Abstract
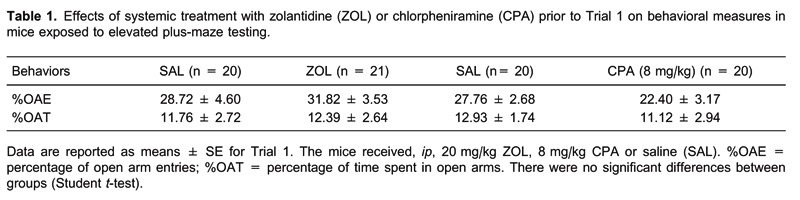
This study investigated the role of H1 and H2 receptors in anxiety and the retrieval of emotional memory using a Trial 1/Trial 2 (T1/T2) protocol in an elevated plus-maze (EPM). Tests were performed on 2 consecutive days, designated T1 and T2. Before T1, the mice received intraperitoneal injections of saline (SAL), 20 mg/kg zolantidine (ZOL, an H2 receptor antagonist), or 8.0 or 16 mg/kg chlorpheniramine (CPA, an H1 receptor antagonist). After 40 min, they were subjected to the EPM test. In T2 (24 h later), each group was subdivided into two additional groups, and the animals from each group were re-injected with SAL or one of the drugs. In T1, the Student t-test showed no difference between the SAL and ZOL or 8 mg/kg CPA groups with respect to the percentages of open arm entries (%OAE) and open arm time (%OAT). However, administration of CPA at the highest dose of 16 mg/kg decreased %OAE and %OAT, but not locomotor activity, indicating anxiogenic-like behavior. Emotional memory, as revealed by a reduction in open arm exploration between the two trials, was observed in all experimental groups, indicating that ZOL and 8 mg/kg CPA did not affect emotional memory, whereas CPA at the highest dose affected acquisition and consolidation, but not retrieval of memory. Taken together, these results suggest that H1 receptor, but not H2, is implicated in anxiety-like behavior and in emotional memory acquisition and consolidation deficits in mice subjected to EPM testing.
Keywords: Chlorpheniramine, Zolantidine, Anxiety, Memory, Elevated plus-maze
Introduction
Histamine is a neurotransmitter present in both the peripheral and central nervous systems that is involved in the modulation of anxiety-related behavior in animals. Furthermore, it has been implicated in cognitive functions, including learning and memory (1, 2). The functions of histamine are mediated through different receptor subtypes: H1, H2, H3, and H4 (3, 4).
It has been suggested that the histaminergic system may exert tonic modulatory control over emotional behavior (5). In addition, some evidence supports the concept that histaminergic neurons influence anxiety-related behavior via H1 and H2 receptor activation (6, 7). For example, administration of the H1 receptor antagonist pyrilamine or H2 receptor antagonist ranitidine in the dorsal hippocampus was found to induce anxiogenic-like behavior in mice in a hole-board test (7). Furthermore, evidence has demonstrated that histamine can facilitate long-term potentiation by activating histamine receptor subtypes (H1 and H2) and consequently modulates synaptic plasticity (2, 8). It is accepted that synaptic plasticity is the cellular basis of emotional memory because long-term potentiation is correlated with memory trace formation (2). Studies have also investigated the effects of H1 and H2 histaminergic receptor activation on emotional memory in animals (9, 10) and humans (11).
The elevated plus-maze (EPM) is a widely used test for animal anxiety (12, 13) and according to Galvis-Alonso et al. (14), animals acquire information about safe and dangerous areas of the maze during EPM testing. Repeated testing provides a measure of the acquisition and retention of memories because experience-dependent behavioral changes can be observed. Our laboratory has performed studies on the histaminergic system addressing anxiety and emotional memory in mice using a Trial 1/Trial 2 (T1/T2) protocol in an EPM (15, 16). Although these studies have indicated that histamine H1 receptors could have a modulatory effect on memory processes, few studies have investigated the effects of histamine mediated by H2 receptors on emotional behavior using repeated testing in an EPM.
Therefore, the objective of the present study was to investigate the effects of a systemically administered selective histamine H2 receptor antagonist, zolantidine (ZOL) and a histamine H1 receptor antagonist, chlorpheniramine (CPA), on the modulation of anxiety-related behaviors and the retrieval and acquisition of emotional memory in mice re-exposed to EPM testing.
Subjects and Methods
Subjects
The experimental subjects were adult male Swiss albino mice supplied by the Animal Facility of Universidade Federal de São Carlos, São Carlos, SP, Brazil, weighing 30-35 g at testing. The mice were housed in groups of 10 per cage (41 x 34 x 16 cm) and maintained under a 12-h light cycle (light on at 7:00 am) in a controlled environment with a temperature of 23 ± 1°C and a relative humidity of 50 ± 5%. The experimental sessions were conducted during the light period of the cycle (9:00 am and 4:00 pm). Food and drinking water were provided ad libitum, except during the brief testing periods. All mice were experimentally naive at the beginning of the study.
Drugs
Zolantidine (an H2 receptor antagonist; Sigma, USA) and chlorpheniramine maleate salt (an H1 receptor antagonist; Sigma) were dissolved in sterile 0.9% saline. The drugs were injected intraperitoneally (ip) at a volume of 2 mL/kg body weight, and the final dose was 20 mg/kg ZOL and 8.0 or 16 mg/kg CPA. The applied doses were based on previous studies (6, 15, 17) and on pilot work performed in our laboratory.
Saline (SAL) was used as control. Both drugs (ZOL and CPA), and SAL were placed in coded Eppendorf tubes under refrigeration. This coding was unknown to the experimenter at the time of the behavioral analysis testing.
Apparatus
The apparatus used for EPM testing was similar to those previously developed and validated for rats (12) and for mice (13). It was constructed from wood, and its enclosed arms had transparent glass walls. The maze consisted of four arms: two open (30 x 5 x 0.25 cm) and two enclosed arms (30 x 15 x 5 cm) extending from a common central platform (5 x 5 cm) and was elevated to a height of 38.5 cm from the floor. All tests were conducted under moderate illumination (77 lx, measured on the central platform of the EPM) during the light phase of the diurnal cycle.
Experimental procedure
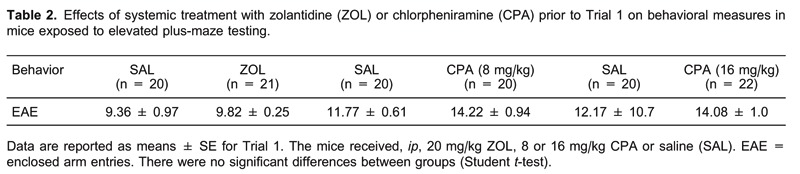
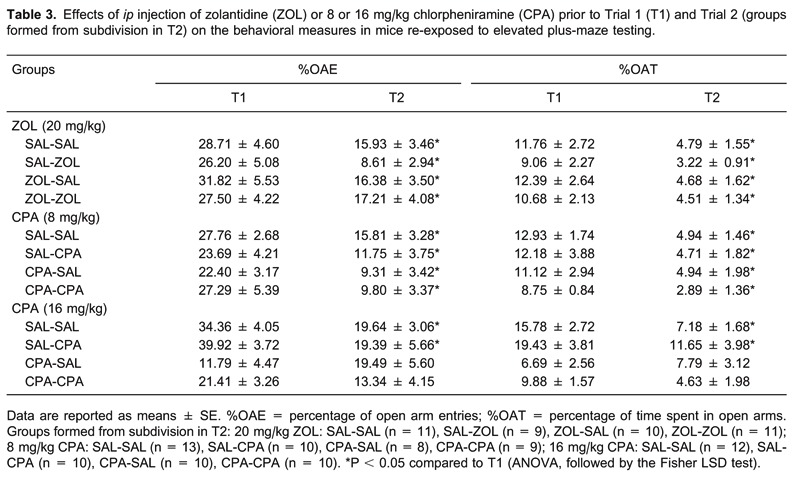
On the day of the experiment, to facilitate adaptation, animals were transported to a dimly lit room and left undisturbed for at least 1 h before testing to facilitate adaptation. The experiments were performed on two consecutive days, designated T1 and T2. In T1, the mice received an ip injection of SAL, ZOL, and 8 or 16 mg/kg CPA. For each drug tested there was a corresponding control group in T1, resulting in the following paired groups: SAL (n = 20) and ZOL (n = 21), SAL (n = 20) and 8 mg/kg CPA (n = 20), and SAL (n = 20) and 16 mg/kg CPA (n = 22). Forty minutes after the injections (6, 18), the mice were exposed to the EPM (T1). In T2 (24 h later), each group was subdivided into two new groups, and the animals from each group were re-injected with SAL or one of the drugs prior to conducting T2. For each drug administered, the animals were randomly assigned to four groups based on the drug treatment: 20 mg/kg ZOL: SAL-SAL (n = 11), SAL-ZOL (n = 9), ZOL-SAL (n = 10), ZOL-ZOL (n = 11); 8 mg/kg CPA: SAL-SAL (n = 13), SAL-CPA (n = 10), CPA-SAL (n = 8), CPA-CPA (n = 9), and 16 mg/kg CPA: SAL-SAL (n = 12), SAL-CPA (n = 10), CPA-SAL (n = 10), CPA-CPA (n = 10).
Each testing session began by placing the subject on the central platform of the maze facing an open arm. The subject was allowed 5 min of free exploration. Between animals, the maze was thoroughly cleaned with 20% alcohol and dry cloths. All sessions were video recorded using a camera positioned above and at a 50° angle with respect to the maze to permit the discrimination and documentation of all behaviors. The video signal was also relayed to a monitor for real-time observation in another room.
Behavioral analysis
Videotapes were scored by a highly trained observer using the ethological analysis software package X-Plot-Rat developed at Laboratório de Comportamento Exploratório, USP, Ribeirão Preto (19). The conventional measures recorded were the frequency of closed arm entries (arm entry = all four paws into an arm), percentage of open arm entries [%OAE = (open / total) x 100] and percentage of time spent (%OAT) in open parts of the maze [(time open / 300) x 100].
Statistical analysis
Initially, all results were subjected to the Levene test for homogeneity of variance. When appropriate, the data were square-root transformed and then analyzed by the Student t-test (T1) or two-way repeated measures ANOVA (T2; factor 1: treatment; factor 2: trial). Significant F tests were followed by post hoc Fisher LSD tests (protected t-tests). In all cases, P values less than 0.05 were considered to be significant.
Ethics
All procedures were approved by the Ethics Committee on Animal Experimentation of Universidade Federal de São Carlos (028/2007) and were consistent with the recommendations of the Brazilian Society of Neuroscience and Behavior (SBNeC), which are based on the US National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Results
Effects of ip injection of SAL or 20 mg/kg ZOL on anxiety and emotional memory retrieval
Table 1 shows the effects of ip SAL or 20 mg/kg ZOL injections on EPM behavioral measures. The Student t-test detected no difference between the SAL and ZOL groups in %OAE (t(20) = 0.53, P > 0.05) or %OAT (t(20) = 0.16, P > 0.05), suggesting that ZOL has no effect on anxiety. Furthermore, no difference was observed in enclosed arm entry (EAE; t(20) = 0.10, P > 0.05) (Table 2) in T1, and the Student t-test showed no differences for the SAL and ZOL groups, indicating that there was no alteration in locomotor activity.
As indicated in Table 3, ANOVA confirmed the effects of the trial factor on %OAE (F(1,40) = 26.3, P < 0.05) and %OAT (F(1,40) = 27.3, P < 0.05). The Fisher LSD test showed that there was decreased open arm activity in T2 in the SAL-SAL, SAL-ZOL, ZOL-SAL, and ZOL-ZOL groups, indicating that ZOL did not affect emotional memory retrieval.
Effects of ip injection of SAL or CPA (8 mg/kg) on anxiety and emotional memory retrieval
The effects of ip injection of SAL or CPA at a dose of 8.0 mg/kg on EPM behavioral measures are shown in Table 1. The Student t-test revealed no significant differences between the SAL and CPA groups for %OAE (t(20) = 1.71, P > 0.05) or %OAT (t(20) = 0.53, P > 0.05) in T1, suggesting that CPA at this dose has no effect on anxiety. Concerning EAE, the Student t-test showed no differences for the SAL and CPA groups, indicating that there was no alteration in locomotor activity (Table 2).
For T2, ANOVA confirmed a statistically significant effect of the trial on %OAE (F(1,39) = 46.39, P < 0.05) and %OAT (F(1,39) = 28.12, P < 0.05). The Fisher LSD test showed decreased open arm exploration (%OAE and %OAT) for all experimental groups (Table 3).
Effects of ip injection of SAL or 16 mg/kg CPA on anxiety and emotional memory retrieval
Figure 1A,B shows the effects of ip injection of SAL or CPA at a dose of 16 mg/kg on EPM behavioral measures. The Student t-test revealed a significant decrease in %OAE (t(20) = 3.74, P < 0.05) and %OAT (t(20) = 2.43, P < 0.05) for the CPA group compared to the SAL group. These results indicate that the highest dose of CPA (16 mg/kg) induced anxiogenic-like effects in mice. The Student t-test detected no differences between the SAL and CPA groups for EAE (t(20) = 1.30, P > 0.05) in T1 (Table 2), indicating that the highest dose of CPA did not affect locomotor activity.
Figure 1. Effects of ip injection of chlorpheniramine (CPA; n = 22) on the percentage of open arm entries (%OAE; A) and the percentage of time spent in the open arms (%OAT; B) during Trial 1 (T1). CPA (16 mg/kg, ip) or saline (SAL) was administered prior to T1. Data are reported as means ± SE. *P < 0.05 for the differences between the SAL and CPA groups in T1 (Student t-test).
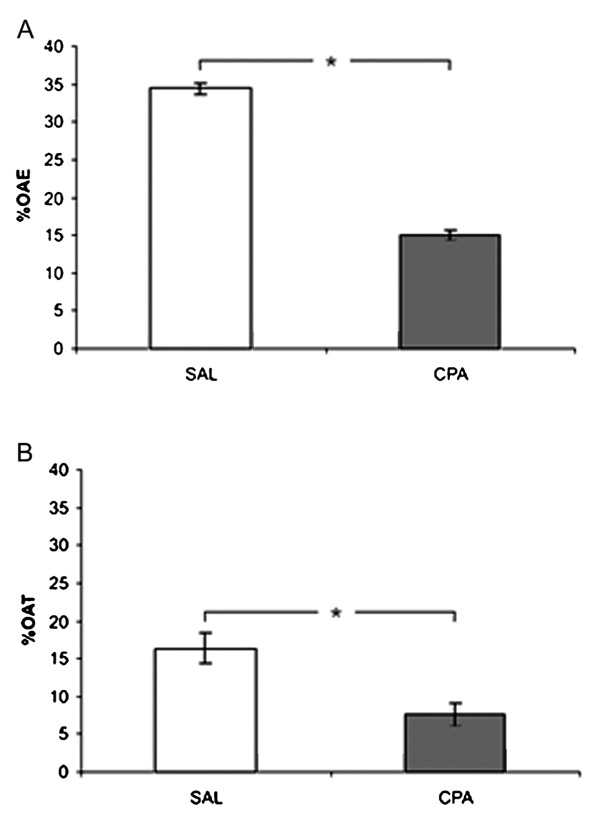
Table 3 presents a comparison between T1 and T2, and repeated measures ANOVA revealed a significant effect of the trials on %OAE (F(1,41) = 21.91, P < 0.05) and %OAT (F(1,41) = 32.53, P < 0.05). The post hoc test indicated a reduction in both variables for the SAL-SAL and SAL-CPA groups, but not for the CPA-SAL and CPA-CPA groups in T2 (ANOVA, P > 0.05).
Discussion
The main results of this study show that systemic administration of 8 mg/kg CPA and 20 mg/kg ZOL did not affect behavioral measures of anxiety. Treatment with CPA at the highest dose induced anxiety-related behavior in mice subjected to the EPM. Importantly, no significant changes were observed in the number of EAE in T1, a parameter considered to be a valid measure of locomotor activity in EPM tests (20).
Our results show that the injection of ZOL or 8 mg/kg CPA ip prior to T1 did not affect anxiety because no significant difference in open arm activity (%OAE and %OAT) was observed between the SAL and ZOL or CPA groups during T1. Our results are consistent with previous studies conducted in our laboratory, which have demonstrated that ip injection of 8 mg/kg CPA and intra-amygdala infusions of CPA (0.016 and 0.052 nmol/0.1 µL) do not alter anxiety levels in mice exposed to the EPM (15, 16). In another study, treatment with 20 mg/kg ip ZOL, an H2 receptor antagonist, also did not affect anxiety in mice subjected to the EPM (6). In the present study, administration of CPA at the highest dose (16 mg/kg) decreased %OAT and %OAE, which are parameters associated with anxiety-related behavior, without locomotor impairment in the EPM, indicating an anxiogenic response, which is in agreement with the data obtained in previous studies (6, 21). It has been proposed that histamine modulates the release of acetylcholine via stimulation of H1 receptors. For example, superfusion with an H1 receptor antagonist was found to decrease the release of acetylcholine in the rat ventral striatum (22). Since acetylcholine may modulate anxiety-related behaviors (23), one may expect that this response of the highest doses of antagonist CPA is mediated through changes in acetylcholine levels, but this hypothesis has not yet been tested. In contrast, other studies have reported an anxiolytic response mediated by H1 receptors in rats and mice (24, 25). The reported discrepancies could be related to experimental differences among the many factors that appear to influence the aversion to open arms, such as the time of day at which testing occurs 26 and the levels of illumination in the testing room (27).
We detected a notable decrease in open arm activity (%OAE and %OAT) in the animals treated with ZOL and CPA at a dose of 8 mg/kg during T2. Our results indicate that learning occurred in these groups during T1 and that emotional memory was evoked in T2, which corroborates the findings of previous studies performed in our laboratory indicating that CPA does not affect emotional memory in mice (15, 16). Behavioral studies have indicated that H1 and H2 receptors enhance the processes of learning and memory in rats (24, 28). Another study has suggested that neither H1 nor H2 receptors alter lithium state-dependent retrieval of memory in mice when administered as pre-test treatment (29). In our study, the animals that received CPA at the highest dose did not show altered activity in the open arms upon a second exposure to the EPM, indicating an emotional memory acquisition and consolidation deficits. These results could primarily be explained by the anxiety responses observed in the group that received 16 mg/kg CPA prior to the first exposure to the EPM, impairing the normal acquisition of configural and contextual characteristics of the maze and inducing the performance impairment observed in the T2 session. However, the anxiety-related behaviors induced by this drug in the EPM are associated with a normal and adaptive anxiety range and it is unlikely that the memory impairment is due to this anxiety. A recent study investigating the relationship between anxiety and cognitive functions found no short-memory or long-memory impairment in mouse strains that display adaptive anxiety (30). Our results agree with other studies showing that administration of CPA impairs learning and memory processes (31, 32). A recent study conducted in our laboratory demonstrated that infusion of CPA (0.16 nmol/0.1 µL) in the amygdala induces emotional memory impairment per se at the highest dose tested (16). Furthermore, behavioral evidence has indicated that the regulatory mechanisms of histamine neural circuits affecting learning and memory are possibly related to their dynamic neural network connections with many structures (33), suggesting tonic modulatory control of this neurotransmitter.
The widespread extension of histaminergic neurons suggests that this system might influence different brain regions; one such area, the locus coeruleus, modulates the attentional state and presents a tight neuromodulatory interaction with the amygdala (34), which play an important regulatory role in the acquisition of emotionally based learning and memory (35). An in vitro study conducted by Korotkova et al. (36) demonstrated that histamine excites noradrenergic neurons in the rat locus coeruleus via H1 receptors. We suggest that ip injection of CPA, an H1 histaminergic antagonist, at the highest dose tested, decreased adrenergic neuron activation in this structure, which impaired emotional memory retrieval in mice during a second exposure to the EPM. Our results suggest that the emotional memory impairment induced by chlorpheniramine in rodents is under the tonic modulatory control of histamine.
We conclude that anxiety-like behavior and emotional memory acquisition and consolidation deficits are mediated by H1 but not H2 receptors in mice re-exposed to EPM testing.
Acknowledgments
Research supported by FAPESP and CNPq (#300543/2010-7).
Footnotes
First published online April 19, 2013.
References
- 1.Brown RE, Stevens DR, Haas HL. The physiology of brain histamine. Prog Neurobiol. 2001;63:637–672. doi: 10.1016/S0301-0082(00)00039-3. [DOI] [PubMed] [Google Scholar]
- 2.Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88:1183–1241. doi: 10.1152/physrev.00043.2007. [DOI] [PubMed] [Google Scholar]
- 3.Leurs R, Smith MJ, Timmerman H. Molecular pharmacological aspects of histamine receptors. Pharmacol Ther. 1995;66:413–463. doi: 10.1016/0163-7258(95)00006-3. [DOI] [PubMed] [Google Scholar]
- 4.Strakhova MI, Nikkel AL, Manelli AM, Hsieh GC, Esbenshade TA, Brioni JD, et al. Localization of histamine h4 receptors in the central nervous system of human and rat. Brain Res. 2009;1250:41–48. doi: 10.1016/j.brainres.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 5.Santos NR, Huston JP, Brandao ML. Escape behavior under tonic inhibitory control of histamine h(2)-receptor mediated mechanisms in the midbrain tectum. Behav Brain Res. 2001;124:167–175. doi: 10.1016/S0166-4328(01)00228-5. [DOI] [PubMed] [Google Scholar]
- 6.Kumar KV, Krishna DR, Palit G. Histaminergic H1 receptors mediate l-histidine-induced anxiety in elevated plus-maze test in mice. Behav Pharmacol. 2007;18:213–217. doi: 10.1097/FBP.0b013e328157f450. [DOI] [PubMed] [Google Scholar]
- 7.Zarrindast MR, Nasehi M, Piri M, Bina P. Anxiety-like behavior induced by histaminergic agents can be prevented by cannabinoidergic WIN55,212-2 injected into the dorsal hippocampus in mice. Pharmacol Biochem Behav. 2010;94:387–396. doi: 10.1016/j.pbb.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 8.Luo T. Endogenous histamine facilitates long-term potentiation in the hippocampus during walking. J Neurosci. 2010;30:7845–7852. doi: 10.1523/JNEUROSCI.1127-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zarrindast MR, Parsaei L, Ahmadi S. Repeated administration of histamine improves memory retrieval of inhibitory avoidance by lithium in mice. Pharmacology. 2008;81:187–194. doi: 10.1159/000111760. [DOI] [PubMed] [Google Scholar]
- 10.Zlomuzica A, Viggiano D, De Souza Silva MA, Ishizuka T, Gironi Carnevale UA, Ruocco LA, et al. The histamine H1-receptor mediates the motivational effects of novelty. Eur J Neurosci. 2008;27:1461–1474. doi: 10.1111/j.1460-9568.2008.06115.x. [DOI] [PubMed] [Google Scholar]
- 11.Kay GG, Harris AG. Loratadine: a non-sedating antihistamine. Review of its effects on cognition, psychomotor performance, mood and sedation. Clin Exp Allergy. 1999;29(Suppl 3):147–150. doi: 10.1046/j.1365-2222.1999.0290s3147.x. [DOI] [PubMed] [Google Scholar]
- 12.Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology. 1987;92:180–185. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- 13.Pellow S, Chopin P, File SE, Briley M. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods. 1985;14:149–167. doi: 10.1016/0165-0270(85)90031-7. [DOI] [PubMed] [Google Scholar]
- 14.Galvis-Alonso OY, Garcia AM, Orejarena MJ, Lamprea MR, Botelho S, Conde CA, et al. A combined study of behavior and Fos expression in limbic structures after re-testing Wistar rats in the elevated plus-maze. Brain Res Bull. 2010;81:595–599. doi: 10.1016/j.brainresbull.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Gianlorenco AC, Canto-de-Souza A, Mattioli R. l-histidine induces state-dependent memory deficit in mice mediated by H(1) receptor. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:91–95. doi: 10.1016/j.pnpbp.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Serafim KR, Gianlorenco AC, Daher FP, Mattioli R. H(1)-histamine receptors in the amygdala are involved in emotional memory but do not mediate anxiety-related behaviors in mice submitted to EPM testing. Brain Res Bull. 2012;89:1–7. doi: 10.1016/j.brainresbull.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Cofiel LP, Mattioli R. Involvement of histamine receptors in the acquisition of inhibitory avoidance in Carassius auratus . Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1246–1250. doi: 10.1016/j.pnpbp.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 18.Serafim KR, Kishi M, Canto-de-Souza A, Mattioli R. L-histidine provokes a state-dependent memory retrieval deficit in mice re-exposed to the elevated plus-maze. Braz J Med Biol Res. 2010;43:100–106. doi: 10.1590/S0100-879X2009007500025. [DOI] [PubMed] [Google Scholar]
- 19.Garcia AM, Cardenas FP, Morato S. Effect of different illumination levels on rat behavior in the elevated plus-maze. Physiol Behav. 2005;85:265–270. doi: 10.1016/j.physbeh.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Cruz AP, Frei F, Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 21.Zarrindast MR, Moghadam AH, Rostami P, Roohbakhsh A. The effects of histaminergic agents in the central amygdala of rats in the elevated plus-maze test of anxiety. Behav Pharmacol. 2005;16:643–649. doi: 10.1097/00008877-200512000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Prast H, Tran MH, Lamberti C, Fischer H, Kraus M, Grass K, et al. Histaminergic neurons modulate acetylcholine release in the ventral striatum: role of H1 and H2 histamine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:552–557. doi: 10.1007/s002109900098. [DOI] [PubMed] [Google Scholar]
- 23.Degroot A, Treit D. Dorsal and ventral hippocampal cholinergic systems modulate anxiety in the plus-maze and shock-probe tests. Brain Res. 2002;949:60–70. doi: 10.1016/S0006-8993(02)02965-7. [DOI] [PubMed] [Google Scholar]
- 24.Privou C, Knoche A, Hasenohrl RU, Huston JP. The H1- and H2-histamine blockers chlorpheniramine and ranitidine applied to the nucleus basalis magnocellularis region modulate anxiety and reinforcement related processes. Neuropharmacology. 1998;37:1019–1032. doi: 10.1016/S0028-3908(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 25.Miyata S, Hirano S, Ohsawa M, Kamei J. Chlorpheniramine exerts anxiolytic-like effects and activates prefrontal 5-HT systems in mice. Psychopharmacology. 2011;213:441–452. doi: 10.1007/s00213-009-1695-0. [DOI] [PubMed] [Google Scholar]
- 26.Griebel G, Moreau JL, Jenk F, Martin JR, Misslin R. Some critical determinants of the behaviour of rats in the elevated plus-maze. Behav Processes. 1993;29:37–48. doi: 10.1016/0376-6357(93)90026-N. [DOI] [PubMed] [Google Scholar]
- 27.Garcia AM, Cardenas FP, Morato S. The effects of pentylenetetrazol, chlordiazepoxide and caffeine in rats tested in the elevated plus-maze depend on the experimental illumination. Behav Brain Res. 2011;217:171–177. doi: 10.1016/j.bbr.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 28.Da Silva WC, Bonini JS, Bevilaqua LR, Izquierdo I, Cammarota M. Histamine enhances inhibitory avoidance memory consolidation through a H2 receptor-dependent mechanism. Neurobiol Learn Mem. 2006;86:100–106. doi: 10.1016/j.nlm.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 29.Zarrindast MR, Fazli-Tabaei S, Khalilzadeh A, Farahmanfar M, Yahyavi SH. Cross state-dependent retrieval between histamine and lithium. Physiol Behav. 2005;86:154–163. doi: 10.1016/j.physbeh.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Salomons AR, Arndt SS, Ohl F. Impact of anxiety profiles on cognitive performance in BALB/c and 129P2 mice. [ September 18, 2012];Cogn Affect Behav Neurosci. doi: 10.3758/s13415-012-0109-7. www.springer.com [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masuoka T, Mikami A, Kamei C. Ameliorative effect of a hippocampal metabotropic glutamate-receptor agonist on histamine H1 receptor antagonist-induced memory deficit in rats. J Pharmacol Sci. 2010;113:41–47. doi: 10.1254/jphs.10022FP. [DOI] [PubMed] [Google Scholar]
- 32.Zarrindast MR, Ahmadi R, Oryan S, Parivar K, Haeri-Rohani A. Effects of alpha-adrenoceptor agonists and antagonists on histamine-induced impairment of memory retention of passive avoidance learning in rats. Eur J Pharmacol. 2002;454:193–198. doi: 10.1016/S0014-2999(02)02497-4. [DOI] [PubMed] [Google Scholar]
- 33.Alvarez EO. The role of histamine on cognition. Behav Brain Res. 2009;199:183–189. doi: 10.1016/j.bbr.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 34.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- 35.Ribeiro AM, Barbosa FF, Munguba H, Costa MS, Cavalcante JS, Silva RH. Basolateral amygdala inactivation impairs learned (but not innate) fear response in rats. Neurobiol Learn Mem. 2011;95:433–440. doi: 10.1016/j.nlm.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Korotkova TM, Sergeeva OA, Ponomarenko AA, Haas HL. Histamine excites noradrenergic neurons in locus coeruleus in rats. Neuropharmacology. 2005;49:129–134. doi: 10.1016/j.neuropharm.2005.03.001. [DOI] [PubMed] [Google Scholar]


