antibodies and analyzed by immunoblotting using an anti-Flag antibody. In addition, anti-Flag immunoprecipitates were analyzed by immunoblotting with an anti-Xpress antibody. The results showed that TNNI3K co-immunoprecipitated cTnI, while no significant bands were detected in the control (Figure 1). Thus, we found that TNNI3K interacts with cTnI in vivo, which is consistent with the results of two-hybrid binding in yeast cells. Our previous results demonstrated that TNNI3K localized in the nucleus and cytoplasm of fetal and adult cardiac myocytes 1. Moreover, cTnI is a sarcomere protein that localizes in the cytoplasm of cardiac myocytes. Therefore, we inferred that TNNI3K and cTnI can co-localize in the myocyte cytoplasm.
Figure 1. Human cardiac troponin I-interacting kinase (TNNI3K) interacts with cardiac troponin I (cTnI) in vitro. HEK293T cells were co-transfected with Xpress-tagged TNNI3K and Flag-tagged cTnI or a Flag control vector. Cell lysates were immunoprecipitated (IP) with an anti-Xpress monoclonal antibody and immunoblotted (IB) with an anti-Flag polyclonal antibody. Conversely, anti-Flag immunoprecipitates were analyzed by immunoblotting with an anti-Xpress (lower). The expression of cTnI and TNNI3K was confirmed by Western blot (upper).
TNNI3K-mediated phosphorylation of cTnI in vitro
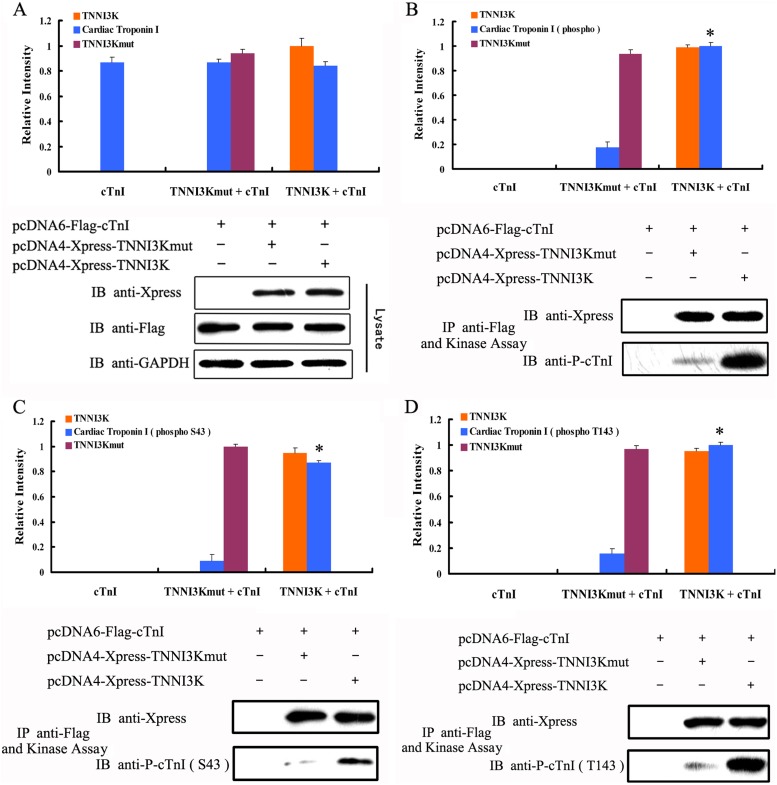
TNNI3K is a cardiac-specific functional PK, but its substrates in vivo have not been elucidated. Phosphorylation of cTnI plays an important role in the regulation of myofilament function. Therefore, we were interested in assessing whether TNNI3K could phosphorylate cTnI. We performed an in vitro kinase assay using wild-type TNNI3K, a TNNI3K mutant where lysine 490 was changed to alanine (TNNI3Kmut), and an empty vector as control. The point mutation of TNNI3Kmut occurs at a conserved lysine residue within the sub-domain II, which plays a crucial role in kinase activity. Therefore, we tested whether a substitution of this lysine residue in TNNI3K, which is reported to block the phosphotransfer reaction in a number of PKs 1,14,15, would lead to the loss of kinase activity in this protein as well. As illustrated in Figure 2A, the expression level of Flag-tagged cTnI (FLAG-cTnI), Xpress-tagged TNNI3K (Xpress-TNNI3K), and Xpress-tagged TNNI3Kmut was similar in each group. As shown in Figure 2B, a 24-kDa band was identified using an anti-p-cTnI antibody, while the phosphorylation of cTnI was sharply reduced with the TNNI3Kmut, and was completely absent in the control group. Therefore, these data show that TNNI3K can phosphorylate cTnI as a functional PK.
Figure 2. Human cardiac troponin I-interacting kinase (TNNI3K) phosphorylates cardiac troponin I (cTnI) at Ser43 (S43) and Thr143 (T143). HEK293T cells were transfected with Xpress-tagged TNNI3K, Xpress-tagged TNNI3Kmut, or Flag-tagged cTnI and incubated for 72 h. A, Western blot using anti-Flag and anti-Xpress antibodies (n = 5). B, Western blot using anti-Xpress and anti-p-cTnI antibodies (n = 5). C, Western blot using anti-P-cTnI (S43) and anti-Xpress antibodies (n = 5). D, Western blot using anti-P-cTnI (Thr143) and anti-Xpress antibodies (n = 5). IP = immunoprecipitated; IB = immunoblotted. Data are reported as means ± SE. *P < 0.01 vs TNNI3Kmut-mediated phosphorylation (one-way ANOVA).
It is known that different phosphorylation sites on cTnI have different roles in the regulation of myofilament function 11,16. Thus, we examined phosphorylation sites on cTnI that were targeted by TNNI3K. We performed Western blots to detect the phosphorylation site on cTnI after a 30-min incubation with TNNI3K. As show in Figure 2C and D, the anti-p-cTnI (Ser43) and anti-p-cTnI (Thr143) antibodies both reacted with a 24-kDa band. These results demonstrated that TNNI3K directly phosphorylates in vitro cTnI at Ser43 and Thr143, which are commonly referred to as the PKC sites 11,17.
TNNI3K-mediated phosphorylation of cTnI in adult rat cardiac myocytes
We next tested whether changes in the expression of rTNNI3K could affect cTnI phosphorylation at Ser43 and Thr143 in isolated myocytes.
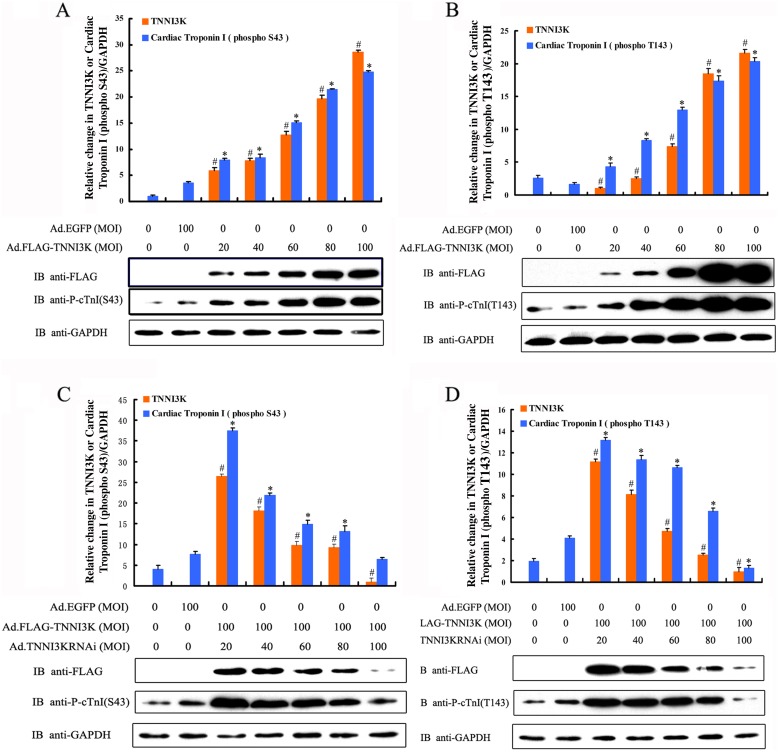
Cardiac myocytes were infected for 24 h with optimal MOIs of 20, 40, 60, 80, or 100 of an adenovirus harboring the rTNNI3K sequence. Phosphorylation of cTnI at Ser43 and Thr143 significantly increased after rTNNI3K was overexpressed in myocytes for 24 h compared to the adenoviral control and blank control (Figure 3A and B; n = 5; P < 0.01). Moreover, the phosphorylation level of cTnI Ser43 and Thr143 sites markedly increased when the MOIs of Ad.Flag-rTNNI3K increased from 20 to 100 (Figure 3A and B; n = 5; P < 0.01). These data indicated that overexpression of TNNI3K can increase the phosphorylation level of cTnI at Ser43 and Thr143 in functional adult rat cardiac myocytes.
Figure 3. The expression of human cardiac troponin I-interacting kinase (TNNI3K) correlates with the phosphorylation of cardiac troponin I (cTnI) at Ser43 (S43) and Thr143 (T143). Rat cardiac myocytes were infected with adenoviral vectors with multiplicity of infections (MOIs) of 20, 40, 60, 80, or 100 for 24 h. Cell lysates were then subjected to Western blot analysis to detect the expression of rTNNI3K as well as the phosphorylation of cTnI at Ser43 and Thr143 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. Overexpression of rTNNI3K produces a significant increase in the phosphorylation of cTnI at Ser43 (A) and Thr143 (B). Knockdown of rTNNI3K with Ad.rTNNI3KRNAi significantly reduces phosphorylation at Ser43 (C) and Thr143 (D) of cTnI. Data were from five independent experiments. Data are reported as means ± SE. *P < 0.01 vs blank control (without virus infection). #P < 0.01 vs cardiomyocytes infected with Ad.EGFP (one-way ANOVA).
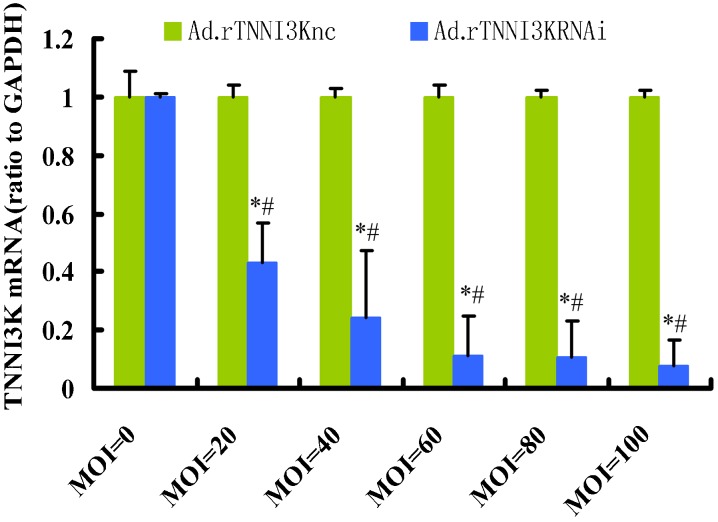
In a reciprocal experiment, rTNNI3K knockdown was performed in adult rat cardiac myocytes using an adenovirus-mediated delivery of a shRNA targeting the rTNNI3K sequence. Ad.rTNNI3Knc was used as a control. We evaluated the expression of the rTNNI3K gene by real-time PCR after shRNA-mediated silencing. As illustrated in Figure 4, Ad.rTNNI3KRNAi efficiently reduced the expression of rTNNI3K in adult rat cardiac myocytes, especially when MOIs of 60, 80, and 100 were used. Based on these results, we knocked down the expression of rTNNI3K using Ad.rTNNI3KRNAi with MOIs of 40, 60, 80, and 100 while concomitantly infecting the same cells with Ad.Flag-rTNNI3K (100 MOI) virus. The Ad.rTNNI3Knc (100 MOI) virus and wild-type adult rat cardiac myocytes were used as the adenovirus vector and blank controls, respectively. After 24 h, the cells were lysed and subjected to Western blot analysis. Compared to the controls, addition of the Ad.rTNNI3KRNAi significantly reduced cTnI phosphorylation at Ser43 and Thr143 (Figure 3C and D). These results demonstrated that a reduction in TNNI3K expression significantly reduced cTnI phosphorylation at Ser43 and Thr143 in adult rat cardiac myocytes. Based on these experiments, we concluded that rTNNI3K can increase the phosphorylation of cTnI at Ser43 and Thr143 and, most importantly, phosphorylate cTnI in adult rat cardiac myocytes.
Figure 4. Knockdown of human cardiac troponin I-interacting kinase (TNNI3K) in isolated rat adult cardiomyocytes. Freshly isolated rat adult cardiomyocytes were infected with an adenovirus carrying shRNA for silencing TNNI3K or scrambling shRNA for 24 h at the indicated multiplicity of infection (MOI). Samples were analyzed by quantitative real-time reverse transcription polymerase chain reaction and normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Data are reported as means ± SE (n = 6). *P < 0.01 vs control (MOI = 0); #P < 0.01 vs green bar at the same MOI value (one-way ANOVA).
Functional consequences of TNNI3K-mediated cTnI phosphorylation
Because kinase-mediated phosphorylation of cTnI can regulate the contractility function of myofilaments, we next determined whether TNNI3K-mediated phosphorylation of cTnI at Ser43 and Thr143 could change the contractility of adult rat cardiomyocytes. Based on the results cited above, we tested Ad.Flag-rTNNI3K or Ad.rTNNI3KRNAi at 100 MOI. The adult rat cardiac myocytes were directly infected with Ad.Flag-rTNNI3K or Ad.EGFP (as vector control) at 100 MOI. We also knocked down the expression of rTNNI3K in adult rat cardiac myocytes using Ad.rTNNI3KRNAi at 100 MOI with Ad.rTNNI3Knc at 100 MOI as the adenovirus vector control. In both experiments, wild-type adult rat cardiac myocytes were used as a blank control.
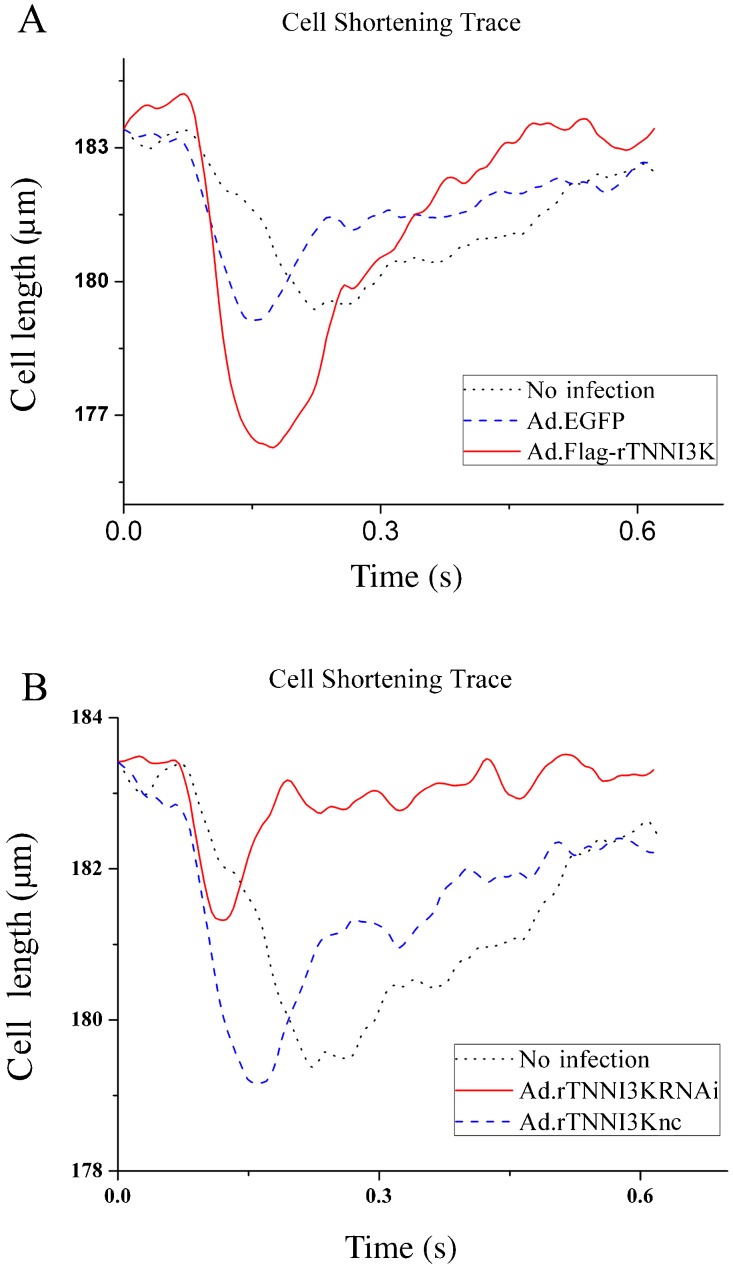
To determine the effect of TNNI3K on cardiomyocyte contractility, we examined contractility of the cells after infection with the indicated adenoviruses using a video-based edge detection system. Isolated adult rat ventricular cardiomyocytes were infected with Ad.GFP, Ad.rTNNI3K, Ad.rTNNI3Knc, or Ad.rTNNI3KRNAi and incubated for 24 h. Cell contractility was then measured in the infected cardiomyocytes as described in Material and Methods. As shown in Figure 5A, TNNI3K overexpression induced a significant increase in cell shortening of cardiomyocytes compared to Ad-GFP-infected cells (2.88 ± 0.087 vs 2.06 ± 0.045%, respectively; n = 20; P < 0.05). In contrast, TNNI3K knockdown caused a decrease in cell shortening compared to control (1.12 ± 0.08 vs 2.13 ± 0.063%, respectively; n = 20; P < 0.05; Figure 5B). Taken together, these data suggest that TNNI3K may regulate cardiomyocyte contraction.
Figure 5. Effects of human cardiac troponin I-interacting kinase (TNNI3K) on cardiomyocyte contraction. A, Representative traces of cell shortening from non-infected cardiomyocytes or cardiomyocytes infected with Ad.GFP or Ad.rTNNI3K. B, Representative traces of cell shortening from non-infected cardiomyocytes or cardiomyocytes infected with Ad.TNNI3Knc or Ad.rTNNI3KRNAi.
Discussion
As an important post-translational modification, phosphorylation of cTnI and other myofilament proteins plays a major role in the dynamic modulation of contractile function and in the regulation of thin filament function in the transition from compensated hypertrophy to heart failure 6,11,18,19. In the present study, we confirmed that TNNI3K, which is a cardiac-specific functional kinase, interacts with cTnI and directly phosphorylates cTnI at Ser43 and Thr143. Furthermore, TNNI3K overexpression not only enhanced cTnI Ser43 and Thr143 phosphorylation, but also increased myocardial contractility. These data also suggest that the effects of TNNI3K are mediated by phosphorylation of cTnI Ser43 and Thr143 and other potential mechanisms, which may participate in the regulation of myocardial contractile function.
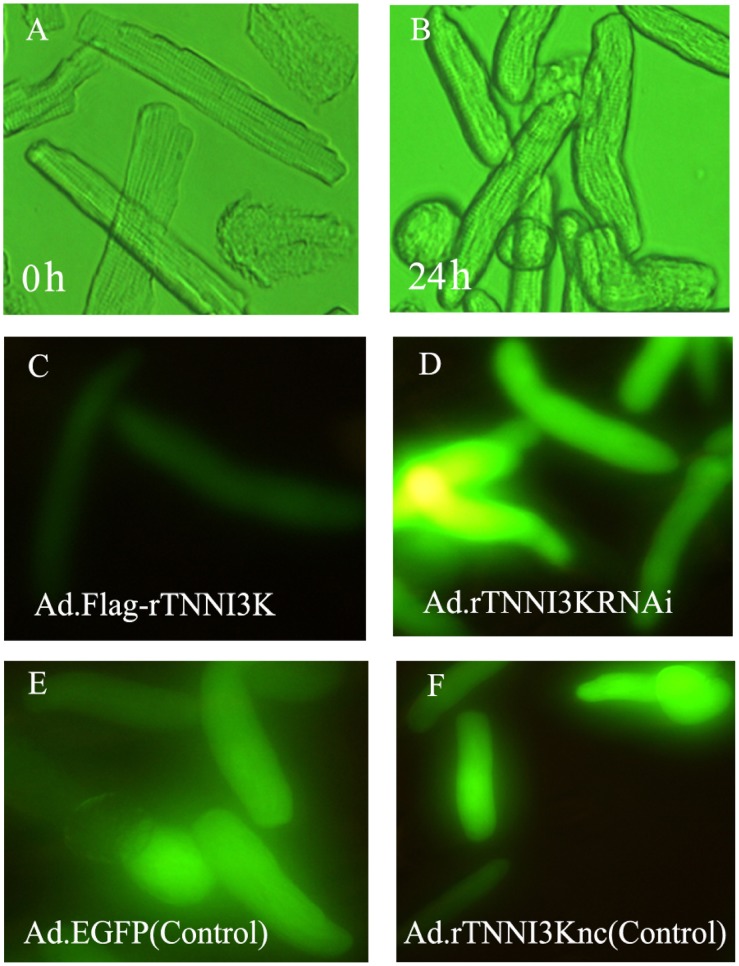
Adult rat cardiomyocytes provide a useful model for gene delivery and contractility studies. However, it is easy for the cells to lose their native rod shape and contractility in an in vitro culture. We first confirmed that TNNI3K can be efficiently overexpressed or knocked down in adult rat cardiac myocytes by infecting the cells with appropriate adenoviruses for 24 h (Figure 3). We also found that adult rat cardiomyocytes still maintained their rod-shaped appearance and contractility after adenoviral infection for 24 h, but lost those features after 48 h post-transfection (Figure 6 and data not shown). Therefore, we chose 24 h post-infection as the optimal time for experimental assessment.
Figure 6. Morphology of rat cardiac myocytes immediately after isolation (0 h) or after 24 h in culture. A and B, Transillumination images. C, D, E, and F, Expression of recombinant adenoviral transgenes in cultured adult rat ventricular myocytes. The rat cardiac myocytes were infected with adenoviral vectors carrying a marker gene, EGFP, at 100 multiplicity of infection after 24 h in culture.
The phosphorylation of cTnI and its regulation of cardiac myofilament contraction have been studied in a variety of systems, ranging from reconstituted myofilament proteins to myocardial cells or transgenic animals with heterologous expression of modified cTnI proteins 11,20-22. cTnI is also a substrate for protein kinase A (PKA) and protein kinase C (PKC). PKA mainly phosphorylates Ser23/24 of cTnI in the N-terminus of the protein (rodent sequence position; Ser22/23 in humans) 6,23. PKC mainly phosphorylates cTnI at the Ser43/45 and Thr143 positions (numbered as in the human cTnI sequence) 6,24. More recently, other kinases have been found to produce functional effects through the regulation of cTnI phosphorylation, such as myosin light-chain kinase, protein kinase D, and mammalian sterile 20-like kinase 1 25-28. In addition, many studies have shown that protein kinase G and p21-activated kinases may also be involved in the modification of cTnI phosphorylation 11,29-31. In the present study, our data demonstrated that TNNI3K specifically targeted cTnI and catalyzed the phosphorylation of cTnI at Ser43 and Thr143 (the PKC sites, numbered as in the human cTnI sequence), which are mostly conserved in cTnI from multiple species, but not at Ser23/24 (the PKA sites; data not shown). Furthermore, we confirmed in adult rat cardiac myocytes that the expression level of TNNI3K positively correlated with the phosphorylation of cTnI at Ser43 and Thr143. These data suggest that active TNNI3K phosphorylates cTnI at Ser43 and Thr143 not only when the substrate protein is used in isolation, but also in isolated myocytes. Thus, cTnI likely represents a physiological substrate for TNNI3K in the myocardium.
It has been reported that PKA phosphorylation of cTnI at Ser23/24 in living myocardium contributes to accelerated relaxation in diastole and increases the rates of force development in systole 32. However, the precise functional effects mediated by PKC-dependent phosphorylation of cTnI that are related to whole heart or muscle contractile function are not yet clear. Evidence suggests that PKC phosphorylation of cTnI at Ser43/45 can reduce maximal Ca2+-activated tension and decrease MgATPase activity as well as the crossbridge cycling rate, which can lead to a reduction in energy consumption and impaired relaxation, while phosphorylation of Thr144 would reduce sliding velocity. Based on these findings, it can be postulated that PKC-mediated phosphorylation of cTnI at Ser43/45 can reduce contractility and prolong relaxation 11. Furthermore, these effects may be relatively beneficial in energetic terms, at least in the non-diseased heart 11. Other studies have shown that there may be significant interdependence between the effects that result from PKC-dependent Ser43/45 phosphorylation and PKA-dependent Ser23/24 phosphorylation. Roman et al. 33 found that phosphorylation of cTnI at Ser23/24 increases in transgenic mice where Ser43/45 is mutated to alanine, which results in enhanced contraction and relaxation under basal conditions 11,33. To further explore the functional effects of TNNI3K-mediated cTnI phosphorylation, we examined the myocardial contractility of cultured adult rat cardiac myocytes in which TNNI3K was overexpressed or knocked down. Our data suggest that overexpression of TNNI3K can enhance myocardial contractility. Taken together, the data support the hypothesis that the phosphorylation of cTnI mediated by TNNI3K, as a novel mechanism, may regulate the functions of the myofilament.
Data from this study also indicate that ventricular myocytes infected with Ad-TNNI3K exhibit increased peak shortening. In contrast, ventricular myocytes infected with Ad-TNNI3KRNAi showed decreased peak shortening. Therefore, our data further strengthen the data presented in an American Heart Association Annual Science Meeting abstract 34, which found that TNNI3K transgenic mice had a marked enhancement of contractility compared to wild-type mice.
TNNI3K-mediated phosphorylation of cTnI and the induction of increased shortening might be related to heart physiology-pathology states. We previously found that TNNI3K had a higher expression when it was stimulated by the pathology factor, ET-1 35, and our data show that the high expression enhanced cardiomyocyte shortening through phosporylation of cTnI. Therefore, the high expression might be a response to compensatory hypertrophy. Moreover, TNNI3K-mediated control of cardiomyocyte shortening through the phosphorylation of cTnI might be a novel mechanism. In conclusion, TNNI3K may be a novel mediator of cTnI phosphorylation and may contribute to the regulation of cardiac myofilament contractile function. Studies of TNNI3K open a new area of research with the goal of developing a better understanding of cardiac contractile dysfunction associated with ischemic heart diseases and heart failure.
Acknowledgments
We thank Prof. Yongli Wang and Prof. Hailin Zhang (Department of Pharmacology, Hebei Medical University, Shijiazhuang, Hebei Province, China) for isolation and culture of adult rat ventricular myocytes as well as for the functional study. We also sincerely thank Prof. Philip R. Mayeux (Department of Pharmacology and Toxicology, University of Arkansas for Medical Sciences, USA) for editing the manuscript. Research supported by grants from the National Natural Science Foundation of China (#30871057 and #30940029).
Footnotes
First published online February 1, 2013.
References
- 1.Zhao Y, Meng XM, Wei YJ, Zhao XW, Liu DQ, Cao HQ, et al. Cloning and characterization of a novel cardiac-specific kinase that interacts specifically with cardiac troponin I. J Mol Med. 2003;81:297–304. doi: 10.1007/s00109-003-0427-x. [DOI] [PubMed] [Google Scholar]
- 2.Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298:1912–1934. doi: 10.1126/science.1075762. [DOI] [PubMed] [Google Scholar]
- 3.Feng Y, Cao HQ, Liu Z, Ding JF, Meng XM. Identification of the dual specificity and the functional domains of the cardiac-specific protein kinase TNNI3K. Gen Physiol Biophys. 2007;26:104–109. [PubMed] [Google Scholar]
- 4.Feng Y, Liu DQ, Wang Z, Liu Z, Cao HQ, Wang LY, et al. AOP-1 interacts with cardiac-specific protein kinase TNNI3K and down-regulates its kinase activity. Biochemistry. 2007;72:1199–1204. doi: 10.1134/s0006297907110053. [DOI] [PubMed] [Google Scholar]
- 5.Ward DG, Cornes MP, Trayer IP. Structural consequences of cardiac troponin I phosphorylation. J Biol Chem. 2002;277:41795–41801. doi: 10.1074/jbc.M206744200. [DOI] [PubMed] [Google Scholar]
- 6.Metzger JM, Westfall MV. Covalent and noncovalent modification of thin filament action: the essential role of troponin in cardiac muscle regulation. Circ Res. 2004;94:146–158. doi: 10.1161/01.RES.0000110083.17024.60. [DOI] [PubMed] [Google Scholar]
- 7.Sadayappan S, Finley N, Howarth JW, Osinska H, Klevitsky R, Lorenz JN, et al. Role of the acidic N′ region of cardiac troponin I in regulating myocardial function. FASEB J. 2008;22:1246–1257. doi: 10.1096/fj.07-9458com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Solaro RJ, Rarick HM. Troponin and tropomyosin: proteins that switch on and tune in the activity of cardiac myofilaments. Circ Res. 1998;83:471–480. doi: 10.1161/01.RES.83.5.471. [DOI] [PubMed] [Google Scholar]
- 9.Solaro RJ, Van Eyk J. Altered interactions among thin filament proteins modulate cardiac function. J Mol Cell Cardiol. 1996;28:217–230. doi: 10.1006/jmcc.1996.0021. [DOI] [PubMed] [Google Scholar]
- 10.Bilchick KC, Duncan JG, Ravi R, Takimoto E, Champion HC, Gao WD, et al. Heart failure-associated alterations in troponin I phosphorylation impair ventricular relaxation-afterload and force-frequency responses and systolic function. Am J Physiol Heart Circ Physiol. 2007;292:H318–H325. doi: 10.1152/ajpheart.00283.2006. [DOI] [PubMed] [Google Scholar]
- 11.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc Res. 2005;66:12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 12.Murphy AM. Heart failure, myocardial stunning, and troponin: a key regulator of the cardiac myofilament. Congest Heart Fail. 2006;12:32–38. doi: 10.1111/j.1527-5299.2006.04320.x. [DOI] [PubMed] [Google Scholar]
- 13.Schluter KD, Schreiber D. Adult ventricular cardiomyocytes: isolation and culture. Methods Mol Biol. 2005;290:305–314. doi: 10.1385/1-59259-838-2:305. [DOI] [PubMed] [Google Scholar]
- 14.Hanks SK, Hunter T. Protein kinases 6. The eukaryotic protein kinase superfamily: kinase (catalytic) domain structure and classification. FASEB J. 1995;9:576–596. [PubMed] [Google Scholar]
- 15.Hanks SK, Quinn AM, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 16.Engel PL, Hinken A, Solaro RJ. Differential effects of phosphorylation of regions of troponin I in modifying cooperative activation of cardiac thin filaments. J Mol Cell Cardiol. 2009;47:359–364. doi: 10.1016/j.yjmcc.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakthivel S, Finley NL, Rosevear PR, Lorenz JN, Gulick J, Kim S, et al. In vivo and in vitro analysis of cardiac troponin I phosphorylation. J Biol Chem. 2005;280:703–714. doi: 10.1074/jbc.M409513200. [DOI] [PubMed] [Google Scholar]
- 18.Westfall MV, Borton AR, Albayya FP, Metzger JM. Myofilament calcium sensitivity and cardiac disease: insights from troponin I isoforms and mutants. Circ Res. 2002;91:525–531. doi: 10.1161/01.RES.0000034710.46739.C0. [DOI] [PubMed] [Google Scholar]
- 19.VanBuren P, Okada Y. Thin filament remodeling in failing myocardium. Heart Fail Rev. 2005;10:199–209. doi: 10.1007/s10741-005-5250-8. [DOI] [PubMed] [Google Scholar]
- 20.Hamdani N, de Waard M, Messer AE, Boontje NM, Kooij V, van Dijk S, et al. Myofilament dysfunction in cardiac disease from mice to men. J Muscle Res Cell Motil. 2008;29:189–201. doi: 10.1007/s10974-008-9160-y. [DOI] [PubMed] [Google Scholar]
- 21.Pi Y, Zhang D, Kemnitz KR, Wang H, Walker JW. Protein kinase C and A sites on troponin I regulate myofilament Ca2+ sensitivity and ATPase activity in the mouse myocardium. J Physiol. 2003;552:845–857. doi: 10.1113/jphysiol.2003.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Knott A, Purcell I, Marston S. In vitro motility analysis of thin filaments from failing and non-failing human heart: troponin from failing human hearts induces slower filament sliding and higher Ca2+ sensitivity. J Mol Cell Cardiol. 2002;34:469–482. doi: 10.1006/jmcc.2002.1528. [DOI] [PubMed] [Google Scholar]
- 23.Kentish JC, McCloskey DT, Layland J, Palmer S, Leiden JM, Martin AF, et al. Phosphorylation of troponin I by protein kinase A accelerates relaxation and crossbridge cycle kinetics in mouse ventricular muscle. Circ Res. 2001;88:1059–1065. doi: 10.1161/hh1001.091640. [DOI] [PubMed] [Google Scholar]
- 24.Noland TA, Jr, Raynor RL, Kuo JF. Identification of sites phosphorylated in bovine cardiac troponin I and troponin T by protein kinase C and comparative substrate activity of synthetic peptides containing the phosphorylation sites. J Biol Chem. 1989;264:20778–20785. [PubMed] [Google Scholar]
- 25.Seguchi O, Takashima S, Yamazaki S, Asakura M, Asano Y, Shintani Y, et al. A cardiac myosin light chain kinase regulates sarcomere assembly in the vertebrate heart. J Clin Invest. 2007;117:2812–2824. doi: 10.1172/JCI30804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haworth RS, Cuello F, Herron TJ, Franzen G, Kentish JC, Gautel M, et al. Protein kinase D is a novel mediator of cardiac troponin I phosphorylation and regulates myofilament function. Circ Res. 2004;95:1091–1099. doi: 10.1161/01.RES.0000149299.34793.3c. [DOI] [PubMed] [Google Scholar]
- 27.Murphy AM. Another new kinase targets troponin I. Circ Res. 2004;95:1043–1045. doi: 10.1161/01.RES.0000150051.81155.88. [DOI] [PubMed] [Google Scholar]
- 28.You B, Yan G, Zhang Z, Yan L, Li J, Ge Q, et al. Phosphorylation of cardiac troponin I by mammalian sterile 20-like kinase 1. Biochem J. 2009;418:93–101. doi: 10.1042/BJ20081340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Layland J, Li JM, Shah AM. Role of cyclic GMP-dependent protein kinase in the contractile response to exogenous nitric oxide in rat cardiac myocytes. J Physiol. 2002;540:457–467. doi: 10.1113/jphysiol.2001.014126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sheehan KA, Ke Y, Wolska BM, Solaro RJ. Expression of active p21-activated kinase-1 induces Ca2+ flux modification with altered regulatory protein phosphorylation in cardiac myocytes. Am J Physiol Cell Physiol. 2009;296:C47–C58. doi: 10.1152/ajpcell.00012.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buscemi N, Foster DB, Neverova I, Van Eyk JE. p21-activated kinase increases the calcium sensitivity of rat triton-skinned cardiac muscle fiber bundles via a mechanism potentially involving novel phosphorylation of troponin I. Circ Res. 2002;91:509–516. doi: 10.1161/01.RES.0000035246.27856.53. [DOI] [PubMed] [Google Scholar]
- 32.Stelzer JE, Patel JR, Walker JW, Moss RL. Differential roles of cardiac myosin-binding protein C and cardiac troponin I in the myofibrillar force responses to protein kinase A phosphorylation. Circ Res. 2007;101:503–511. doi: 10.1161/CIRCRESAHA.107.153650. [DOI] [PubMed] [Google Scholar]
- 33.Roman BB, Goldspink PH, Spaite E, Urboniene D, McKinney R, Geenen DL, et al. Inhibition of PKC phosphorylation of cTnI improves cardiac performance in vivo . Am J Physiol Heart Circ Physiol. 2004;286:H2089–H2095. doi: 10.1152/ajpheart.00582.2003. [DOI] [PubMed] [Google Scholar]
- 34.Wang XJ, Yang X, Chen JZ, Song XD, Yi Zhen, Wang SX, et al. TNNI3K, a cardiac-specific kinase, promotes cardiac hypertrophy in vivo . Circulation. 2006;114:II–5. doi: 10.1161/CIRCULATIONAHA.106.643940. [DOI] [Google Scholar]
- 35.Wang L, Wang H, Ye J, Xu RX, Song L, Shi N, et al. Adenovirus-mediated overexpression of cardiac troponin I-interacting kinase promotes cardiomyocyte hypertrophy. Clin Exp Pharmacol Physiol. 2011;38:278–284. doi: 10.1111/j.1440-1681.2011.05499.x. [DOI] [PubMed] [Google Scholar]