Abstract
One of the challenges of the postgenomic era is characterizing the function and regulation of specific genes. For various reasons, the early chick embryo can easily be adopted as an in vivo assay of gene function and regulation. The embryos are robust, accessible, easily manipulated, and maintained in the laboratory. Genomic resources centered on vertebrate organisms increase daily. As a consequence of optimization of gene transfer protocols by electroporation, the chick embryo will probably become increasingly popular for reverse genetic analysis. The challenge of establishing chick embryonic electroporation might seem insurmountable to those who are unfamiliar with experimental embryological methods. To minimize the cost, time, and effort required to establish a chick electroporation assay method, we describe and illustrate in great detail the procedures involved in building a low-cost electroporation setup and the basic steps of electroporation.
Keywords: Electroporation, In vivo gene expression, Electroporator, Vertebrate embryo
Introduction
In the era of high-throughput genomic analysis, we are faced with a staggering number of gene sequences whose cellular functions have not been analyzed. Furthermore, it is clear that characterizing noncoding cis-regulatory sequences is essential for understanding species-specific differences and similarities in expression (1). For both scientific and practical reasons, the chick embryo comes forward as a powerful model system that can be used for posttranscriptomic analysis of gene function and regulation.
The chick embryo is historically one of the first experimental embryos used in developmental biology research. Scientifically, the research results obtained from this embryo are among the most important and most numerous in this field (2,3). In addition, because of its economic importance, much effort has been directed toward sequencing, assembly, and mapping of the chicken genome. The haploid chicken genome contains 1.2×109 bp (4). The diploid karyotype is distributed in 9 pairs of large chromosomes and 30 pairs of microchromosomes (5). This genome has been extensively characterized by linkage mapping, and expression sequence tag resources abound (2,6,7). Comparative mapping analysis of the chicken, human and mouse genomes revealed a high degree of synteny conservation between chickens and mammals (8), and that the orthologous gene expression profile between chickens and other vertebrates is similar (9). Finally, the number of identified pseudogenes in chickens (96) is significantly less than in humans (1069) or mice (1351) (10).
The breakthrough in chick episomal gene misexpression came in 1997 with the introduction of in ovo electroporation (11). Briefly, in ovo electroporation uses a pulsed electric field to transiently permeabilize the plasma membrane, driving DNA entry toward the positively charged pole. Adjusting the values of the voltage applied and delivering the current as square wave pulses results in maximal delivery of DNA with minimal cell death. Although viral infection is the method of choice for transgenesis (12,13), electroporation provides the strongest gene expression with no limit for the maximally transfectable amount (11). Also, because the electrodes can be positioned at various sites on the embryo, the number of tissues that can be targeted by electroporation is higher than with viral infection or liposomal transfection. Moreover, the expression of transgenes through electroporation occurs much faster than with viral infection, which is a significant advantage for analysis of early phenotypes (14). Finally, as a reflection of its versatility, electroporation is being used in an increasing number of animal models, including zebrafish and mice (15,16).
Although the process of electroporation itself is relatively simple and has been described previously (17-19), its setup is still daunting to those who are unfamiliar with the chick embryo or with the basic procedures used to construct electrical devices. Moreover, most previous articles recommend the purchase of commercially available electroporators, which can be a considerable investment for the laboratory that is considering the chick embryo for in vivo gene function analysis.
We describe here in detail the steps required to build a low-cost, working electroporation station using common electrical parts and laboratory equipment. The electroporator described here was made by one of us (J.T. Vidal) following the electrical diagram presented.
Material and Methods
Electroporator (electric square pulse generator)
The electroporator is a device capable of generating square pulses of electric current. Our electroporator was built according to the electric diagram shown in Figure 1. For convenience, our current switch was activated through a pedal connected to an external trigger plug. This pedal allows the hands to be free during the electroporation process. Pedal sources and their pricing vary. Suggested items are the Harvard Apparatus injection foot switch (part Nos. 450211 or 450214, Harvard Apparatus Company, USA), a simple electric bass/amplifier foot switch, or the low-cost alternative adopted in the lab - a sewing machine foot switch.
Figure 1. Electrical diagram for an in ovo electroporator.
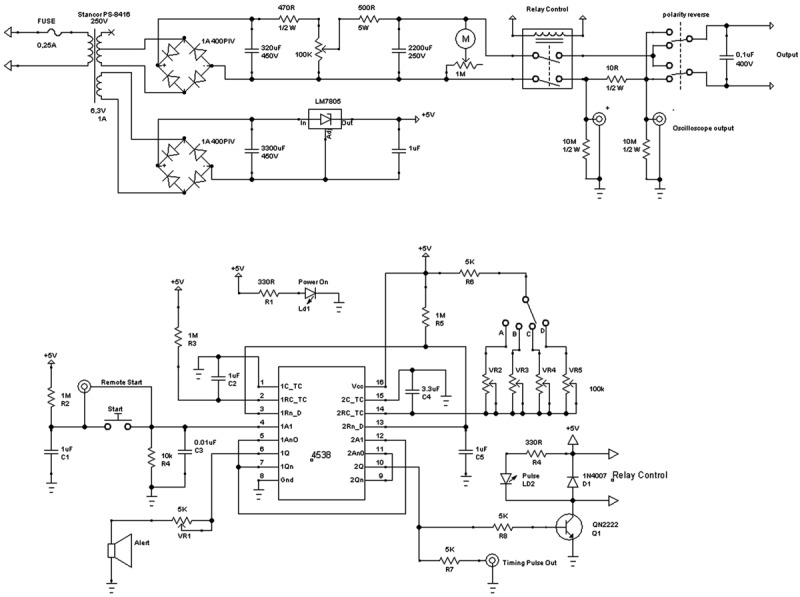
The electroporator diagram presented here allows variation of pulse voltage (V), pulse duration time (L), interval time between pulses (I), and number of pulses (Figure 2A). All of these parameters have to be optimized for different target tissues. There is already a plethora of publications indicating the parameters for various targets (17-22). The electroporator that we used in the experiments demonstrated here has preset pulse duration times and interval times, but an alternative model can be made where these values are set by the user.
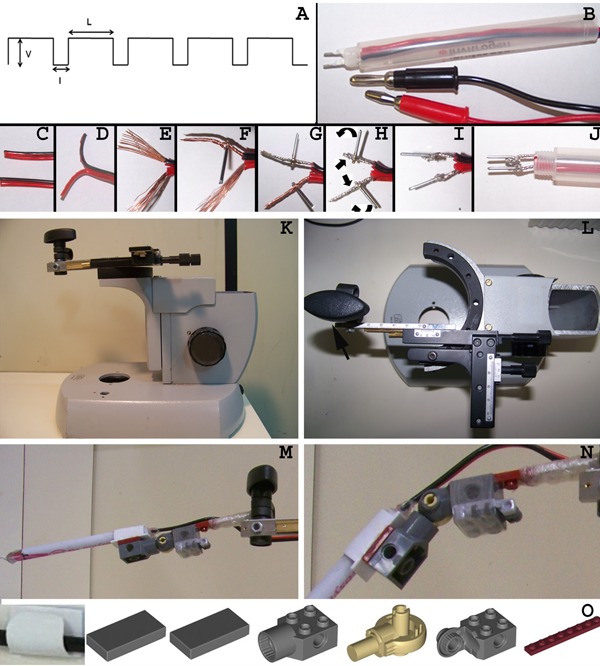
Figure 2. Description of electrode and manipulator building process. A, Electroporator pulse variable parameters: V: pulse voltage value; I: interval between pulses; L: pulse length. B, Completed electrode with the platinum poles and the connector plugs. C, Both ends of the copper speaker cables must have a clean cut. D, Separation of the positive and negative cables at a single end. E, The positive and negative cables were stripped for 1 cm revealing the naked copper wire. F, Insertion of a platinum rod into the midsection of the negative pole's twisted copper wire. G, Insertion of the second platinum rod into the positive pole. H, Both copper wires were further twisted and bent over to secure the platinum rods and to increase contact surface. I, The contact between the platinum rods and copper wires was further stabilized by adding a drop of solder to the interface. J, The wires were threaded through an empty ball point pen cartridge, which acts as the solid outer shell of the electrode. K, Lateral view of the microscope base with the modified slide holder mechanical stage attached at the top. L, Top view of the adapted microscope base with emphasis on the mechanical stage. Note that the slide holder attachment was removed and substituted for a screw (arrow) that secures the arm of the LEGO electrode holder (M). N, Detailed view of the electrode holder hinge. O, Components that comprise the electrode holder hinge. The LEGO parts can be acquired separately through the LEGO website or are included in the 4507 set. The wire holder (available through 3M, Brazil) was glued to the top of the flat pieces and the electrode outer shell was inserted in the wire holder.
Electrodes
We manufactured our own electrodes with platinum wires shaped to fit the target tissue using the following components: one bicolor copper cable for speakers (or other flexible electronic cable), 0.5 mm2 cross-section×1800 mm length (or any workable length to connect the electroporator to the embryo, which is placed under the dissecting microscope); two banana-type plugs to be inserted at the connection with the electroporator (black for negative pole and red for positive pole) - there is a diversity of banana-type plugs and diameter sets available, and the only restriction is that the diameter of the male plug has to fit the electroporator socket snugly (female plug); two platinum wires, 0.5 mm diameter×10 mm length each, for the short electrode and 0.5 mm diameter×15 mm for the long electrode; one empty pen body; soldering iron and solder; sharp pliers for stripping the cables; screwdriver for banana-plug screws; hot glue gun and hot glue cartridge.
A 1-cm long piece of insulation was stripped from each end of both copper speaker cables (Figure 2C-E). The platinum wire was placed perpendicular to end of one of the copper cables (Figure 2F), and the junction was soldered (Figure 2I). Both red and black soldered cables and platinum wire were inserted through an empty pen body, leaving a 2-mm stretch protruding from the pen body (Figure 2J). This allows visualization of the polarity and correct positioning of the electrodes when electroporating. Both platinum wires were fixed in this position by applying hot glue from the pen body base up to the platinum/speaker cable junctions (Figure 2B). At the other end of the pen body, the banana plugs were soldered to their color-matched copper cables. Alternatively, the platinum electrodes can be constructed solder-free, with epoxy resin (15).
Other materials can be used for the electrodes. For example, tungsten wire is often used for microelectroporations (21-23). The main advantage of platinum over tungsten is its stability over time. Platinum will not oxidize with repeated use. On the other hand, tungsten wires can be sharpened by electrolysis. The sharp point can be used for microelectroporation, i.e., fine spatial definition of target tissue (23).
To sharpen tungsten electrodes, we used a 12-V AC/DC converter and soldered one of the poles to a copper rod of approximately 5 cm in length and 2 mm in diameter. To the other wire, we soldered an electric clamp to hold the tungsten needle during the electrolysis procedure. After inserting the needle in the clamp, we immersed the copper rod in 5 M NaOH. Thereafter, we briefly dipped the tungsten needle tip into the sodium hydroxide to erode the tungsten needle. The appearance of tiny bubbles at the tip signaled successful electrolysis. The shape of the tip was monitored under a dissecting scope. The resulting sharpened tip was extensively washed prior to use.
Micromanipulator
In some cases, such as electroporation of the neural tube, the whole procedure can be performed freehand. However, in other cases (e.g., lens placode), the injection of the plasmid and the triggering of the pulses have to be done simultaneously. Then, an electrode holder is necessary to maintain electrode stability and to free the hands. There are many commercially available micromanipulators that can be used (e.g., Narishige, Japan; Sutter Instrument, USA; Harvard Apparatus Company).
We used a homemade, modified and recycled microscope base with its mechanical stage translational control device attached (Figure 2K and L). The microscope base and arm contained the former focus knob and provided the vertical movement necessary to position the electrode. The x-y movement of the electrode was controlled by the translational control knobs. A holder with a screw [Figure 2K and L (arrow)] was attached to the extremity of the mechanical stage to secure the electrode holder. Alternatively, a simpler micromanipulator can be made with a burette stand (Supplementary Figure S1). This latter setup also allows gross adjustments in the z- and x-axes.
Electrode holder
A holder facilitates adjustment of the angle of the electrode when it approaches the embryo and minimizes fatigue during a long electroporation procedure. We have also manufactured an alternative electrode holder made with LEGO¯ articulated pieces (LEGO, Denmark; Figure 2M-O). These articulated joints permit variations of the angle between electrode and target tissue. The final setup was positioned under the dissecting microscope (Supplementary Figure S1).
Electroporation procedure
Plasmids for microinjection were purified by columns (Qiagen, USA, or Invitrogen, USA) and resuspended in molecular biology grade water at concentrations ranging from 2 to 4 mg/mL in a 0.05% Fast Green solution. The Fast Green aids the visualization of the solution during injection. This was then aspirated into glass needles made from pulled glass capillary tubes. A popular source for capillary glass is A-M Systems (Cat. #626000; USA). Heparin-free glass capillary tubes for microhematocrit assays (1.1 mm internal diameter/1.5 mm external diameter/75 mm length) from a local biomedical supplier work as well as those from A-M Systems.
The key to the injection process is the shape of the pulled capillary needle. The neck of the needle must taper slowly to a long thin needle (Supplementary Figure S1). This shape allows access to the embryonic tissues with minimal damage. And, if the needle tip needs to be broken repeatedly due to clogging, the long thin shape minimizes variations of bore size. This shape is better attained on a microelectrode puller but can also be made over a Bunsen burner after some practice.
DNA injection was done by mouth pipetting. The glass needle was inserted into an aspirator tube (A5177; Sigma, USA) and the mouthpiece was plugged with cotton to minimize contamination of the embryo. The DNA was injected in ovo into previously contrasted embryos (18) (Supplementary Figure S2).
Results and Discussion
To express enhanced green fluorescence protein (eGFP) in the neural tube and neural crest cells, we used plasmid pMES, a bicistronic plasmid containing an internal ribosomal entry site that allows expression of the gene of interest together with eGFP (20). pMES was injected into the neural tube lumen of HH10 embryos. Immediately after injection of the DNA, we added 1 mL Ringer's saline to hydrate the embryo and lowered the electrodes onto the amniotic membrane that covers the embryo, aligning them to the injected neural tube with the positive pole on the side to be electroporated. The ideal shape for the electrode in this case was a double “L”, where the whole lower leg of the “L” contacted the extraembryonic membrane at the sides of the embryo. This shape ensures that a broad segment of the neural tube is electroporated. The distance between the two electrodes was 4 mm. Thereafter, we triggered a series of electric pulses (4 pulses at 18 V with 30 ms duration and intervals of 500 ms). We added 2-3 mL more of Ringer's solution, sealed the eggs with packing tape, and reincubated them in an incubator at 37.7°C with 50-55% humidity for 2 days. The resulting neural tube expressed cytoplasmic GFP unilaterally, corresponding to the side where the positive electrode was positioned (Figure 3A). Fluorescent cells in the neural tube and dorsal root ganglion cells indicated successful targeting of neural tube and neural crest cells. No signal was observed in other embryonic tissues. Alternatively, the electroporation can also be performed in slightly older embryos (HH12) with different parameters (5 pulses at 20 V with 50 ms duration and intervals of 100 ms).
Figure 3. Electroporation results. A, Cross-section view of an electroporated neural tube after cryosections and immunofluorescence detection of enhanced green fluorescent protein (eGFP). The electroporated GFP-positive cells are restricted to the right half of the neural tube and the right dorsal root ganglion (DRG). GFP can also be detected in the commissural neurites that cross to the contralateral neural tube and in the motor neuron projections. Dorsal is up. B, Neural crest cells electroporated with a plasmid encoding the fusion protein Tomato-2A-H2GFP, which is cleaved intracellularly to label the cell membrane with red fluorescence and the cell nucleus with green fluorescence. C, Fluorescence microscope view of a live embryo (embryonic day 3) with the electroporated right lens placode expressing GFP. D, Visualization of GFP (green) and actin (red) in the lens placode after processing cryosections for immunofluorescence with anti-GFP antibody (Abcam, UK) followed by exposure to rhodamine-labeled phalloidin.
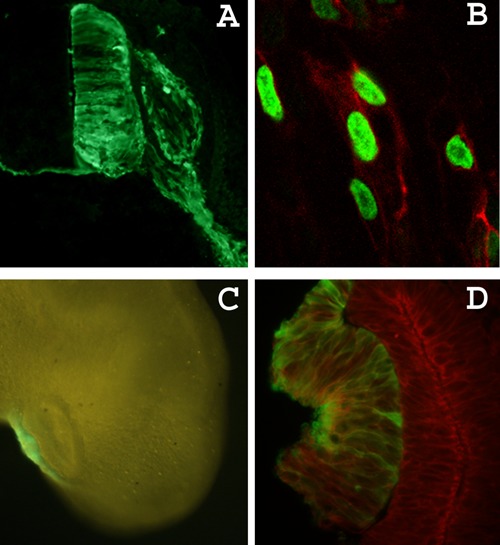
Most of the regular eukaryotic expression vectors driven by a cytomegalovirus or simian virus 40 promoter will induce robust expression. Here, with the same procedure described earlier, we labeled neural crest cell nuclei and cell membranes after electroporation with pCS2-TdTomato-2A-H2bGFP (Figure 3B). This expression vector encodes for a single fusion protein that generates a membrane-targeted myristoylated red fluorescent protein and a nuclear-accumulated histone-GFP fusion protein (24) (Figure 3B).
One can also change the configuration of the electrodes according to the target-tissue anatomy and localization. For instance, when the target was the preplacodal lens ectoderm, we used straight platinum electrodes placed perpendicularly to the underlying optic vesicle and externally to the embryo. The plasmid was injected externally, close to the preplacodal ectoderm of HH9-10 embryos. As the plasmid solution formed a cloud over the ectoderm, we triggered the electric current concomitantly with the injection (5 pulses at 9 V with 50 ms duration and 100-ms intervals). This procedure provided specific labeling of the lens placode, which could be visualized directly in whole mount embryos (Figure 3C) or indirectly by immunofluorescence in cryosections (Figure 3D).
In summary, these results show that introducing embryonic electroporation and procedures in a laboratory can be a low-cost setup that requires minimal effort with maximal results.
Supplementary material
Acknowledgments
The authors would like to thank Drs. Shankar Srinivas (Oxford University) and Cathy Krull (University of Michigan Medical School) for sharing the plasmids pCS2-TdTomato-2A-H2bGFP and pMES, respectively. Research partially supported by FAPESP and CNPq.
Footnotes
First published online September 16, 2013.
References
- 1.Lelli KM, Slattery M, Mann RS. Disentangling the many layers of eukaryotic transcriptional regulation. Annu Rev Genet. 2012;46:43–68. doi: 10.1146/annurev-genet-110711-155437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern CD. The chick embryo - past, present and future as a model system in developmental biology. Mech Dev. 2004;121:1011–1013. doi: 10.1016/j.mod.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Stern CD. The chick; a great model system becomes even greater. Dev Cell. 2005;8:9–17. doi: 10.1016/j.devcel.2004.11.018. [DOI] [PubMed] [Google Scholar]
- 4.Brown WR, Hubbard SJ, Tickle C, Wilson SA. The chicken as a model for large-scale analysis of vertebrate gene function. Nat Rev Genet. 2003;4:87–98. doi: 10.1038/nrg998. [DOI] [PubMed] [Google Scholar]
- 5.Burt DW. The chicken genome and the developmental biologist. Mech Dev. 2004;121:1129–1135. doi: 10.1016/j.mod.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 6.Groenen MA, Cheng HH, Bumstead N, Benkel BF, Briles WE, Burke T, et al. A consensus linkage map of the chicken genome. Genome Res. 2000;10:137–147. doi: 10.1101/gr.10.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallis JW, Aerts J, Groenen MA, Crooijmans RP, Layman D, Graves TA, et al. A physical map of the chicken genome. Nature. 2004;432:761–764. doi: 10.1038/nature03030. [DOI] [PubMed] [Google Scholar]
- 8.Burt DW, Bruley C, Dunn IC, Jones CT, Ramage A, Law AS, et al. The dynamics of chromosome evolution in birds and mammals. Nature. 1999;402:411–413. doi: 10.1038/46555. [DOI] [PubMed] [Google Scholar]
- 9.Nie H, Crooijmans RP, Lammers A, van Schothorst EM, Keijer J, Neerincx PB, et al. Gene expression in chicken reveals correlation with structural genomic features and conserved patterns of transcription in the terrestrial vertebrates. PLoS One. 2010;5:e11990. doi: 10.1371/journal.pone.0011990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burt DW. Emergence of the chicken as a model organism: implications for agriculture and biology. Poult Sci. 2007;86:1460–1471. doi: 10.1093/ps/86.7.1460. [DOI] [PubMed] [Google Scholar]
- 11.Muramatsu T, Mizutani Y, Ohmori Y, Okumura J. Comparison of three nonviral transfection methods for foreign gene expression in early chicken embryos in ovo . Biochem Biophys Res Commun. 1997;230:376–380. doi: 10.1006/bbrc.1996.5882. [DOI] [PubMed] [Google Scholar]
- 12.McGrew MJ, Sherman A, Ellard FM, Lillico SG, Gilhooley HJ, Kingsman AJ, et al. Efficient production of germline transgenic chickens using lentiviral vectors. EMBO Rep. 2004;5:728–733. doi: 10.1038/sj.embor.7400171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishii Y, Reese DE, Mikawa T. Somatic transgenesis using retroviral vectors in the chicken embryo. Dev Dyn. 2004;229:630–642. doi: 10.1002/dvdy.10484. [DOI] [PubMed] [Google Scholar]
- 14.Ogura T. In vivo electroporation: a new frontier for gene delivery and embryology. Differentiation. 2002;70:163–171. doi: 10.1046/j.1432-0436.2002.700406.x. [DOI] [PubMed] [Google Scholar]
- 15.Hoegler KJ, Horne JH. Targeting the zebrafish optic tectum using in vivo electroporation. Cold Spring Harb Protoc. 2010;2010:pdb.prot5463. doi: 10.1101/pdb.prot5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calegari F, Marzesco AM, Kittler R, Buchholz F, Huttner WB. Tissue-specific RNA interference in post-implantation mouse embryos using directional electroporation and whole embryo culture. Differentiation. 2004;72:92–102. doi: 10.1111/j.1432-0436.2004.07202002.x. [DOI] [PubMed] [Google Scholar]
- 17.Croteau LP, Kania A. Optimisation of in ovo electroporation of the chick neural tube. J Neurosci Methods. 2011;201:381–384. doi: 10.1016/j.jneumeth.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 18.Sauka-Spengler T, Barembaum M. Gain- and loss-of-function approaches in the chick embryo. Methods Cell Biol. 2008;87:237–256. doi: 10.1016/S0091-679X(08)00212-4. [DOI] [PubMed] [Google Scholar]
- 19.Krull CE. A primer on using in ovo electroporation to analyze gene function. Dev Dyn. 2004;229:433–439. doi: 10.1002/dvdy.10473. [DOI] [PubMed] [Google Scholar]
- 20.Swartz M, Eberhart J, Mastick GS, Krull CE. Sparking new frontiers: using in vivo electroporation for genetic manipulations. Dev Biol. 2001;233:13–21. doi: 10.1006/dbio.2001.0181. [DOI] [PubMed] [Google Scholar]
- 21.Scaal M, Gros J, Lesbros C, Marcelle C. In ovo electroporation of avian somites. Dev Dyn. 2004;229:643–650. doi: 10.1002/dvdy.10433. [DOI] [PubMed] [Google Scholar]
- 22.Yasuda K, Momose T, Takahashi Y. Applications of microelectroporation for studies of chick embryogenesis. Dev Growth Differ. 2000;42:203–206. doi: 10.1046/j.1440-169x.2000.00502.x. [DOI] [PubMed] [Google Scholar]
- 23.Momose T, Tonegawa A, Takeuchi J, Ogawa H, Umesono K, Yasuda K. Efficient targeting of gene expression in chick embryos by microelectroporation. Dev Growth Differ. 1999;41:335–344. doi: 10.1046/j.1440-169X.1999.413437.x. [DOI] [PubMed] [Google Scholar]
- 24.Trichas G, Begbie J, Srinivas S. Use of the viral 2A peptide for bicistronic expression in transgenic mice. BMC Biol. 2008;6:40. doi: 10.1186/1741-7007-6-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]


