Abstract
Bone morphogenetic protein 2 (BMP2) and basic fibroblast growth factor (bFGF) have been shown to exhibit a synergistic effect to promote bone repair and healing. In this study, we constructed a novel adenovirus with high coexpression of BMP2 and bFGF and evaluated its effect on osteogenic differentiation of goat bone marrow progenitor cells (BMPCs). Recombinant adenovirus Ad-BMP2-bFGF was constructed by using the T2A sequence. BMPCs were isolated from goats by density gradient centrifugation and adherent cell culture, and were then infected with Ad-BMP2-bFGF or Ad-BMP2. Expression of BMP2 and bFGF was detected by ELISA, and alkaline phosphatase (ALP) activity was detected by an ALP assay kit. In addition, von Kossa staining and immunocytochemical staining of collagen II were performed on BMPCs 21 days after infection. There was a high coexpression of BMP2 and bFGF in BMPCs infected with Ad-BMP2-bFGF. Twenty-one days after infection, ALP activity was significantly higher in BMPCs infected with Ad-BMP2-bFGF than in those infected with Ad-BMP2. Larger and more mineralized calcium nodules, as well as stronger collagen II staining, were observed in BMPCs infected with Ad-BMP2-bFGF than in those infected with Ad-BMP2. In summary, we developed a novel adenovirus vector Ad-BMP2-bFGF for simultaneous high coexpression of BMP2 and bFGF, which could induce BMPCs to differentiate efficiently into osteoblasts.
Keywords: BMP2, bFGF, Adenovirus, T2A sequence, BMPCs, Osteogenic differentiation
Introduction
Several studies have shown that bone morphogenetic protein (BMP) and basic fibroblast growth factor (bFGF) induce bone formation in ectopic and orthotopic sites in vivo (1,2). In addition, systemic coadministration of BMP and bFGF in preclinical models has been shown to stimulate bone deposition in skeletal tissues (3,4). BMP2 is an important stimulator of bone formation by controlling the proliferation and differentiation of osteoblasts (5,6). A recent study reported that BMP2 could modulate osteogenic differentiation of adipose-derived stem cells (7). Notably, BMP2 and bFGF exhibited a synergistic effect on promotion of bone repair and healing (8). The osteogenic activity of bone marrow stromal cells (BMSCs) induced by both BMP2 and bFGF was greater than that induced by either bFGF or BMP2 alone (9).
Unfortunately, exogenously administered bone induction factors easily lose activity due to hydrolysis by proteases. Thus, it is necessary to provide a consistent supply of these factors in vivo. Chen et al. (10) successfully induced osteoblastic differentiation of BMSCs using recombinant adenovirus Ad-BMP2, which consistently produced BMP2, and the adenovirus did not cause a strong immune response in the body. Weng et al. (11) constructed a recombinant adenovirus coexpressing truncated human prostate-specific membrane antigen and mouse 4-1BBL genes using the internal ribosome entry site (IRES) sequence. However, the expression of more than two genes within a single vector using conventional approaches has several limitations, such as imbalanced protein expression and large size of the transgene cassette. Small T2A peptide sequences, when placed between the genes, allow for efficient and stoichiometric production of discrete protein products within a single vector through a novel “cleavage” event within the T2A peptide sequence. The separation of genes placed between 2A peptide sequences is nearly 100%, which allows for stoichiometric and concordant expression of the genes (12-14).
In this study, we employed T2A to link the BMP2 and bFGF genes, and constructed an Ad-BMP2-bFGF recombinant adenovirus vector for coexpression of BMP2 and bFGF. Next, we isolated goat bone marrow progenitor cells (BMPCs) and infected them with the recombinant adenovirus vector. We then evaluated the expression of BMP2 and bFGF and osteogenesis in the BMPCs.
Material and Methods
Isolation and culture of goat BMPCs
This study was approved by the Ethics Committee of Guangzhou Medical University. Two 2-year-old goats each weighing 25 to 30 kg were provided by Guangzhou Longgui Xingke Experimental Animal Center. BMPCs were isolated from goat bone marrow by density gradient centrifugation and adherent cell culture as described previously (15). With the use of a cell counting chamber, cell density was adjusted to 2×105/mL in high-glucose DMEM (Gibco BRL, USA) supplemented with fetal bovine serum (FBS; Hyclone, USA). The cells were seeded in a 25-cm2 plastic culture flask and placed in 5% CO2, in a humidified incubator at 37°C. The medium was changed at 48 h and then every 2 to 3 days.
Construction of adenovirus vectors Ad-BMP2-bFGF and Ad-BMP2
BMP2 and bFGF cDNAs were amplified from RNA isolated from 293A cells by RT-PCR with the following primers: BMP2, forward: 5′-ATGGTGGCCGGGACCC-3′ and reverse: 5′-GCGACACCCACAACCCTC-3′; bFGF, forward: 5′-ATGGTGGGTGTGGGGGGT-3′ and reverse: 5′-ATCAGCTCTTAGCAGACATTGG-3′. PCR products (BMP2 1200 bp and bFGF 860 bp) were separated by 0.8% agarose gel electrophoresis and collected through gel recovery. Next, the connection sequence for T2A (GAGGGCAGAGGAAGTCTTCTAACATGCGGTGACGTGGAGGAGAATCCCGGCCCT) was added to the C-terminal of BMP2 cDNA and the N-terminal of bFGF cDNA, and the full sequences of both cDNAs were obtained through the complementary extension of the T2A sequence. Then, the BMP2-bFGF dual gene was amplified by PCR using an upstream primer containing the BglII site and a downstream primer containing the EcoRV site, and subcloned into the pShuttle-IRES-hrGFP-1 (green fluorescent protein) vector to make pShuttle-BMP2-bFGF and pShuttle-BMP2 vectors, respectively. Next, the BMP2-bFGF dual gene and the BMP2 gene were subcloned from shuttle plasmids to the Ad-GFP adenovirus vector. The recombinant Ad-BMP2-bFGF and Ad-BMP2 vectors were confirmed by DNA sequencing (Biosune Company, China).
Package and amplification of Ad-BMP2-bFGF and Ad-BMP2
The 293A cells used in this procedure were cultured in 25-cm2 flasks for 24 h to 50-70% confluence and then transfected with Ad-BMP2-bFGF and Ad-BMP2 vectors linearized by PacI using lipofection. After the cytopathic effect had occurred, the cells were frozen in liquid nitrogen and thawed at 37°C three times. The supernatant was collected and adenovirus was purified by CsCl gradient centrifugation. The absorbance at 260 nm (A260) of purified virus was measured to calculate the number of virus particles (VP), with one A260 corresponding to 1.1×1012 VP/mL.
MTT assay
Cell proliferation was determined using a cell counting kit (CCK-8, Beyotime Institute of Biotechnology, China) according to the manufacturer's instructions. Briefly, cells were seeded at a density of 5×103 cells/well on 96-well plates and grown for the indicated time. Then, 20 µL MTT solution (5 g/L) was added into each well. After incubation for 4 h, the supernatant was replaced with 150 µL dimethyl sulfoxide (Sigma, USA). The absorbance was determined at 490 nm using a microplate reader.
Infection of BMPCs by adenovirus
BMPCs were seeded on 12-well plates (8×104 cells/well) and infected with Ad-BMP2-bFGF or Ad-BMP2. The multiplicity of infection (MOI) was 5000. After a 2-h incubation at 37°C, 40 µL FBS and 500 µL complete medium were added into each well and the medium was changed every 2 days. Cell supernatants were frozen at -80°C.
ELISA
Twenty days after infection, the BMPC culture supernatants were collected. The concentrations of BMP2 and bFGF in the supernatants were determined with a human BMP2 ELISA kit (Boster, China) and a human bFGF ELISA kit (Boster, China), according to the manufacturers' instructions.
Alkaline phosphatase (ALP) and von Kossa staining
BMPCs were infected with Ad-BMP2-bFGF or Ad-BMP2 and collected 7, 14, and 21 days after infection. The cells were lysed and ALP activity in the lysate was detected with the use of an alkaline phosphatase assay kit (Abcam, USA) according to the manufacturer's protocol. Absorbance of the lysates was measured at a wavelength of 405 nm. The corresponding activity was calculated based on a standard curve according to a sample absorbance value. ALP activity was also evaluated in cells stained using an ALP staining kit (Genmed, China) according to the manufacturer's protocol. BMPCs were rinsed twice with PBS and immersed in an alkaline-dye mixture for 10 min at room temperature. Next, the dyed cells were rinsed twice with PBS and observed with a light microscope.
To evaluate cell-mediated calcium deposition, von Kossa staining was performed. Twenty-one days after infection, BMPCs were fixed in 4% paraformaldehyde, stained with 5% silver nitrate, placed under a UV lamp for 10 min, and rinsed with distilled water. The cells were then stained with 5% sodium thiosulfate, rinsed with distilled water, and washed with alcohol, and von Kossa-positive deposits were observed with a light microscope.
Immunocytochemistry
The cells were seeded on slides, permeabilized in 0.2% (v/v) Triton X-100 in PBS at pH 7.4, for 5 min at room temperature, post-fixed in paraformaldehyde, and blocked for 1 h at room temperature in 5% normal goat serum. Next, the slides were incubated with rabbit anti-collagen 2A1 (Santa Cruz Biotechnology, USA) overnight at 4°C, followed by incubation with goat anti-rabbit IgG conjugated with FITC for 2 h at room temperature. Finally, the slides were washed several times in PBS and mounted for observation with a confocal microscope.
Statistical analysis
Data are reported as means±SD and were analyzed using the SPSS 17.0 (USA) statistical software. The differences were analyzed by ANOVA followed by a post hoc test; P<0.05 indicated significant difference.
Results
Isolation and culture of goat BMPCs
After isolation, a small number of adherent spindle-shaped cells could be seen. Nearly all nonadhering cells had been removed after three or four changes of the culture medium. After an incubation period of 3 to 4 days, adherent cells grew rapidly to form colonies. BMPCs showed a fibroblast-like morphology with a small cell body and a few long and thin cell processes (Figure 1). After about 1 week, the cells reached 80-90% confluence.
Figure 1. Colony-like growth of second generation of bone marrow progenitor cells. Bar = 100 μm.

Transduction of BMPCs by recombinant adenovirus in vitro
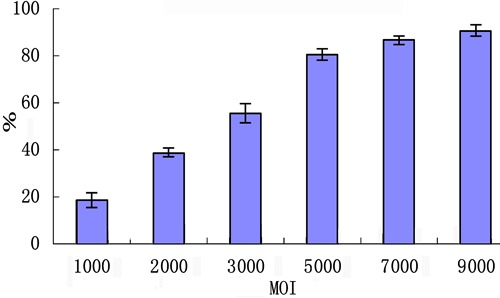
BMPCs were infected with Ad-BMP2-bFGF or Ad-BMP2 at a MOI value of 5000 and 48 h later green fluorescence was observed in most BMPCs (Figure 2A and B). We calculated the infection efficiency of Ad-BMP2-bFGF into BMPCs based on the number of GFP-positive cells per total number of nuclei stained with 4′,6-diamidino-2-phenylindole (DAPI), and found that the efficiency reached as high as 80% at a MOI of 5000 (Figure 3).
Figure 2. Morphology of bone marrow progenitor cells 48 h after infection with Ad-BMP2-bFGF-GFP at a multiplicity of infection (MOI) value of 5000. A, Image under light microscope. B, Image under fluorescence microscope showing the expression of green fluorescent protein-positive cells. Bar = 50 μm.

Figure 3. Infection efficiency of Ad-BMP2-bFGF in bone marrow progenitor cells. The infection efficiency was calculated by the number of green fluorescent protein-positive cells/total number of nuclei stained with DAPI x100 (%) (n=3). MOI: multiplicity of infection.
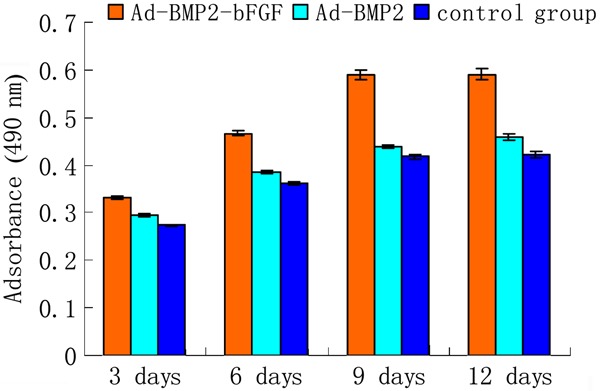
To exclude the possibility that virus infection has adverse effects on the viability of BMPCs, we performed an MTT assay. The results showed that infection by Ad-BMP2-bFGF led to a small increase in cell viability, but there were no significant differences in cell viability between BMPCs infected with Ad-BMP2-bFGF or Ad-BMP2 and control (Figure 4).
Figure 4. Effect of infection on the viability of bone marrow progenitor cells (BMPCs). BMPCs were infected with Ad-BMP2-bFGF or Ad-BMP2 and cell viability was determined by MTT assay (n=3).
High expression of BMP2 and bFGF in BMPCs infected with Ad-BMP2-bFGF
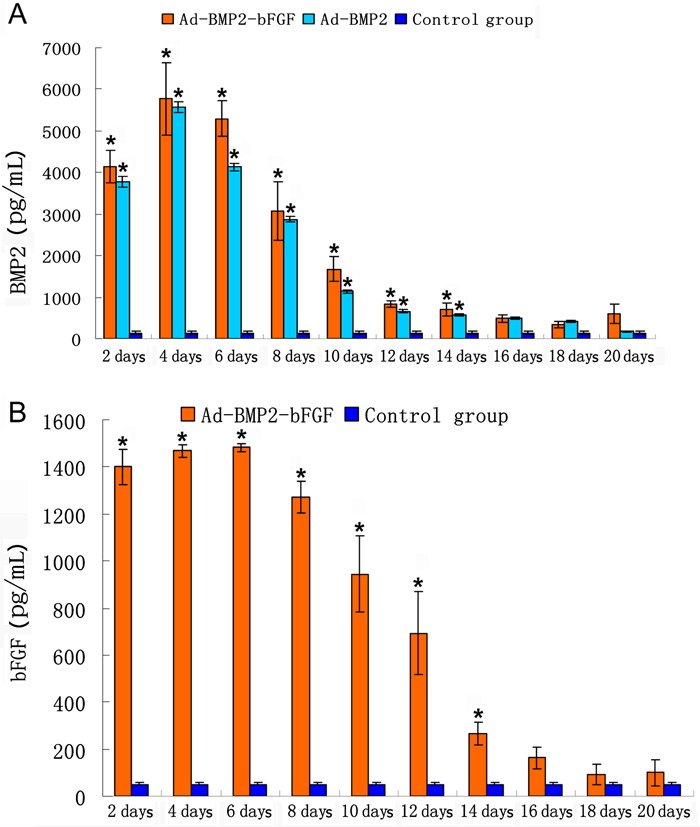
An ELISA assay showed that, at 48 h after infection, the BMP2 secretion level in Ad-BMP2-bFGF- or Ad-BMP2-infected BMPCs was significantly higher than that in control BMPCs (P<0.05), and the bFGF secretion level in Ad-BMP2-bFGF-infected BMPCs was also significantly higher than that in control BMPCs (P<0.05). After that, the secretion levels of BMP2 and bFGF in Ad-BMP2-bFGF-infected BMPCs remained high until the 6th day, and then decreased gradually over time, but were still higher than in control BMPCs 16 days later (Figure 5A and B).
Figure 5. Expression of bone morphogenetic protein 2 (BMP-2) and basic fibroblast growth factor (bFGF) in bone marrow progenitor cells (BMPCs). A, ELISA assay of BMP2 level secreted by BMPCs at different days after infection. B, ELISA assay of bFGF level secreted by BMPCs at different days after infection. Data are reported as means±SD (n=3). *P<0.05 vs control (ANOVA).
Characterization of BMPCs infected with Ad-BMP2-bFGF
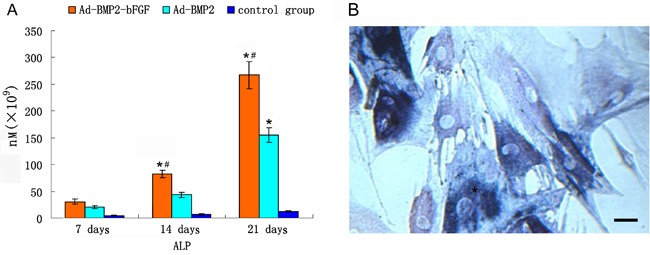
To examine the effects of the high expression of BMP2 and bFGF on BMPCs, we detected ALP activity, and the results showed that ALP activity in the Ad-BMP2-bFGF group was significantly higher than in the Ad-BMP2 group and the uninfected group on the 7th, 14th, and 21st day after infection (P<0.05, Figure 6A). In addition, ALP staining showed that, on the 18th day after infection, BMPCs showed positive ALP staining and many brown-black particles appeared in the cytoplasm, indicating strong secretion of ALP and typical osteoblast differentiation (Figure 6B).
Figure 6. Alkaline phosphatase (ALP) activity in bone marrow progenitor cells (BMPCs). A, ALP activity detected in BMPCs at different days after infection. *P<0.05 vs control. #P<0.05 vs Ad-BMP2-infected BMPCs. B, ALP staining of BMPCs 21 days after infection with Ad-BMP2-bFGF. Many brown-black particles appeared in the cytoplasm, and cells were stained blue. Bar = 100 μm.
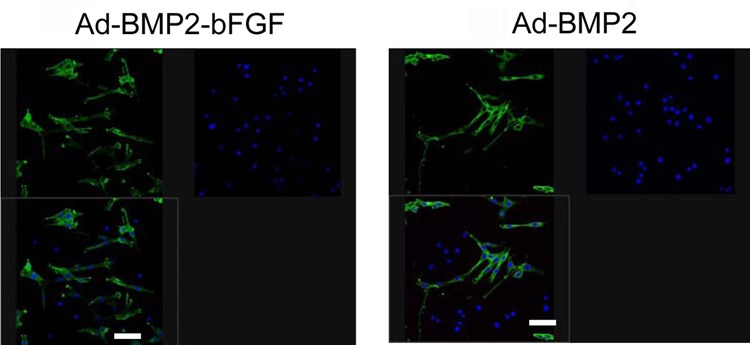
Next, we performed von Kossa staining and found more and larger mineralized calcium nodules in the Ad-BMP2-bFGF group than in the Ad-BMP2 group (Figure 7). In contrast, no mineralization was observed in the control group. Furthermore, immunocytochemical staining showed that the staining of collagen II was much stronger in the Ad-BMP2-bFGF group than in the Ad-BMP2 group (Figure 8).
Figure 7. Kossa staining of bone marrow progenitor cells 21 days after infection with Ad-BMP2-bFGF or Ad-BMP2. Mineralized calcium deposits were stained as dark nodules. Bar = 400 μm.
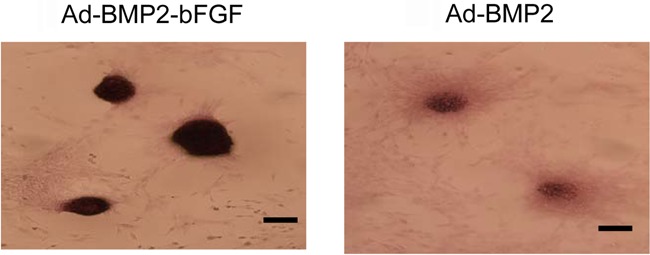
Figure 8. Immunocytochemical staining of collagen II in bone marrow progenitor cells 21 days after infection with Ad-BMP2-bFGF or Ad-BMP2. The expression of collagen II is indicated by green (upper left panels), the nuclei are indicated by blue (upper right panels), and the overlapping is shown in lower left panels. Bar = 200 μm.
Discussion
A variety of bone growth factors participate in the regulation of cell proliferation, differentiation, and bone metabolism. New bone formation involves the recruitment of osteoprogenitor cells, with the rate of mature bone formation dependent on the commitment and proliferation of these cells, their differentiation into functional osteoblasts, and the lifespan of mature osteoblasts. Osteoblast differentiation and matrix mineralization are regulated by signaling factors such as BMP2 and bFGF (16).
In the present study, BMPCs were isolated by density gradient centrifugation and adherent culture. GFP staining was apparent in BMPCs 48 h after infection by recombinant adenovirus, and infection efficiency was as high as 80% at a MOI of 5000. Consistent with the expression of GFP, we detected significant expression of BMP2 and bFGF in Ad-BMP2-bFGF-infected but not in uninfected control BMPCs. In addition, we found that BMP2 and bFGF expression were not sustained after prolonged culture, which may be due to the transient infection. Strikingly, the expression level of BMP2 in Ad-BMP2-bFGF-infected BMPCs was a little more than that in Ad-BMP2-infected BMPCs, but the difference was not significant. Taken together, these data suggest that Ad-BMP2-bFGF adenovirus has a high efficiency to coexpress BMP2 and bFGF. The use of the T2A link could successfully avoid the interference of expression between the individual genes.
Virus infection is known to have effects on host cell viability. To evaluate the safety of the adenovirus we constructed, we examined the viability of BMPCs after adenovirus infection. An MTT assay showed that the cell viability was not significantly changed, although Ad-BMP2-bFGF adenovirus infection led to a small increase in cell viability, perhaps due to the growth-promoting effects of bFGF. These data prove that the recombinant adenovirus we employed had no obvious toxicity on BMPCs.
Increased ALP activity, mineralized calcium nodules, and high expression of collagen II have been accepted as the markers of osteogenesis (17-20). Characterization of infected BMPCs showed that, 21 days after infection, ALP activity was significantly higher in BMPCs infected with Ad-BMP2-bFGF than in BMPCs infected with Ad-BMP2. Moreover, more and larger mineralized calcium nodules were apparent in BMPCs infected with Ad-BMP2-bFGF than in those infected with Ad-BMP2. Furthermore, immunostaining of collagen II showed that the collagen II level was higher in BMPCs infected with Ad-BMP2-bFGF than in those infected with Ad-BMP2. Collectively, these results showed that BMPCs infected with Ad-BMP2-bFGF exhibit the characteristics of osteogenic differentiation.
In summary, we developed a novel adenovirus vector Ad-BMP2-bFGF for simultaneous high coexpression of BMP2 and bFGF, and demonstrated that it could induce BMPCs to differentiate efficiently into osteoblasts.
Acknowledgments
Research supported by grants from Science and Technology Projects of Guangdong Province (#2010B031500005) and Science and Technology Projects of Guangzhou (#2010J-E021-1 and #201102A212029).
Footnotes
First published online September 16, 2013.
References
- 1.Huang Z, Nelson ER, Smith RL, Goodman SB. The sequential expression profiles of growth factors from osteoprogenitors to osteoblasts in vitro . Tissue Eng. 2007;13:2311–2320. doi: 10.1089/ten.2006.0423. [DOI] [PubMed] [Google Scholar]
- 2.Yamagiwa H, Endo N, Tokunaga K, Hayami T, Hatano H, Takahashi HE. In vivo bone-forming capacity of human bone marrow-derived stromal cells is stimulated by recombinant human bone morphogenetic protein-2. J Bone Miner Metab. 2001;19:20–28. doi: 10.1007/s007740170056. [DOI] [PubMed] [Google Scholar]
- 3.Akita S, Fukui M, Nakagawa H, Fujii T, Akino K. Cranial bone defect healing is accelerated by mesenchymal stem cells induced by coadministration of bone morphogenetic protein-2 and basic fibroblast growth factor. Wound Repair Regen. 2004;12:252–259. doi: 10.1111/j.1067-1927.2004.012118.x. [DOI] [PubMed] [Google Scholar]
- 4.Lan J, Wang Z, Wang Y, Wang J, Cheng X. The effect of combination of recombinant human bone morphogenetic protein-2 and basic fibroblast growth factor or insulin-like growth factor-I on dental implant osseointegration by confocal laser scanning microscopy. J Periodontol. 2006;77:357–363. doi: 10.1902/jop.2006.050016. [DOI] [PubMed] [Google Scholar]
- 5.Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, et al. BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet. 2006;38:1424–1429. doi: 10.1038/ng1916. [DOI] [PubMed] [Google Scholar]
- 6.Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Genet. 2006;2:e216. doi: 10.1371/journal.pgen.0020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang X, Gong P, Lin Y, Zhang L, Li X, Yuan Q, et al. Cyclic tensile stretch modulates osteogenic differentiation of adipose-derived stem cells via the BMP-2 pathway. Arch Med Sci. 2010;6:152–159. doi: 10.5114/aoms.2010.13886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam S, Ueki K, Marukawa K, Ohara T, Hase T, Takazakura D, et al. Expression of bone morphogenetic protein 2 and fibroblast growth factor 2 during bone regeneration using different implant materials as an onlay bone graft in rabbit mandibles. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:16–26. doi: 10.1016/j.tripleo.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Huang Y, Pan K, Jiang X, Liu C. Osteogenic responses to different concentrations/ratios of BMP-2 and bFGF in bone formation. Ann Biomed Eng. 2010;38:77–87. doi: 10.1007/s10439-009-9841-8. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y, Luk KD, Cheung KM, Lu WW, An XM, Ng SS, et al. Combination of adeno-associated virus and adenovirus vectors expressing bone morphogenetic protein-2 produces enhanced osteogenic activity in immunocompetent rats. Biochem Biophys Res Commun. 2004;317:675–681. doi: 10.1016/j.bbrc.2004.03.098. [DOI] [PubMed] [Google Scholar]
- 11.Weng X, Kuang Y, Liu X, Chen Z, Zhu H, Chen H, et al. Construction of a recombinant adenovirus co-expressing truncated human prostate-specific membrane antigen and mouse 4-1BBL genes and its effect on dendritic cells. Braz J Med Biol Res. 2011;44:186–192. doi: 10.1590/S0100-879X2011007500002. [DOI] [PubMed] [Google Scholar]
- 12.Szymczak-Workman AL, Vignali KM, Vignali DA. Design and construction of 2A peptide-linked multicistronic vectors. Cold Spring Harb Protoc. 2012;2012:199–204. doi: 10.1101/pdb.ip067876. [DOI] [PubMed] [Google Scholar]
- 13.Osborn MJ, Panoskaltsis-Mortari A, McElmurry RT, Bell SK, Vignali DA, Ryan MD, et al. A picornaviral 2A-like sequence-based tricistronic vector allowing for high-level therapeutic gene expression coupled to a dual-reporter system. Mol Ther. 2005;12:569–574. doi: 10.1016/j.ymthe.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 14.Chinnasamy D, Milsom MD, Shaffer J, Neuenfeldt J, Shaaban AF, Margison GP, et al. Multicistronic lentiviral vectors containing the FMDV 2A cleavage factor demonstrate robust expression of encoded genes at limiting MOI. Virol J. 2006;3:14. doi: 10.1186/1743-422X-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X, Bao C, Hu J, Yin G, Luo E. Effects of clodronate combined with hydroxyapatite on multi-directional differentiation of mesenchymal stromal cells. Arch Med Sci. 2010;6:670–677. doi: 10.5114/aoms.2010.17079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jager M, Fischer J, Dohrn W, Li X, Ayers DC, Czibere A, et al. Dexamethasone modulates BMP-2 effects on mesenchymal stem cells in vitro . J Orthop Res. 2008;26:1440–1448. doi: 10.1002/jor.20565. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa R, Mizuno H, Watanabe A, Migita M, Shimada T, Hyakusoku H. Osteogenic and chondrogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice. Biochem Biophys Res Commun. 2004;313:871–877. doi: 10.1016/j.bbrc.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Dragoo JL, Choi JY, Lieberman JR, Huang J, Zuk PA, Zhang J, et al. Bone induction by BMP-2 transduced stem cells derived from human fat. J Orthop Res. 2003;21:622–629. doi: 10.1016/S0736-0266(02)00238-3. [DOI] [PubMed] [Google Scholar]
- 19.Derubeis AR, Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32:160–165. doi: 10.1023/B:ABME.0000007800.89194.95. [DOI] [PubMed] [Google Scholar]
- 20.Maegawa N, Kawamura K, Hirose M, Yajima H, Takakura Y, Ohgushi H. Enhancement of osteoblastic differentiation of mesenchymal stromal cells cultured by selective combination of bone morphogenetic protein-2 (BMP-2) and fibroblast growth factor-2 (FGF-2) J Tissue Eng Regen Med. 2007;1:306–313. doi: 10.1002/term.41. [DOI] [PubMed] [Google Scholar]


